Deformed Mediated Larval Incisor Lobe Development Causes Differing Feeding Behavior between Oriental Armyworm and Fall Armyworm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
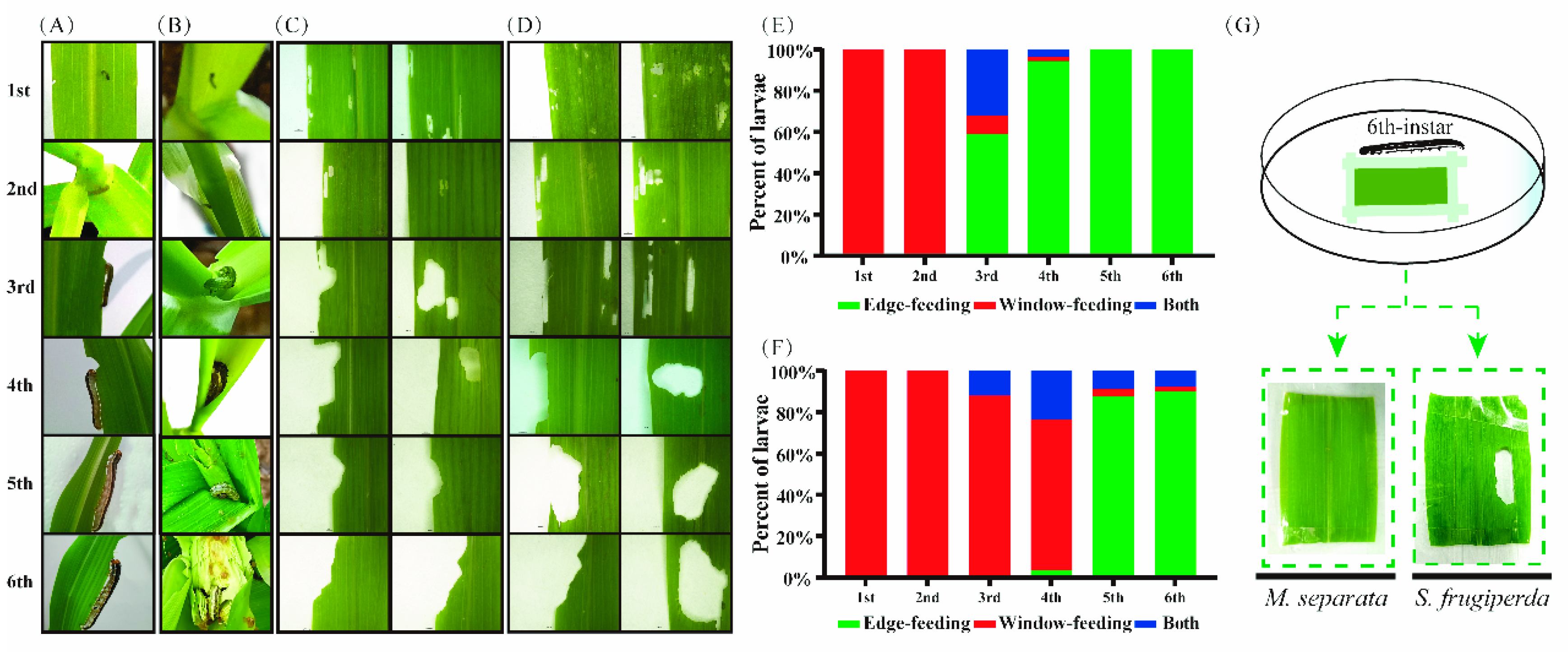
2.2. Feeding Behavior of M. separata and S. frugiperda Larvae
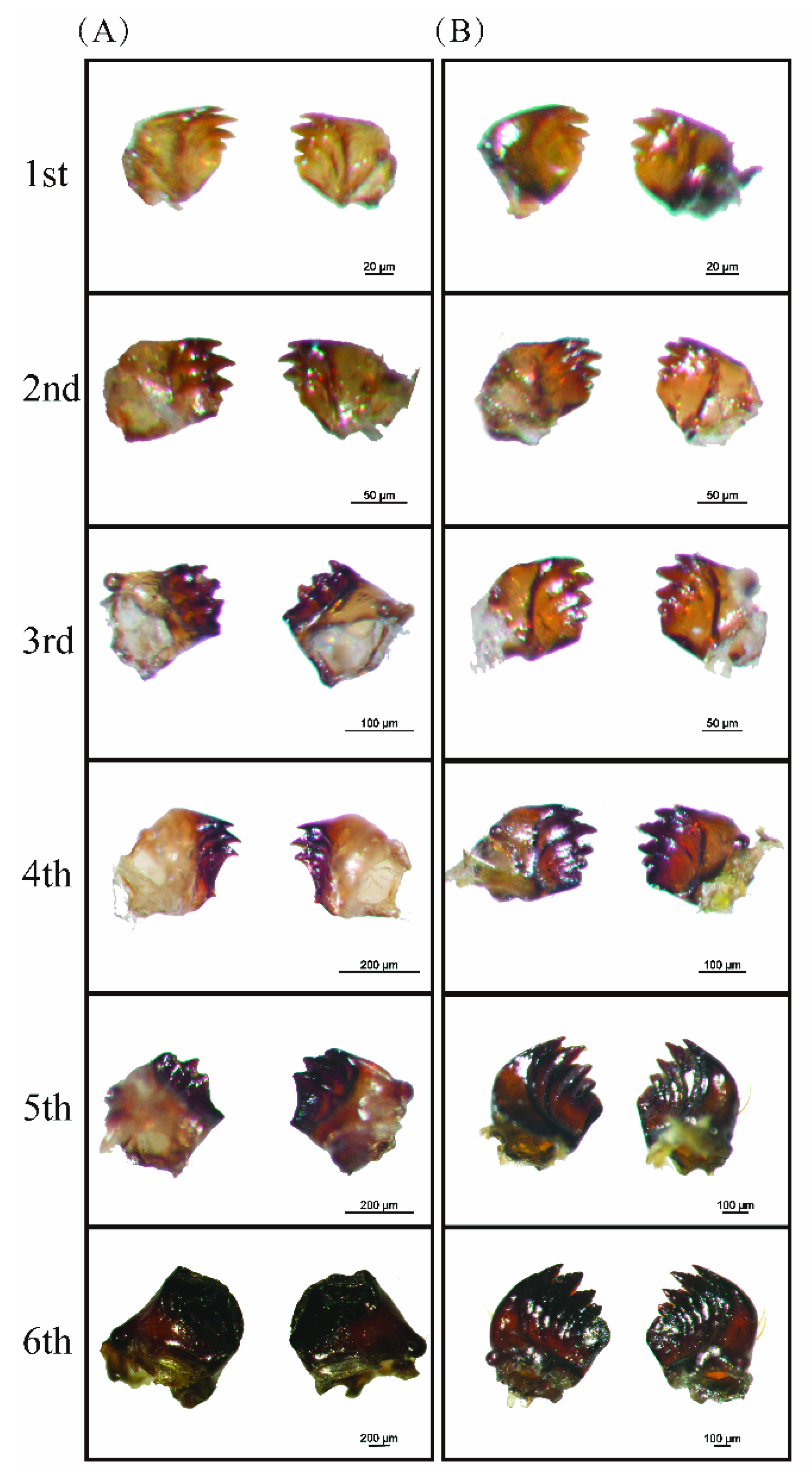
2.3. Morphology of Larval Mandibles
2.4. Characterizations of Dfd from M. feparata and S. frugiperda
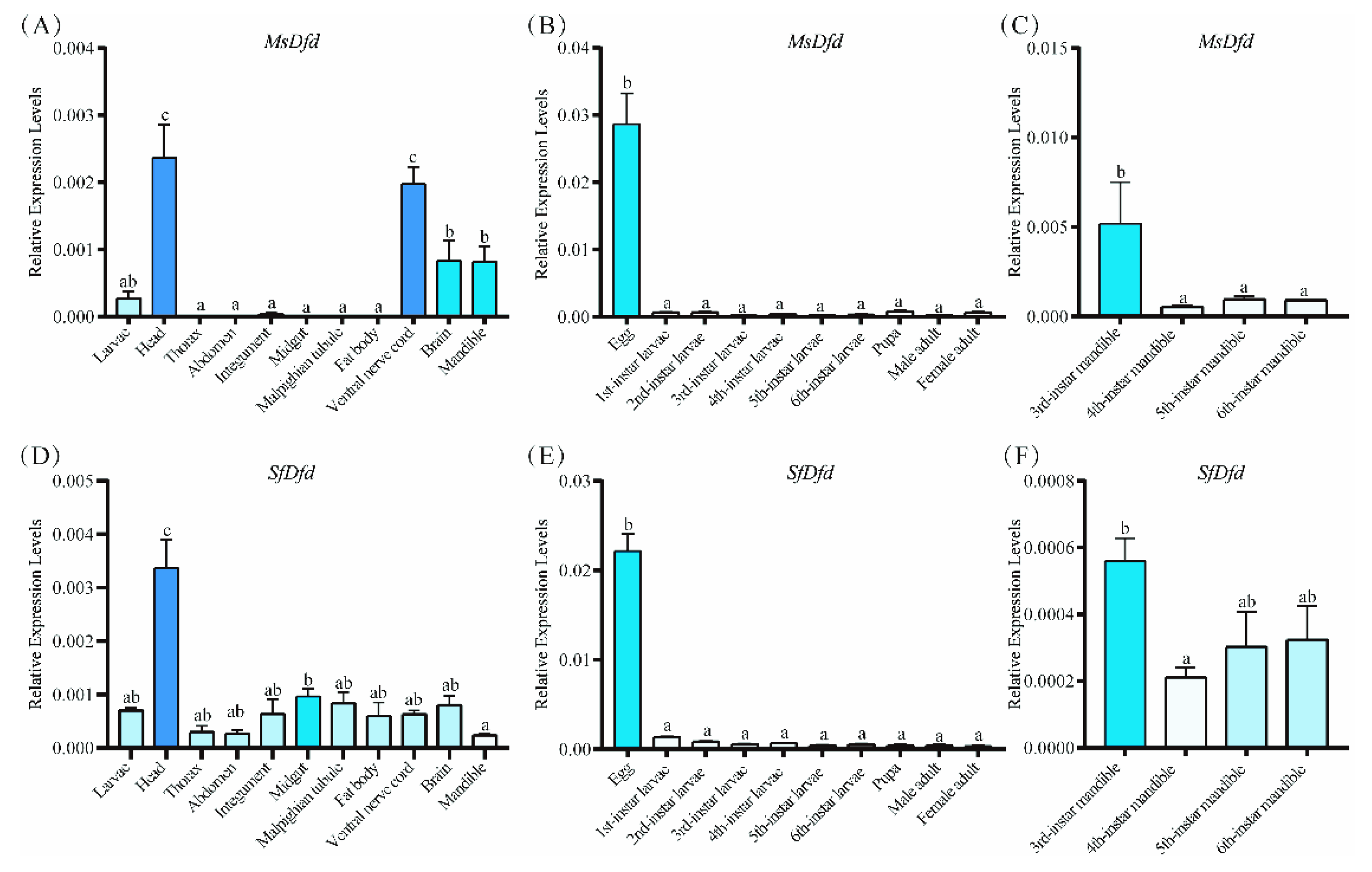
2.5. Spatiotemporal Expression Patterns of MsDfd and SfDfd
2.6. Function of Dfd in M. separata and S. frugiperda
2.7. Data Analysis
3. Results
3.1. Different Feeding Behavior between M. separata and S. frugiperda Larvae
3.2. Morphological Differences between M. separata and S. frugiperda Larval Mandibles
3.3. Characterizations and Expressions of MsDfd and SfDfd
3.4. Functions of Dfds in Mandible Development and Larval Feeding
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malook, S.; Xu, Y.X.; Qi, J.F.; Li, J.; Wang, L.; Wu, J.Q. Mythimna separata herbivory primes maize resistance in systemic leaves. J. Exp. Bot. 2021, 72, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.A.; Pellegaud, J.G.; Nava-Camberos, U.; Lugo-Barrera., D.; Vega-Aquino., P.; Coello., J.; Terán-Vargas., A.P.; Vargas-Camplis., J. Maize pests in Mexico and challenges for the adoption of integrated pest management programs. J. Integ. Pest Mngmt. 2014, 5, E1–E9. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; He, L.M.; Shen, X.J.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Estimation of the potential infestation area of newly-invaded fall armyworm Spodoptera frugiperda in the Yangtze River valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Qi, M.G.; Chi, Y.C.; Wuriyanghan, H. De novo assembly of the transcriptome for oriental armyworm Mythimna separata (Lepidoptera: Noctuidae) and analysis on insecticide resistance-related genes. J. Insect Sci. 2016, 16, 92. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 3710. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef]
- Niveyro, S.; Benítez, H.A. Interspecific larvae competence and mandible shape disparity in cutworm pest complex (Lepidoptera: Noctuidae). Zool. Anz. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Song, Y.F.; Yang, X.M.; Zhang, H.W.; Zhang, D.D.; He, W.; Wyckhuys, K.A.G.; Wu, K.M. Interference competition and predation between invasive and native herbivores in maize. J. Pest Sci. 2021, 94, 1053–1063. [Google Scholar] [CrossRef]
- Oliveira, C.; Orozco-Restrepo, S.M.; Alves, A.C.; Pinto, B.S.; Miranda, M.S.; Barbosa, M.H.; Picanço, M.C.; Pereira, E.J. Seed treatment for managing fall armyworm as defoliator and cutworm on maize: Plant protection, residuality, and the insect life history. Pest Manag. Sci. 2022, 78, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Keathley, C.P.; Potter, D.A. Behavioral plasticity of a grass-feeding caterpillar in response to spiny-or smooth-edged leaf blades. Arthropod-Plant Inte. 2011, 5, 339–349. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Hippodamia variegata (Coleoptera: Coccinellidae), with reference to their feeding mechanism. Zoomorphology 2019, 139, 199–212. [Google Scholar] [CrossRef]
- Heffer, A.; Pick, L. Conservation and variation in Hox genes: How insect models pioneered the evo-devo field. Annu. Rev. Entomol. 2013, 58, 161–179. [Google Scholar] [CrossRef]
- Percival-Smith, A. Non-specificity of transcription factor function in Drosophila melanogaster. Dev. Genes Evol. 2017, 227, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.L.; Kaufman, T.C. RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: Novel roles for Hox genes in the Hemipteran head. Development 2000, 127, 3683–3694. [Google Scholar] [CrossRef]
- Sorge, S.; Ha, N.; Polychronidou, M.; Friedrich, J.; Bezdan, D.; Kaspar, P.; Schaefer, M.H.; Ossowski, S.; Henz, S.R.; Mundorf, J.; et al. The cis-regulatory code of Hox function in Drosophila. EMBO J. 2012, 31, 3323–3333. [Google Scholar] [CrossRef]
- Kumar, R.; Chotaliya, M.; Vuppala, S.; Auradkar, A.; Palasamudrum, K.; Joshi, R. Role of Homothorax in region specific regulation of Deformed in embryonic neuroblasts. Mech. Develop. 2015, 138, 190–197. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Gopinathan, K.P. Comparative analysis of the development of the mandibular salivary glands and the labial silk glands in the mulberry silkworm, Bombyx mori. Gene Expr. Patterns 2005, 5, 323–339. [Google Scholar] [CrossRef]
- Coulcher, J.F.; Telford, M.J. Cap’n’collar differentiates the mandible from the maxilla in the beetle Tribolium castaneum. EvoDevo 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Dearden, P.K.; Wilson, M.J.; Sablan, L.; Osborne, P.W.; Havler, M.; McNaughton, E.; Kimura, K.; Milshina, N.V.; Hasselmann, M.; Gempe, T.; et al. Patterns of conservation and change in honey bee developmental genes. Genome Res. 2006, 16, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Sugime, Y.; Oguchi, K.; Gotoh, H.; Hayashi, Y.; Matsunami, M.; Shigenobu, S.; Koshikawa, S.; Miura, T. Termite soldier mandibles are elongated by dachshund under hormonal and Hox gene controls. Development 2019, 146, dev171942. [Google Scholar] [CrossRef]
- Lohmann, I.; McGinnis, N.; Bodmer, M.; McGinnis, W. The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell 2002, 110, 457–466. [Google Scholar] [CrossRef]
- Yin, X.; Yang, G.F.; Niu, D.B.; Chen, J.; Liao, M.; Cao, H.Q.; Sheng, C.W. Identification and pharmacological characterization of histamine-gated chloride channels in the fall armyworm, Spodoptera frugiperda, Insect Biochem. Mol. Biol. 2022, 140, 103698. [Google Scholar]
- Huang, Y.; Shi, S.; Wu, H.L.; Yue, S.L.; Liao, M.; Cao, H.Q. CYP4G7 and CYP4G14 mediate cuticle formation and pigmentation in Tribolium confusum. J. Stored Prod. Res. 2021, 94, 101881. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, H.Q.; Zhu, Z.; Jiang, X.C.; Li, X.X.; Dong, Y.C.; Sheng, C.W.; Liao, M.; Cao, H.Q. Expression and functional analysis of cytochrome P450 genes in the integument of the oriental armyworm, Mythimna separata (Walker). Pest Manag. Sci. 2021, 77, 577–587. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Yoshida, K.; Murakami, M. Larval morphology and feeding behavior in Notodontidae (Lepidoptera) in relation to leaf toughness of host plants. Eurasian J. For. Res. 2012, 15, 45–52. [Google Scholar]
- Dolinskaya, I.V. Taxonomic variation in larval mandibular structure in Palaearctic Notodontidae (Noctuoidea). Nota. Lipid 2008, 31, 165–177. [Google Scholar]
- Magruga, J.; Spechi, A.; Salik, L.M.G.; Casagrande, M.M. The external morphology of Mythimna (Pseudaletia) sequax (Lepidoptera: Noctuidae). Neotrop. Entomol. 2019, 48, 834–852. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Sorge, S.; Bujupi, F.; Eichenlaub, M.P.; Schulz, N.G.; Wittbrodt, J.; Lohmann, I. Hox function is required for the development and maintenance of the Drosophila feeding motor unit. Cell Reports 2016, 14, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Tiberghien, M.A.; Lebreton, G.; Cribbs, D.; Benassayag, C.; Suzanne, M. The Hox gene Dfd controls organogenesis by shaping territorial border through regulation of basal DE-Cadherin distribution. Dev. Biol. 2015, 405, 183–188. [Google Scholar] [CrossRef]
- Regulski, M.; McGinnis, N.; Chadwick, R.; McGinnis, W. Developmental and molecular analysis of Deformed; a homeotic gene controlling Drosophila head development. EMBO J. 1987, 6, 767–777. [Google Scholar] [CrossRef]
- Merrill, V.; Turner, F.R.; Kaufman, T.C. A genetic and developmental analysis of mutations in the Deformed locus in Drosophila melanogaster. Dev. Biol. 1987, 122, 379–395. [Google Scholar] [CrossRef]
- Abzhanov, A.; Kaufman, T.C. Homeotic genes and the arthropod head: Expression patterns of the labial, proboscipedia, and deformed genes in crustaceans and insects. Proc. Natl. Acad. Sci. USA 1999, 96, 10224–10229. [Google Scholar] [CrossRef]
- Toga, K.; Saiki, R.; Maekawa, K. Hox gene Deformed is likely involved in mandibular regression during presoldier differentiation in the nasute termite Nasutitermes takasagoensis. J. Exp. Zool. Part B 2013, 320, 385–392. [Google Scholar] [CrossRef]
- Hughes, C.L.; Kaufman, T.C. Hox genes and the evolution of the arthropod body plan. Evol. Dev. 2002, 4, 459–499. [Google Scholar] [CrossRef]
- Hou, S.H.; Tao, C.C.; Yang, H.G.; Cheng, T.C.; Liu, C. Sage controls silk gland development by regulating Dfd in Bombyx mori. Insect Biochem. Mol. Biol. 2021, 132, 103568. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhu, Z.; Xing, G.; Li, Y.; Zhou, X.; Wang, J.; Li, G.; Cao, H.; Huang, Y. Deformed Mediated Larval Incisor Lobe Development Causes Differing Feeding Behavior between Oriental Armyworm and Fall Armyworm. Insects 2022, 13, 594. https://doi.org/10.3390/insects13070594
Zhao H, Zhu Z, Xing G, Li Y, Zhou X, Wang J, Li G, Cao H, Huang Y. Deformed Mediated Larval Incisor Lobe Development Causes Differing Feeding Behavior between Oriental Armyworm and Fall Armyworm. Insects. 2022; 13(7):594. https://doi.org/10.3390/insects13070594
Chicago/Turabian StyleZhao, Hailong, Zeng Zhu, Gaoliang Xing, Yiyu Li, Xue Zhou, Jingjing Wang, Guiting Li, Haiqun Cao, and Yong Huang. 2022. "Deformed Mediated Larval Incisor Lobe Development Causes Differing Feeding Behavior between Oriental Armyworm and Fall Armyworm" Insects 13, no. 7: 594. https://doi.org/10.3390/insects13070594
APA StyleZhao, H., Zhu, Z., Xing, G., Li, Y., Zhou, X., Wang, J., Li, G., Cao, H., & Huang, Y. (2022). Deformed Mediated Larval Incisor Lobe Development Causes Differing Feeding Behavior between Oriental Armyworm and Fall Armyworm. Insects, 13(7), 594. https://doi.org/10.3390/insects13070594





