Parasitism by the Tachinid Parasitoid Exorista japonica Leads to Suppression of Basal Metabolism and Activation of Immune Response in the Host Bombyx mori
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Parasitization of B. mori by E. japonica
2.3. Transcriptome Analysis
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.5. Reverse Transcription-Quantitative PCR (RT-qPCR) Analyses
2.6. Statistical Analysis
3. Results
3.1. Global Transcriptomic Changes in the Fat Body of B. mori after E. japonica Parasitization
3.2. Functional Annotation of DEGs and Pathway Enrichment Analysis
3.3. Down-Regulation of Genes Involved in Host Energy and Nutrient Metabolism
3.4. Regulation of Host Development-Related Genes
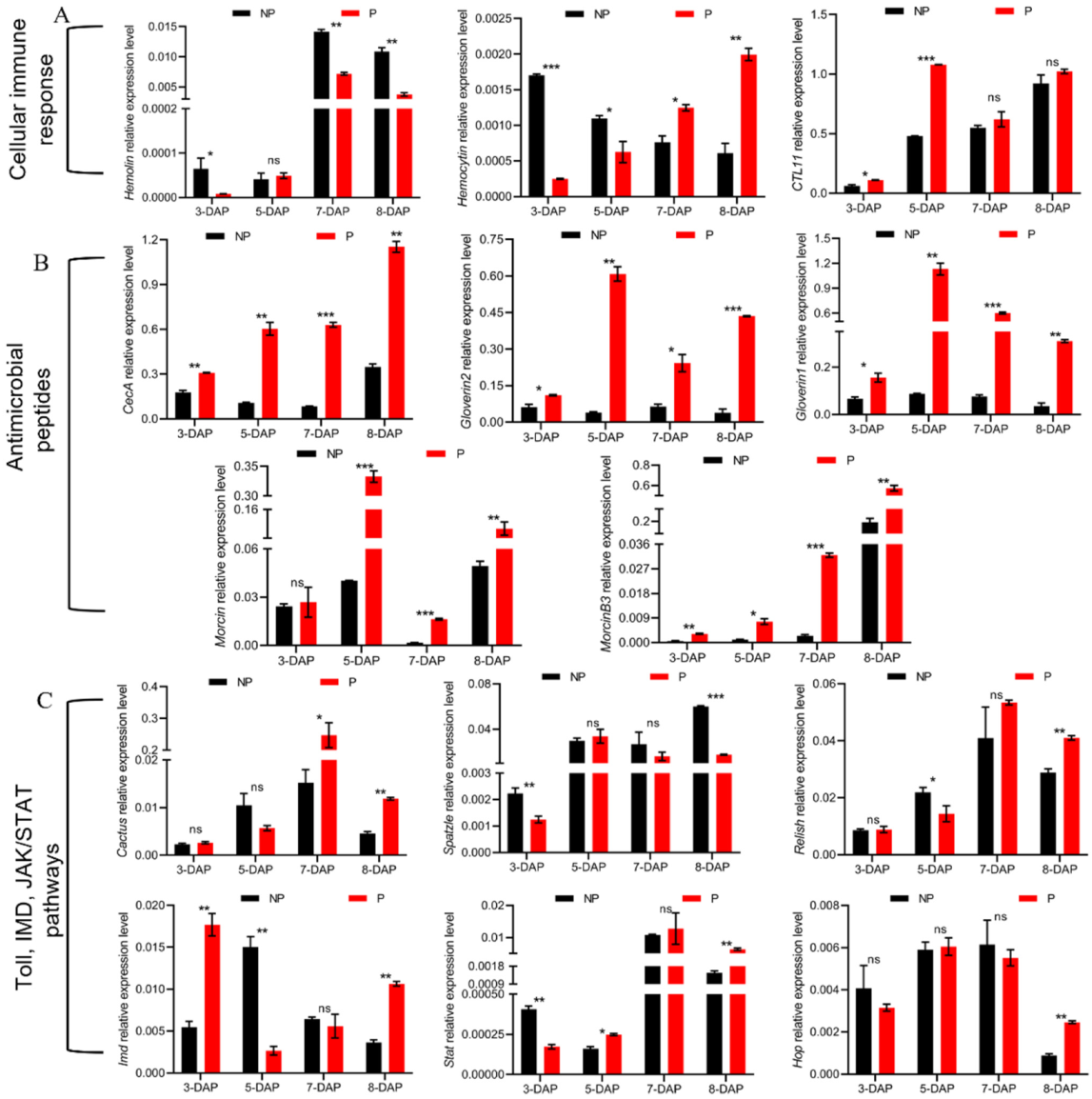
3.5. Manipulation of Host Cellular Immune Responses by E. japonica Parasitization
3.6. Induction of Humoral-Immune-Response-Related Genes
3.6.1. Melanization
3.6.2. Antimicrobial Peptides
3.6.3. Immune-Related Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Cerretti, P.; O’Hara, J.E.; Blaschke, J.D.; Moulton, J.K. Molecular phylogeny and evolution of world Tachinidae (Diptera). Mol. Phylogenet Evol. 2019, 139, 106358. [Google Scholar] [CrossRef] [PubMed]
- Stireman, J.O., III; Singer, M.S. Determinants of parasitoid–host associations: Insights from a natural tachinid–lepidopteran community. Ecology 2003, 84, 296–310. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Yang, H.; Sappington, T.W.; Cheng, Y. Biocontrol of the oriental armyworm, Mythimna separata, by the tachinid fly Exorista civilis is synergized by Cry1Ab protoxin. Sci. Rep. 2016, 6, 26873. [Google Scholar] [CrossRef]
- Cherry, A.; Cock, M.; van den Berg, H.; Kfir, R. Biological control of Helicoverpa armigera in Africa. In Biological Ontrol in IPM Systems in Africa; CAB International: Wallingford, UK, 2003; pp. 329–346. [Google Scholar]
- Broadley, H.J.; Kelly, E.A.; Elkinton, J.S.; Kula, R.R.; Boettner, G.H. Identification and impact of hyperparasitoids and predators affecting Cyzenis albicans (Tachinidae), a recently introduced biological control agent of winter moth (Operophtera brumata L.) in the northeastern USA. Biol. Control 2018, 121, 99–108. [Google Scholar] [CrossRef]
- Dindo, M.L. Tachinid parasitoids: Are they to be considered as koinobionts? BioControl 2011, 56, 249–255. [Google Scholar] [CrossRef]
- Hayakawa, Y. Inhibition of lipid transport in insects by a factor secreted by the parasite, Blepharipa sericariae. FEBS Lett. 1986, 195, 122–124. [Google Scholar] [CrossRef]
- Dai, M.L.; Ye, W.T.; Jiang, X.J.; Feng, P.; Zhu, Q.Y.; Sun, H.N.; Li, F.C.; Wei, J.; Li, B. Effect of Tachinid Parasitoid Exorista japonica on the larval development and pupation of the host silkworm Bombyx mori. Front. Physiol. 2022, 13, 824203. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, L.M.; Fang, Q.; Stanley, D.W.; Ye, G.Y. Cellular and humoral immune interactions between Drosophila and its parasitoids. Insect Sci. 2021, 28, 1208–1227. [Google Scholar] [CrossRef]
- Valigurová, A.; Michalková, V.; Koník, P.; Dindo, M.L.; Gelnar, M.; Vaňhara, J. Penetration and encapsulation of the larval endoparasitoid Exorista larvarum (Diptera: Tachinidae) in the factitious host Galleria mellonella (Lepidoptera: Pyralidae). B. Entomol. Res. 2014, 104, 203–212. [Google Scholar] [CrossRef]
- Yamashita, K.; Zhang, K.; Ichiki, R.; Nakamura, S.; Furukawa, S. Novel host immune evasion strategy of the endoparasitoid Drino inconspicuoides. B. Entomol. Res. 2019, 109, 643–648. [Google Scholar] [CrossRef]
- Makwana, P.; Dubey, H.; Pradeep, A.N.R.; Sivaprasad, V.; Ponnuvel, K.M.; Mishra, R.K. Dipteran endoparasitoid infestation actively suppressed host defense components in hemocytes of silkworm Bombyx mori for successful parasitism. Anim. Gene 2021, 22, 200118. [Google Scholar] [CrossRef]
- Pradeep, A.N.R.; Anitha, J.; Awasthi, A.K.; Babu, M.A.; Geetha, M.N.; Arun, H.K.; Chandrashekhar, S.; Rao, G.C.; Vijayaprakash, N.B. Activation of autophagic programmed cell death and innate immune gene expression reveals immuno-competence of integumental epithelium in Bombyx mori infected by a dipteran parasitoid. Cell Tissue Res. 2013, 352, 371–385. [Google Scholar] [CrossRef]
- Xu, P.Z.; Zhang, M.R.; Gao, L.; Wu, Y.C.; Qian, H.Y.; Li, G.; Xu, A.Y. Comparative proteomic analysis reveals immune competence in hemolymph of Bombyx mori pupa parasitized by silkworm maggot Exorista sorbillans. Insects 2019, 10, 413. [Google Scholar] [CrossRef]
- Stireman III, J.O.; O’Hara, J.E.; Wood, D.M. Tachinidae: Evolution, behavior, and ecology. Annu. Rev. Entomol. 2006, 51, 525–555. [Google Scholar] [CrossRef]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Molec. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Seo, B.Y.; Cho, J.; Lee, G.S.; Park, J.; Park, J. The complete mitochondrial genome of Exorista japonica (Townsend, 1909) (Diptera: Tachinidae). Mitochondrial DNA B 2019, 4, 2244–2245. [Google Scholar] [CrossRef]
- Dindo, M.L.; Nakamura, S. Oviposition strategies of tachinid parasitoids: Two Exorista species as case studies. Int. J. Insect Sci. 2018, 10, 1179543318757491. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Andersen, L.H.; Kristensen, T.N.; Loeschcke, V.; Toft, S.; Mayntz, D. Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. J. Insect Physiol. 2010, 56, 336–340. [Google Scholar] [CrossRef]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Rosales, C. Phagocytosis, a cellular immune response in insects. Invert. Surviv. J. 2011, 8, 109–131. [Google Scholar]
- Nakhleh, J.; El Moussawi, L.; Osta, M.A. The melanization response in insect immunity. Adv. Insect Physiol. 2017, 52, 83–109. [Google Scholar] [CrossRef]
- Lavine, M.; Strand, M. Insect hemocytes and their role in immunity. Insect Biochem. Molec. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Hu, Y.; Wang, Y.; Chen, Y.R.; Bryant, B.; Clem, R.J.; Schwartz, L.M.; Blissard, G.; Jiang, H. The immune signaling pathways of Manduca sexta. Insect Biochem. Molec. 2015, 62, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Woodcock, K.J.; Geissmann, F.; Trouillet, C.; Dionne, M.S. Multiple TGF-β superfamily signals modulate the adult Drosophila immune response. Curr. Biol. 2011, 21, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 2020, 62, 22–30. [Google Scholar] [CrossRef]
- Kleino, A.; Myllymäki, H.; Kallio, J.; Vanha-aho, L.M.; Oksanen, K.; Ulvila, J.; Hultmark, D.; Valanne, S.; Rämet, M. Pirk is a negative regulator of the Drosophila Imd pathway. J. Immunol. 2008, 180, 5413–5422. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to Smads. CSH Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Massagué, J.; Chen, Y.G. Controlling TGF-β signaling. Gene. Dev. 2000, 14, 627–644. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef]
- Cuny, M.A.C.; Poelman, E.H. Evolution of koinobiont parasitoid host regulation and consequences for indirect plant defence. Evol. Ecol. 2022, 36, 299–319. [Google Scholar] [CrossRef]
- Parra, J.R.P. Mass rearing of egg parasitoids for biological control programs. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Springer: Berlin/Heidelberg, Germany, 2009; pp. 267–292. [Google Scholar] [CrossRef]
- Visser, B.; Le Lann, C.; Den Blanken, F.J.; Harvey, J.A.; van Alphen, J.J.; Ellers, J. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 8677–8682. [Google Scholar] [CrossRef]
- Caccia, S.; Leonardi, M.; Casartelli, M.; Grimaldi, A.; De Eguileor, M.; Pennacchio, F.; Giordana, B. Nutrient absorption by Aphidius ervi larvae. J. Insect Physiol. 2005, 51, 1183–1192. [Google Scholar] [CrossRef]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wang, Z.; Chen, T.; Zhou, S.; Chen, J.; Pang, L.; Ye, X.; Shi, M.; Huang, J.; et al. Symbiotic bracovirus of a parasite manipulates host lipid metabolism via tachykinin signaling. PLoS Pathog. 2021, 17, e1009365. [Google Scholar] [CrossRef]
- Bajgar, A.; Kucerova, K.; Jonatova, L.; Tomcala, A.; Schneedorferova, I.; Okrouhlik, J.; Dolezal, T. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol. 2015, 13, e1002135. [Google Scholar] [CrossRef]
- Glatz, R.V.; Asgari, S.; Fau Schmidt, O.; Schmidt, O. Evolution of polydnaviruses as insect immune suppressors. Trends Microbiol. 2004, 12, 545–554. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Mulero, I.; Noga, E.J.; Meseguer, J.; García-Ayala, A.; Mulero, V. The antimicrobial peptides piscidins are stored in the granules of professional phagocytic granulocytes of fish and are delivered to the bacteria-containing phagosome upon phagocytosis. Dev. Comp. Immunol. 2008, 32, 1531–1538. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Xu, J.; Yang, W.; Li, Q.; Zhong, Y.; Cao, Y.; Yu, X.Q.; Deng, X. Regulation of antimicrobial peptide genes via insulin-like signaling pathway in the silkworm Bombyx mori. Insect Biochem. Mol. 2018, 103, 12–21. [Google Scholar] [CrossRef]
- Benassi, V.; Coustau, C.; Carton, Y. Insect immunity: A genetic factor (hrtp) is essential for antibacterial peptide expression in Drosophila after infection by parasitoid wasps. Arch. Insect Biochem. 2000, 43, 64–71. [Google Scholar] [CrossRef]
- Dostálová, A.; Rommelaere, S.; Poidevin, M.; Lemaitre, B.A.O. Thioester-containing proteins regulate the Toll pathway and play a role in Drosophila defence against microbial pathogens and parasitoid wasps. BMC Biol. 2017, 15, 79. [Google Scholar] [CrossRef]
- Schlenke, T.A.; Morales, J.; Govind, S.; Clark, A.G. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007, 3, 1486–1501. [Google Scholar] [CrossRef]
- Louradour, I.; Sharma, A.; Morin-Poulard, I.; Letourneau, M.; Vincent, A.A.O.; Crozatier, M.; Vanzo, N.A.O. Reactive oxygen species-dependent Toll/NF-κB activation in the Drosophila hematopoietic niche confers resistance to wasp parasitism. Elife 2017, 6, e25496. [Google Scholar] [CrossRef]
- Wertheim, B.; Kraaijeveld, A.R.; Schuster, E.; Blanc, E.; Hopkins, M.; Pletcher, S.D.; Strand, M.R.; Partridge, L.; Godfray, H.C.J. Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 2005, 6, R94. [Google Scholar] [CrossRef]
- Yang, H.; Kronhamn, J.; Ekström, J.O.; Korkut, G.G.; Hultmark, D. JAK/STAT signaling in Drosophila muscles controls the cellular immune response against parasitoid infection. EMBO Rep. 2015, 16, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-immunity trade-offs in Insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hultmark, D. Drosophila muscles regulate the immune response against wasp infection via carbohydrate metabolism. Sci. Rep. 2017, 7, 15713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Com. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef] [Green Version]



| Category | KEGG Term | KO ID | Input/Background Number | p Value |
|---|---|---|---|---|
| Up-regulated DEGs | Antigen processing and presentation | ko04612 | 7/24 | 1.19 × 10−5 |
| Estrogen signaling pathway | ko04915 | 6/38 | 0.001819 | |
| Isoquinoline alkaloid biosynthesis | ko00950 | 2/5 | 0.011286 | |
| MAPK signaling pathway | ko04010 | 6/60 | 0.017507 | |
| Protein processing in endoplasmic reticulum | ko04141 | 9/122 | 0.026165 | |
| Indole alkaloid biosynthesis | ko00901 | 1/1 | 0.034861 | |
| Arginine biosynthesis | ko00220 | 3/23 | 0.044288 | |
| Down-regulated DEGs | Oxidative phosphorylation | ko00190 | 34/131 | 8.86 × 10−11 |
| Phagosome | ko04145 | 15/65 | 7.89 × 10−5 | |
| Thermogenesis | ko04714 | 28/177 | 0.000146 | |
| Cardiac muscle contraction | ko04260 | 11/42 | 0.000216 | |
| Gap junction | ko04540 | 10/41 | 0.00077 | |
| Valine, leucine and isoleucine biosynthesis | ko00290 | 4/7 | 0.000961 | |
| Carbon metabolism | ko01200 | 16/144 | 0.011988 | |
| Biosynthesis of amino acids | ko01230 | 11/69 | 0.014215 | |
| Fatty acid biosynthesis | ko00061 | 5/21 | 0.018282 | |
| Pyruvate metabolism | ko00620 | 6/32 | 0.031156 | |
| Cutin, suberine and wax biosynthesis | ko00073 | 5/24 | 0.03165 | |
| Two-component system | ko02020 | 5/24 | 0.03165 | |
| Collecting duct acid secretion | ko04966 | 4/17 | 0.035501 | |
| Retrograde endocannabinoid signaling | ko04723 | 10/71 | 0.041068 |
| Category | Sequence ID | Gene Name | DEGs (log2 Value) | p Value |
|---|---|---|---|---|
| Oxidative phosphorylation | BMSK0000124 | probable DH dehydrogenase | −3.11178 | 6.97 × 10−14 |
| BMSK0000321 | DH-ubiquinone oxidoreductase subunit 8 | −4.38754 | 1.23 × 10−23 | |
| BMSK0000411 | DH-ubiquinone oxidoreductase B18 subunit | −11.2037 | 2.38 × 10−15 | |
| BMSK0000424 | cytochrome b-c1 complex subunit Rieske | −4.3818 | 2.22 × 10−29 | |
| BMSK0000434 | V-type proton ATPase 116 kDa subunit a isoform 1-like | −5.4141 | 1.12 × 10−21 | |
| BMSK0000861 | cytochrome c oxidase subunit 7A1 | −1.37335 | 1.76 × 10−5 | |
| BMSK0000858 | cytochrome c oxidase subunit 7C | −11.654 | 4.86 × 10−13 | |
| BMSK0000687 | ATP synthase subunit gamma | −2.69535 | 1.22 × 10−11 | |
| BMSK0000635 | succinate dehydrogenase | −5.56853 | 1.17 × 10−39 | |
| BMSK0006733 | cytochrome oxidase c subunit Vib | −4.17749 | 6.85 × 10−25 | |
| BMSK0005384 | probable DH dehydrogenase | −5.3812 | 6.98 × 10−30 | |
| BMSK0003855 | cytochrome c oxidase subunit 5B | −5.06134 | 1.02 × 10−33 | |
| BMSK0002658 | DH dehydrogenase [ubiquinone] iron-sulfur protein 3 | −4.08797 | 9.99 × 10−19 | |
| BMSK0002109 | DH-ubiquinone oxidoreductase 49 kDa subunit | −4.49602 | 1.29 × 10−23 | |
| BMSK0008624 | cytochrome c oxidase subunit 6A2 | −4.38976 | 8.49 × 10−38 | |
| BMSK0007592 | cytochrome b-c1 complex subunit 8-like | −4.60053 | 3.50 × 10−35 | |
| Nitrogen metabolism | BMSK0000310 | glutamine synthetase 2 cytoplasmic isoform X2 | 1.236855 | 4.63 × 10−5 |
| BMSK0004749 | carbonic anhydrase 1 isoform X1 | −4.69514 | 2.09 × 10−23 | |
| BMSK0004746 | putative carbonic anhydrase | −4.88355 | 8.85 × 10−16 | |
| Amino acid metabolism | BMSK0006194 | alanine aminotransferase 1 | 9.384746 | 1.72 × 10−71 |
| BMSK0005711 | inducible nitric oxide synthase-like protein | 2.269884 | 2.81 × 10−11 | |
| BMSK0005320 | glutamine synthetase 1 | −9.03892 | 4.01 × 10−9 | |
| BMSK0003612 | glutamate dehydrogenase | −4.376969 | 2.28 × 10−25 | |
| BMSK0007733 | malate dehydrogenase 1 | −5.259740 | 3.16 × 10−41 | |
| Carbohydrate metabolism | BMSK0005441 | multiple inositol polyphosphate phosphatase 1 | −1.011 | 0.001744 |
| BMSK0004863 | pyruvate kinase-like isoform X4 | −3.59947 | 7.08 × 10−10 | |
| BMSK0000610 | aldose 1-epimerase isoform X1 | −4.00298 | 1.71 × 10−32 | |
| BMSK0000507 | enolase | −4.57115 | 9.42 × 10−36 | |
| BMSK0014846 | alcohol dehydrogenase | −4.72435 | 2.26 × 10−33 | |
| BMSK0014852 | 1,5-anhydro-D-fructose reductase | −3.14956 | 3.56 × 10−12 | |
| BMSK0003272 | malate dehydrogenase isoform X1 | −1.35844 | 1.10 × 10−5 | |
| BMSK0004863 | pyruvate kinase-like isoform X4 | −3.59947 | 7.08 × 10−10 | |
| BMSK0004862 | pyruvate kinase, alpha/beta domain | −4.3419 | 4.16 × 10−12 | |
| BMSK0001495 | glyoxylate reductase/hydroxypyruvate reductase | −2.55827 | 3.15 × 10−14 | |
| BMSK0012704 | glutamine synthetase 2 cytoplasmic-like | −6.05855 | 2.51 × 10−28 | |
| BMSK0000686 | glycine cleavage system H protein-like | −3.09021 | 1.93 × 10−17 | |
| BMSK0000636 | succinate dehydrogenase cytochrome b560 subunit | −6.39877 | 6.41 × 10−21 | |
| BMSK0004075 | chitinase isoform X1 | 1.200746 | 0.000638 | |
| BMSK0004232 | beta-N-acetylglucosaminidase 2 precursor | 1.370167 | 4.74 × 10−5 | |
| BMSK0007975 | glucosamine-6-phosphate isomerase isoform X1 | 1.569734 | 4.96 × 10−7 | |
| BMSK0006682 | cysteine sulfinic acid decarboxylase | 5.528822 | 1.15 × 10−26 | |
| BMSK0016012 | UDP-glycosyltransferase UGT33D8 | 1.353298 | 0.000243 | |
| BMSK0014767 | uridine diphosphate glucosyltransferase | 6.283757 | 1.71 × 10−28 | |
| Lipid metabolism | BMSK0009526 | fatty acid synthase | −2.72055 | 3.34 × 10−13 |
| BMSK0009516 | acyl transferase domain | −2.04429 | 1.07 × 10−9 | |
| BMSK0013704 | fatty acyl-CoA reductase wat-like | −2.961012 | 1.66 × 10−10 | |
| BMSK0004409 | gamma-glutamyl transpeptidase isoform X1 | −3.172825 | 5.65 × 10−21 | |
| BMSK0004474 | lysophospholipid acyltransferase 7 isoform X2 | −5.22114 | 2.42 × 10−23 | |
| BMSK0007012 | non-lysosomal glucosylceramidase | −2.26115 | 9.35 × 10−10 | |
| BMSK0013693 | fatty-acyl CoA reductase 2 | −1.07664 | 0.003835 | |
| BMSK0005884 | UDP-glycosyltransferase UGT48C1 precursor | 2.373975 | 7.55 × 10−7 | |
| BMSK0009441 | phospholipase A2-like | 2.619790 | 1.01 × 10−14 | |
| BMSK0003963 | uridine diphosphate glucosyltransferase precursor | 6.808911 | 5.44 × 10−42 |
| Sequence ID | Gene Name | DEGs (log2 Value) | p Value |
|---|---|---|---|
| BMSK0005887 | facilitated trehalose transporter Tret1-like | 3.53769 | 2.27 × 10−8 |
| BMSK0014493 | juvenile hormone esterase-like isoform X2 | 2.9759 | 3.63 × 10−8 |
| BMSK0014862 | ecdysteroid-phosphate phosphatase | 1.958563 | 2.08 × 10−9 |
| BMSK0013050 | juvenile hormone binding protein an-0128 precursor | 1.777608 | 2.12 × 10−6 |
| BMSK0010481 | ecdysteroid-regulated 16 kDa protein | −1.74256 | 3.15 × 10−7 |
| BMSK0013317 | hemolymph juvenile hormone binding protein precursor | −1.01676 | 0.000499 |
| BMSK0008902 | juvenile hormone binding protein brP-1649 precursor | −3.73864 | 2.83 × 10−10 |
| Sequence ID | Gene Name | DEGs (log2 Value) | p Value |
|---|---|---|---|
| BMSK0014004 | hemolin isoform X1 | 2.879532 | 1.24 × 10−9 |
| BMSK0005301 | hemocytin | −1.54542 | 1.14 × 10−6 |
| BMSK0015652 | scavenger receptor type C precursor | −1.55632 | 3.58 × 10−6 |
| BMSK0013731 | scavenger receptor class B member 1 isoform X2 | −1.69213 | 1.99 × 10−7 |
| BMSK0002618 | very low-density lipoprotein receptor | 1.666729 | 3.83 × 10−8 |
| BMSK0001793 | integrin beta4 | 1.163103 | 0.001039 |
| BMSK0001792 | integrin beta3 | −1.78043 | 9.31 × 10−5 |
| BMSK0008195 | intraflagellar transport protein 46 homolog isoform X3 | −3.71239 | 3.53 × 10−12 |
| BMSK0001621 | dynein intermediate chain 3 | −4.69828 | 1.14 × 10−22 |
| BMSK0005812 | tetratricopeptide repeat protein 30A | −3.65764 | 1.69 × 10−14 |
| BMSK0007120 | cytoplasmic dynein 2 light intermediate chain 1 isoform X1 | −3.93271 | 1.86 × 10−17 |
| BMSK0014572 | intraflagellar transport protein 20 homolog | −2.88381 | 2.26 × 10−11 |
| BMSK0009828 | dynein assembly factor 5 | −2.04465 | 5.92 × 10−10 |
| BMSK0014854 | dynein beta chain | −2.94315 | 1.05 × 10−20 |
| BMSK0011063 | dynein intermediate chain 2 | −4.95565 | 6.14 × 10−33 |
| BMSK0015762 | tektin-4 | −4.38482 | 6.12 × 10−29 |
| BMSK0015667 | heat shock protein 83 | 1.229242 | 6.17 × 10−7 |
| BMSK0009364 | centromere protein J | −1.20679 | 6.56 × 10−5 |
| BMSK0002250 | intraflagellar transport protein 80 homolog isoform X1 | −3.18285 | 1.22 × 10−14 |
| BMSK0000038 | actin-85C | −3.57324 | 1.38 × 10−21 |
| BMSK0009907 | cytoplasmic A3 | 1.491502 | 6.72 × 10−9 |
| BMSK0015598 | tubulin beta chain isoform X1 | −1.04303 | 0.004396 |
| BMSK0009003 | beta-tubulin | −4.27001 | 6.91 × 10−44 |
| BMSK0015598 | tubulin beta chain | −4.74837 | 4.3 × 10−45 |
| BMSK0000091 | tubulin alpha-1 chain | −4.34691 | 9.12 × 10−44 |
| BMSK0003474 | tektin-B1 | −4.70582 | 6.68 × 10−38 |
| Sequence ID | Gene Name | DEGs (log2 Value) | p Value |
|---|---|---|---|
| BMSK0009350 | peptidoglycan-recognition protein LB-like | 11.52585 | 1.75 × 10−15 |
| BMSK0004848 | peptidoglycan recognition protein S2 | 6.973384 | 2.01 × 10−58 |
| BMSK0009349 | peptidoglycan recognition protein S6 precursor | 6.245274 | 1.51 × 10−47 |
| BMSK0006299 | beta-1,3-glucan recognition protein 3 isoform X2 | 1.40262 | 1.33 × 10−5 |
| BMSK0012017 | serine protease 7 precursor | 7.447781 | 5.12 × 10−51 |
| BMSK0009527 | thioesterase domain | −2.45384 | 1.44 × 10−10 |
| BMSK0012018 | serine protease snake | 5.39302 | 3.76 × 10−39 |
| BMSK0015991 | serpin 5 | 1.5216 | 2.88 × 10−6 |
| BMSK0008651 | serine protease inhibitor 6 isoform X1 | 5.430386 | 2.04 × 10−42 |
| BMSK0003816 | serine protease inhibitor 12 isoform X1 | 1.555923 | 1.16 × 10−6 |
| BMSK0003812 | serine protease inhibitor 3 isoform X1 | 1.338882 | 3.74 × 10−5 |
| BMSK0003441 | angiotensin-converting enzyme | −1.99032 | 6.07 × 10−7 |
| BMSK0015864 | lysozyme-like | 1.688813 | 2.42 × 10−8 |
| BMSK0013244 | moricin | 6.174524 | 1.58 × 10−51 |
| BMSK0009812 | gloverin 2 isoform X1 | 4.685764 | 1.64 × 10−35 |
| BMSK0003513 | cecropin A | 3.788939 | 8.94 × 10−26 |
| BMSK0016018 | gloverin 4 precursor | 3.367737 | 2.31 × 10−21 |
| BMSK0003511 | cecropin family | 3.283748 | 6.29 × 10−21 |
| BMSK0016016 | gloverin1 | 3.227077 | 4.27 × 10−20 |
| BMSK0016017 | gloverin-like protein | 2.986507 | 5.72 × 10−15 |
| BMSK0015407 | antibacterial peptide enbocin 2 precursor | 2.910463 | 3.97 × 10−17 |
| BMSK0003627 | attacin-like | 2.88477 | 2.35 × 10−17 |
| BMSK0015405 | antibacterial peptide enbocin 3 precursor | 2.879573 | 7.74 × 10−17 |
| BMSK0005463 | ebocin 3 | 2.729055 | 6.01 × 10−16 |
| BMSK0015969 | gloverin 3 isoform X1 | 2.474034 | 2.23 × 10−13 |
| BMSK0001742 | a pirk homolog | 1.564452 | 6.54 × 10−7 |
| BMSK0006299 | beta-1,3-glucan recognition protein 3 isoform X2 | 1.40262 | 1.33 × 10−5 |
| BMSK0012472 | eukaryotic initiation factor 4E-1 | 1.420455 | 6.20 × 10−5 |
| BMSK0002354 | signal transducing adapter molecule 2 | 1.034003 | 0.000234 |
| BMSK0000175 | epidermal growth factor receptor isoform X2 | −1.20559 | 0.000214 |
| BMSK0003517 | heat shock protein 68 | −4.28148 | 4.89 × 10−34 |
| BMSK0007712 | cAMP-dependent protein kinase catalytic subunit alpha-like | −5.71941 | 1.47 × 10−41 |
| BMSK0007713 | protein kinase domain | −4.55635 | 2.52 × 10−27 |
| BMSK0015669 | heat shock protein 70 | 4.108944 | 1.04 × 10−23 |
| BMSK0015756 | growth arrest and DNA-damage-inducible protein GADD45 alpha | 1.222944 | 0.00032 |
| BMSK0001919 | protein 60A | 1.695163 | 3.1 × 10−7 |
| BMSK0001708 | Tgif2 | −5.366 | 9.01 × 10−45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.; Yang, J.; Liu, X.; Gu, H.; Li, F.; Li, B.; Wei, J. Parasitism by the Tachinid Parasitoid Exorista japonica Leads to Suppression of Basal Metabolism and Activation of Immune Response in the Host Bombyx mori. Insects 2022, 13, 792. https://doi.org/10.3390/insects13090792
Dai M, Yang J, Liu X, Gu H, Li F, Li B, Wei J. Parasitism by the Tachinid Parasitoid Exorista japonica Leads to Suppression of Basal Metabolism and Activation of Immune Response in the Host Bombyx mori. Insects. 2022; 13(9):792. https://doi.org/10.3390/insects13090792
Chicago/Turabian StyleDai, Minli, Jin Yang, Xinyi Liu, Haoyi Gu, Fanchi Li, Bing Li, and Jing Wei. 2022. "Parasitism by the Tachinid Parasitoid Exorista japonica Leads to Suppression of Basal Metabolism and Activation of Immune Response in the Host Bombyx mori" Insects 13, no. 9: 792. https://doi.org/10.3390/insects13090792







