Ultra-Morphology and Mechanical Function of the Trichoideum Sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Samples
2.2. Preparation of Samples for Scanning Electron Microscopy
2.3. Preparation of Samples for Light and Transmission Electron Microscopy
2.4. Image Stacking and Surface Generation
2.5. Nomenclature and Measuring of Sensilla
| Types, Length, and Arrangement of the Sensilla | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| shorter | longer | longer | shorter | |||||||
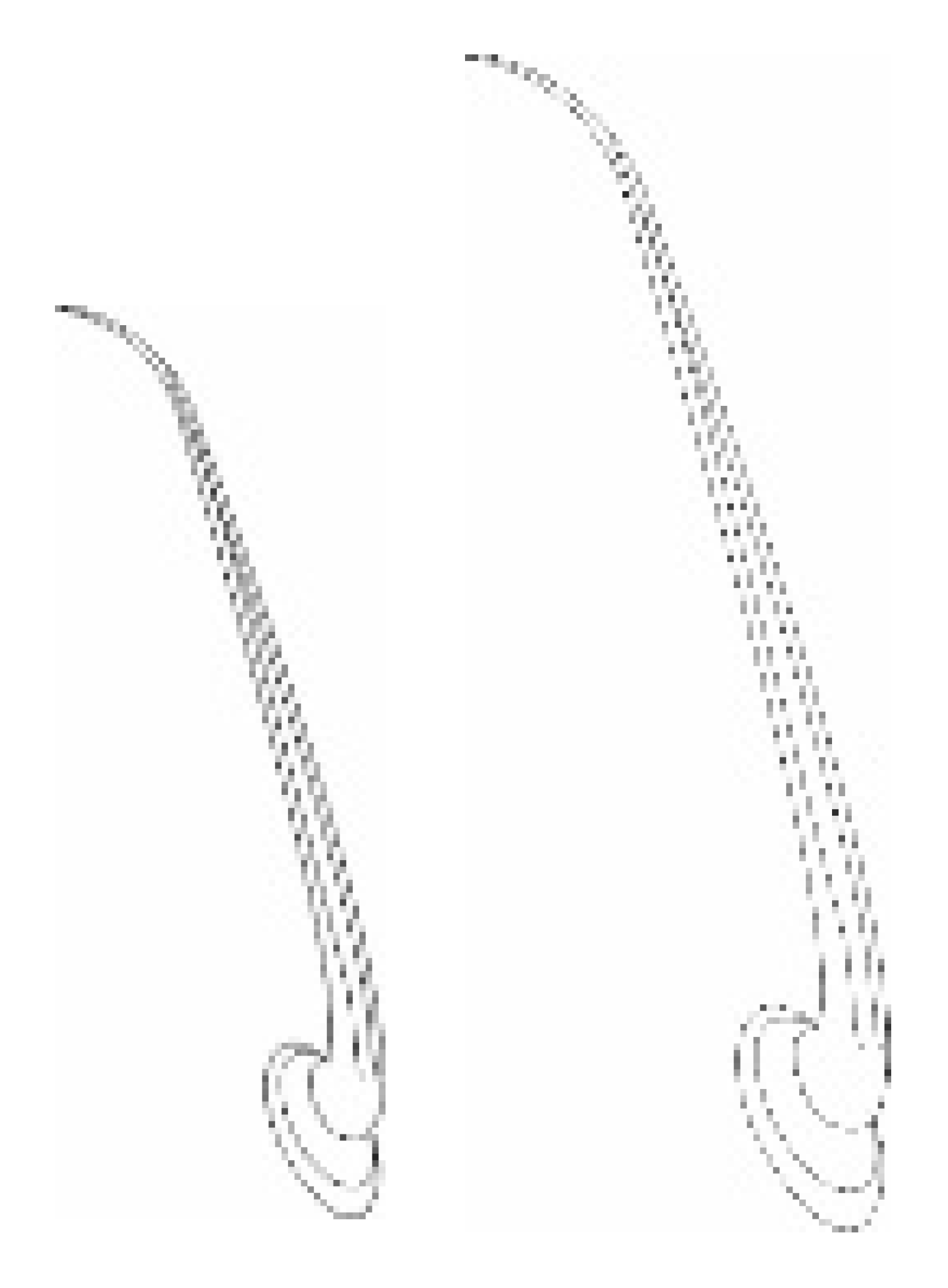 | 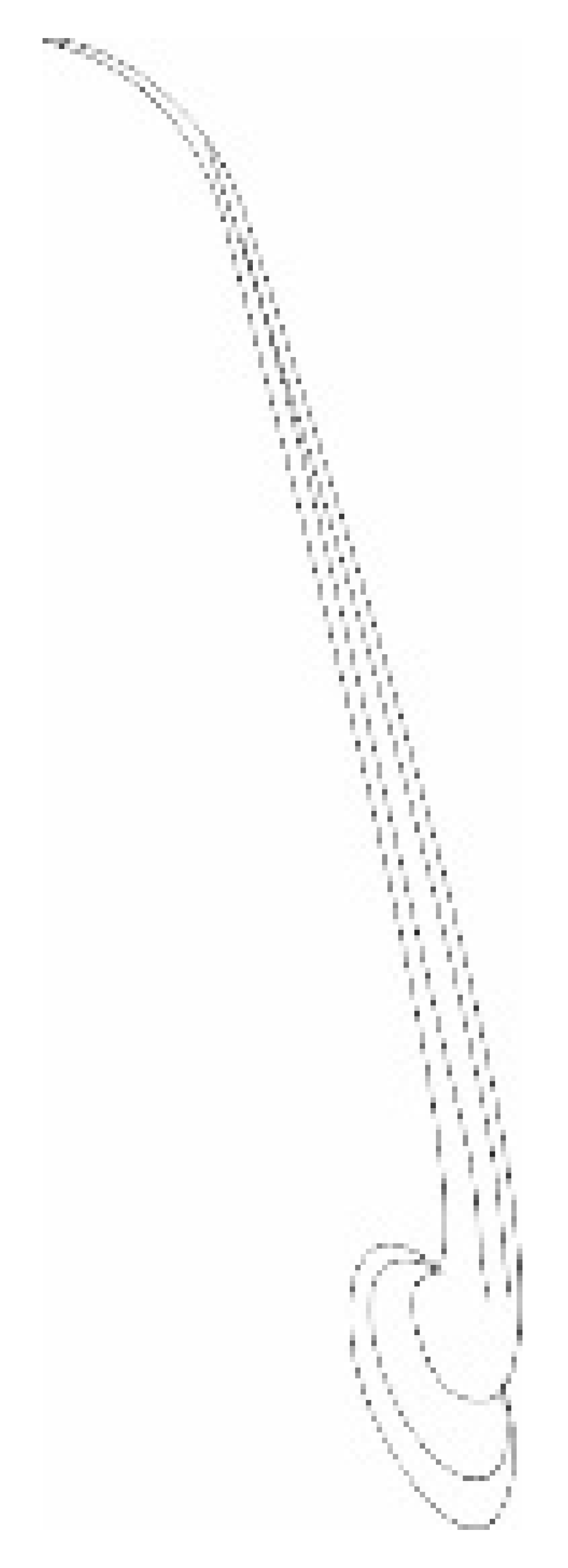 | 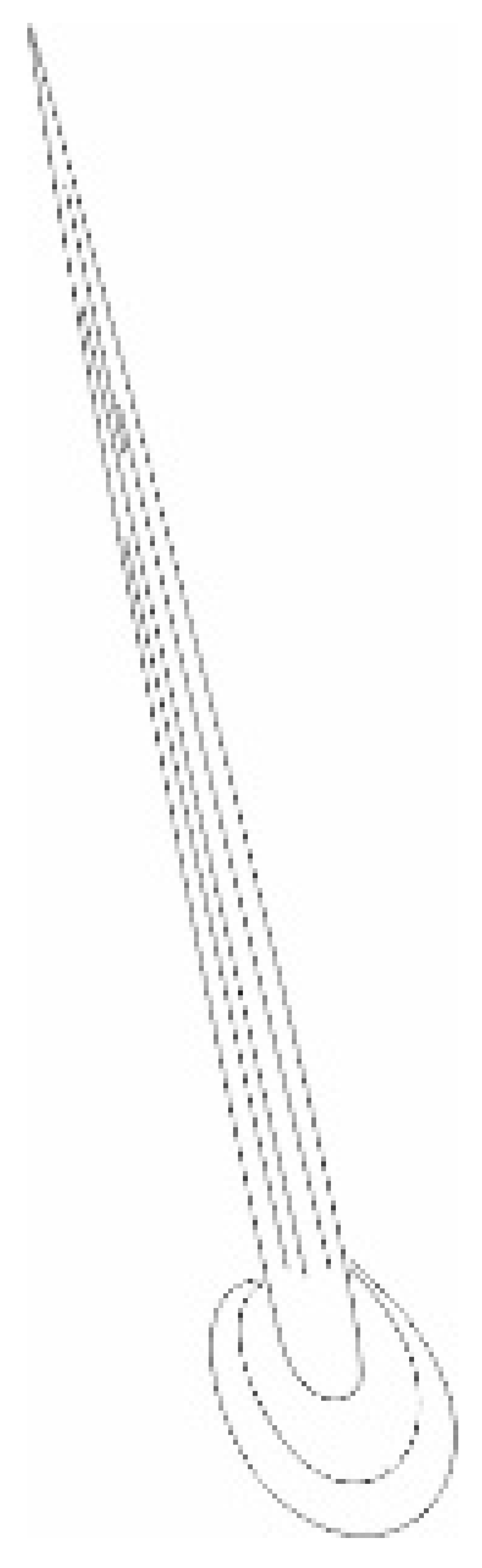 |  |  | 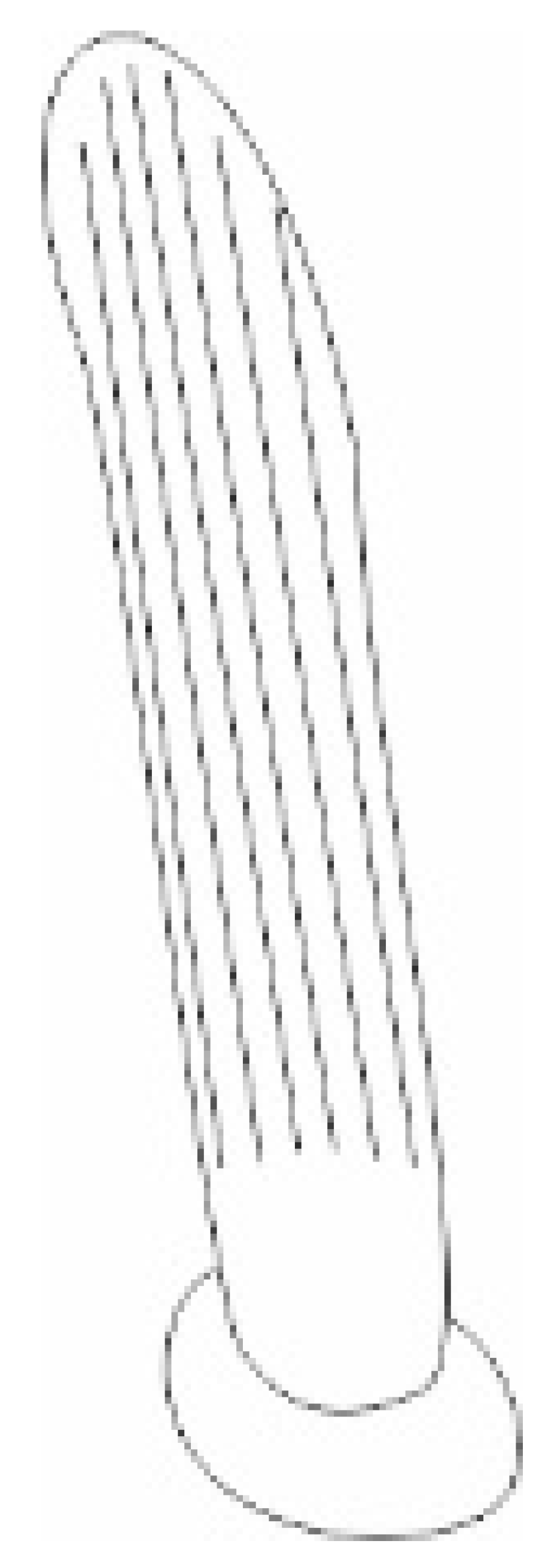 |  |  |  | ||
| Types sensilla | TSa | TSa | TSa | ChS | TSp1 | TSp2 | BSp | CS | BSa (pair) | CoS |
| Length (µm) | 22.2 23.5 24.7 27.2 28.0 | 34.2 36.0 42.2 44.8 45.5 | 47.6 52.1 58.1 63.7 65.9 | 53.2 57.8 57.8 62.8 67.9 | 61.2 65.3 68.5 69.7 66.3 | 46.2 46.7 46.3 45.6 47.6 | 14.4 14.7 11.2 11.0 10.0 | 8.5 7.9 | 12.8 12.1 | 4.6 |
| Scapus (s) | + | + | + | +4 | - | - | - | +2 | + | - |
| Pedicel (p) | ++ | ++ | + | +4 | - | - | - | +2 | + | - |
| Flagellomere (f1) | - | - | + | +11 | +++ | +++ | ++8 | +3 | + | +1 |
| Flagellomere (f2) | - | - | + | ++18 | +++ | +++ | +++16 | +4 | + | +1 |

3. Results
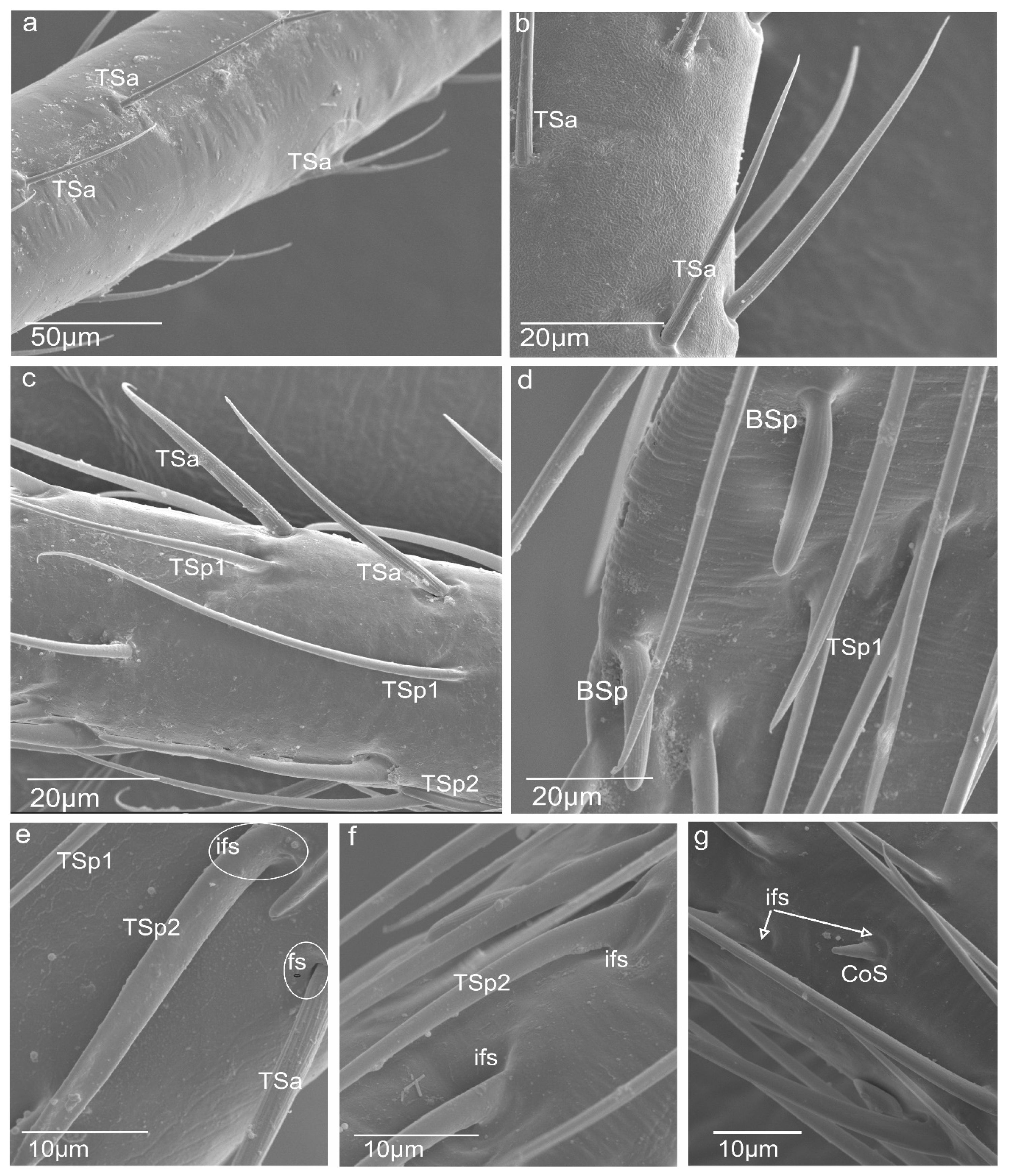
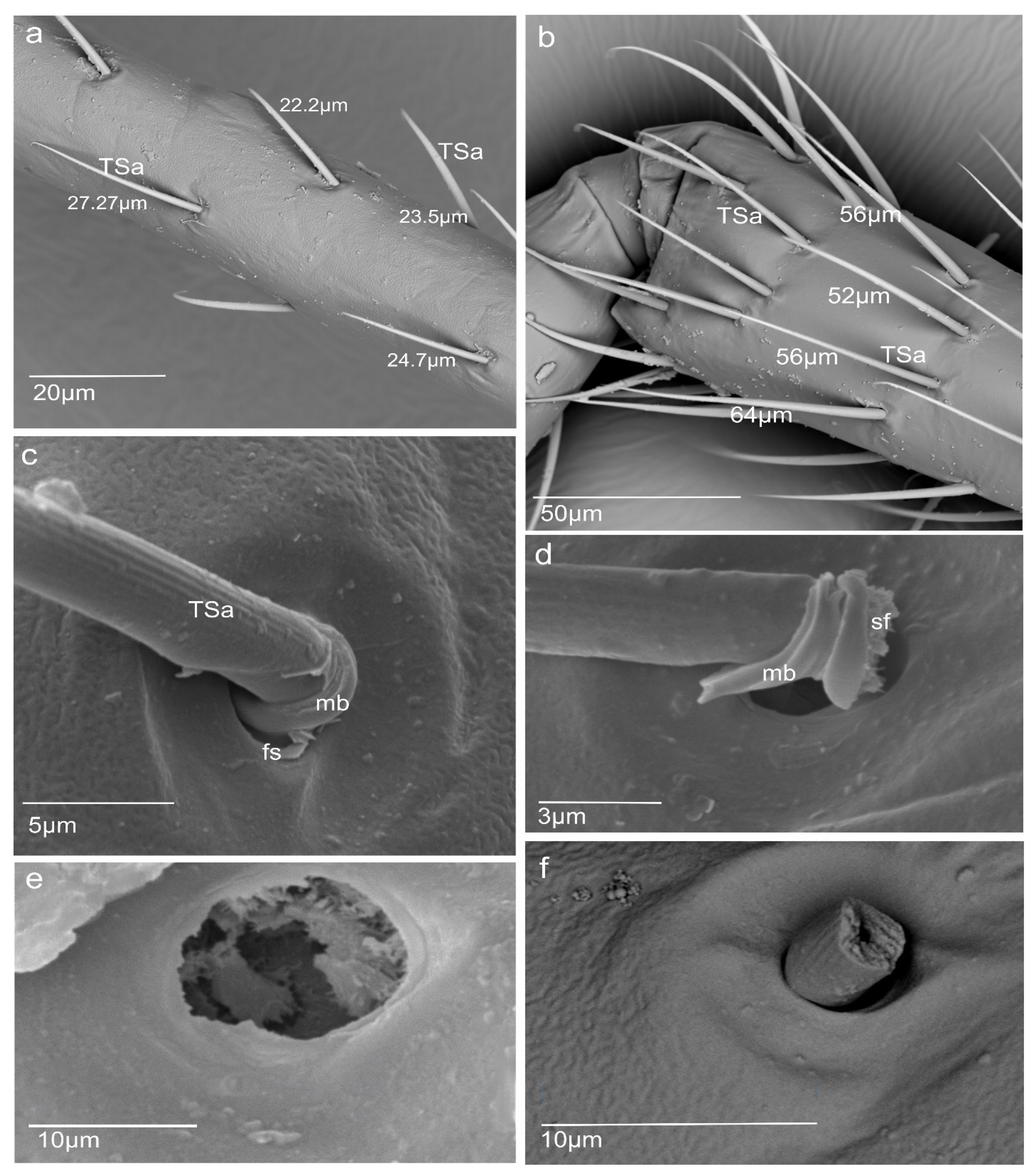
3.1. Morphology of N. rugosus Antennae
- Sensilla trichodea (TS) are hair-like structures. The stem is generally wider in the base and narrow in the apex, which frequently is bent. These sensilla of different lengths are found in larger or fewer numbers on different antennomeres. Their essential character is they are positioned to the surface at an angle (e.g., 25.5–35.1°), laying parallel according to the long axis of the antennae, and slightly raised over their surface.
- 2.
- Sensilla chaetica (ChS) are long (53 to 70 µm), rigid structures that are ribbed and sharpened at the tip and arise from a flexible socket. These sensilla are not numerous, and they were only found as singles in the distal part of the scapus (Figure 1b) and proximal portion of the pedicel (Figure 1b). Still, there are slightly more on the lateral sides of the first and second flagellomeres (Figure 1d,e). The sensilla are positioned on the antennal surface at a larger angle (42°, 48°) than the trichoid sensilla; therefore, they are visible, as they stick out (Figure 1e).
- 3.
- Sensilla campaniformia (CS) are oval-shaped structures with a single pore in the middle embedded in the flexible sockets. They are present only a few (2–4) in different places of each of the antennomeres. Their presence is shown on the scapus and pedicel (Figure 1b,c).
- 4.
- Sensilla basiconica (BS) represents two subtypes. One of the subtypes (BSa) is short (length approximately 5 µm), smooth, aporous, cone-shaped structures embedded in the flexible socket, occurring in one pair on the edge of the adjacent segments (Figure 1b).
- 5.
- Sensilla coeloconica (CoS) are peg-like and short, with smooth surfaces embedded in a shallow cavity of cuticles in inflexible sockets. Only one such sensillum was observed on each flagellomere (Figure 2g).
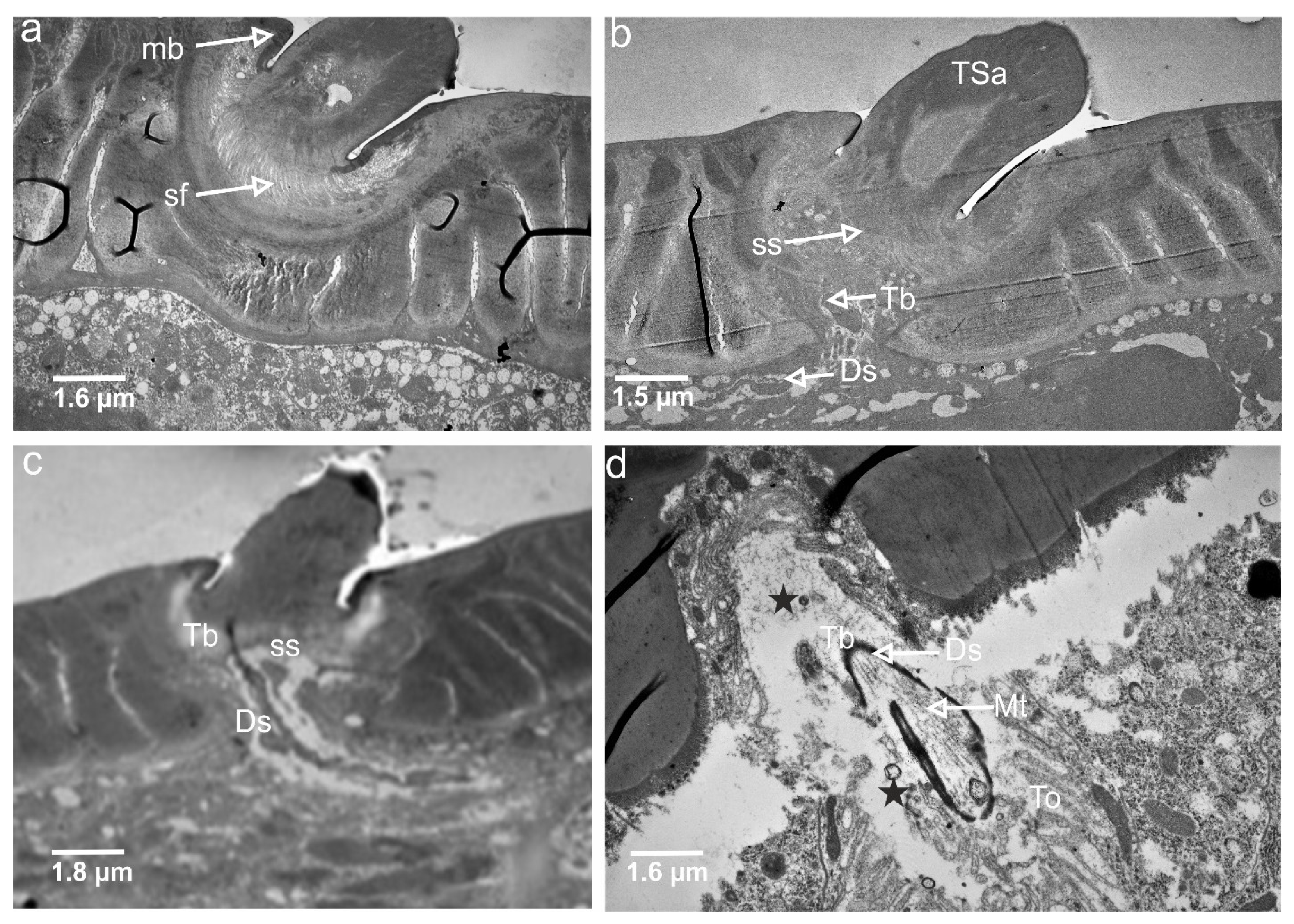
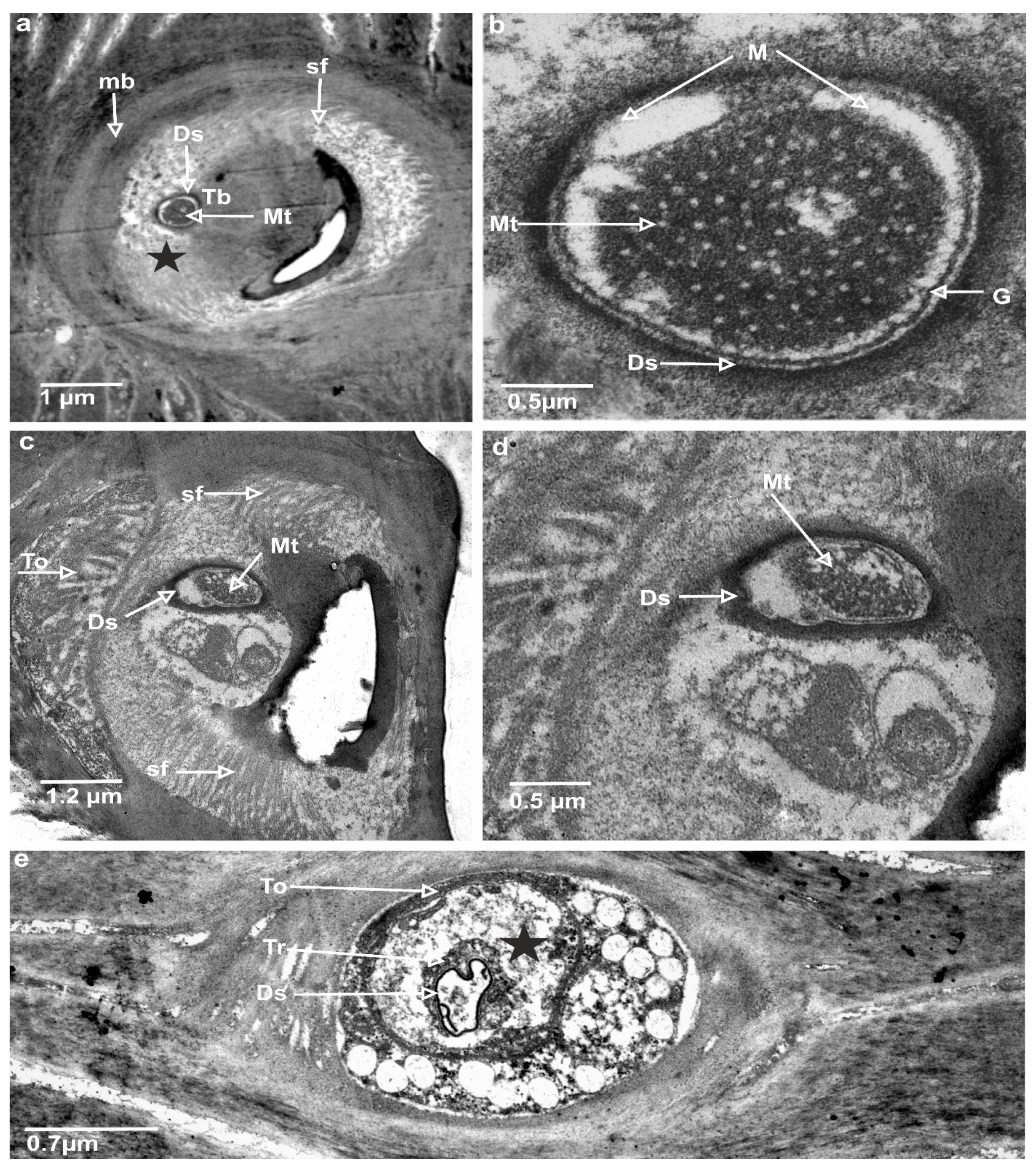
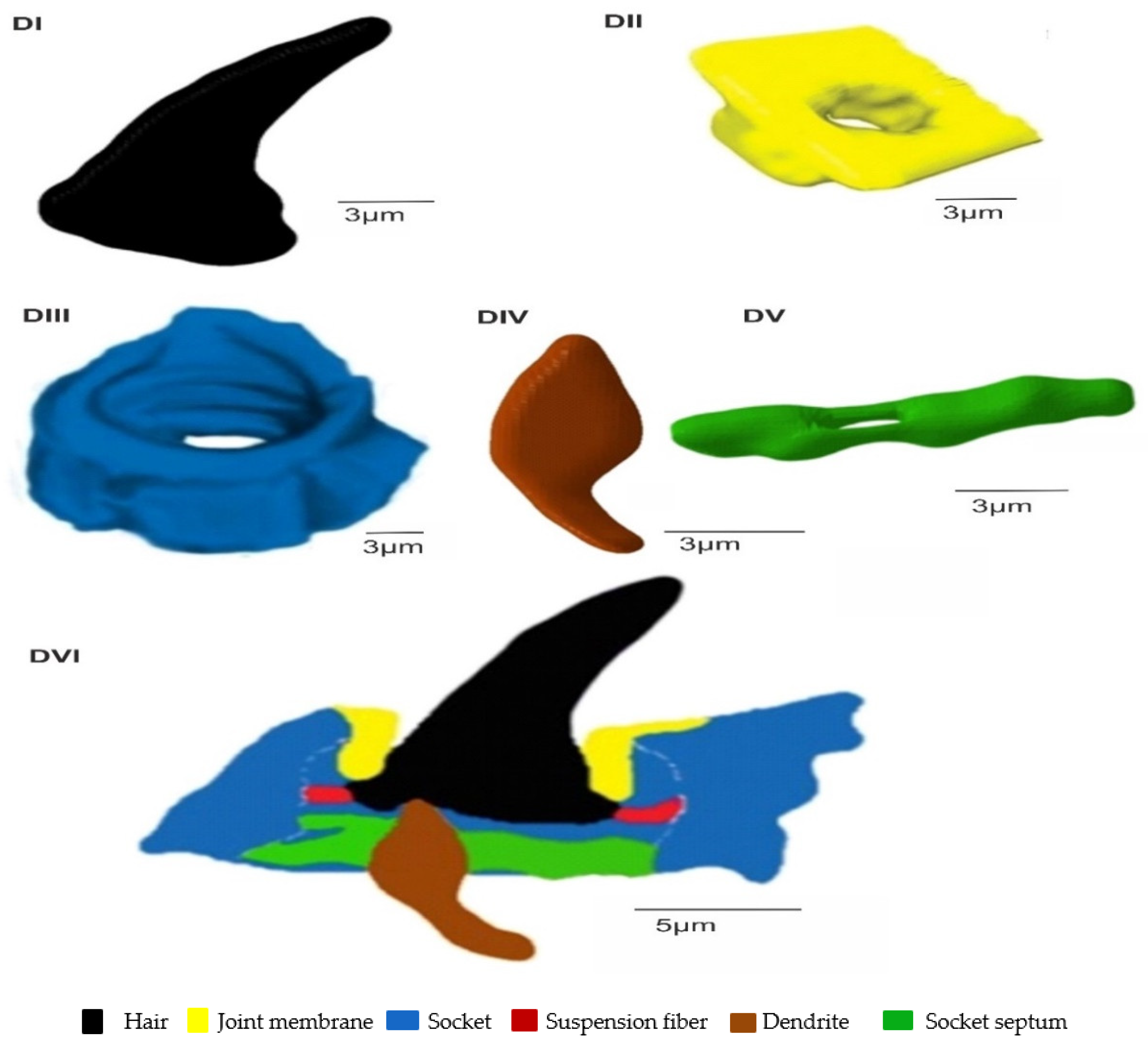
3.2. A Detailed Description of the Trichoideum sensillum on the Pedicel with the Structure of the Socket
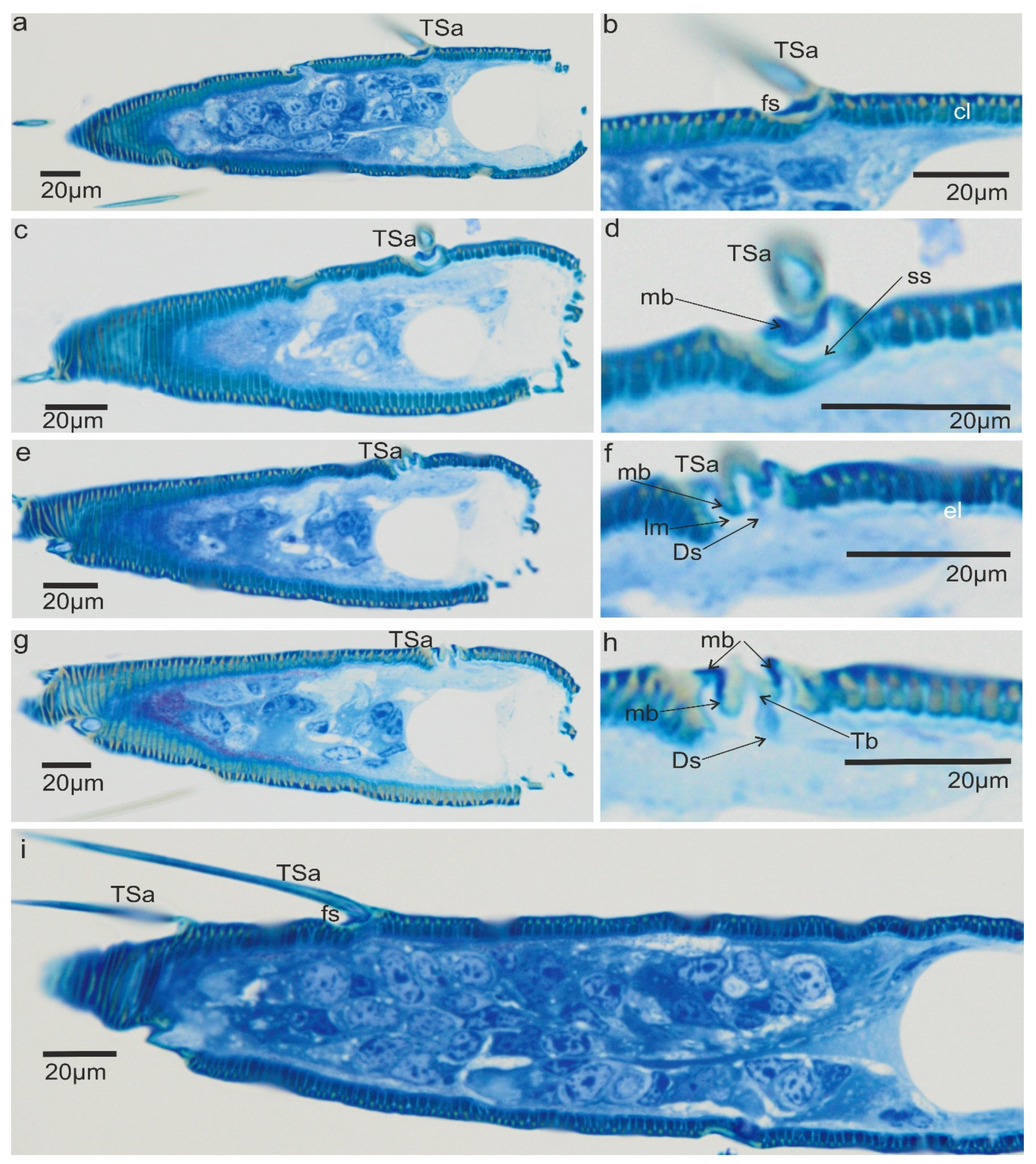
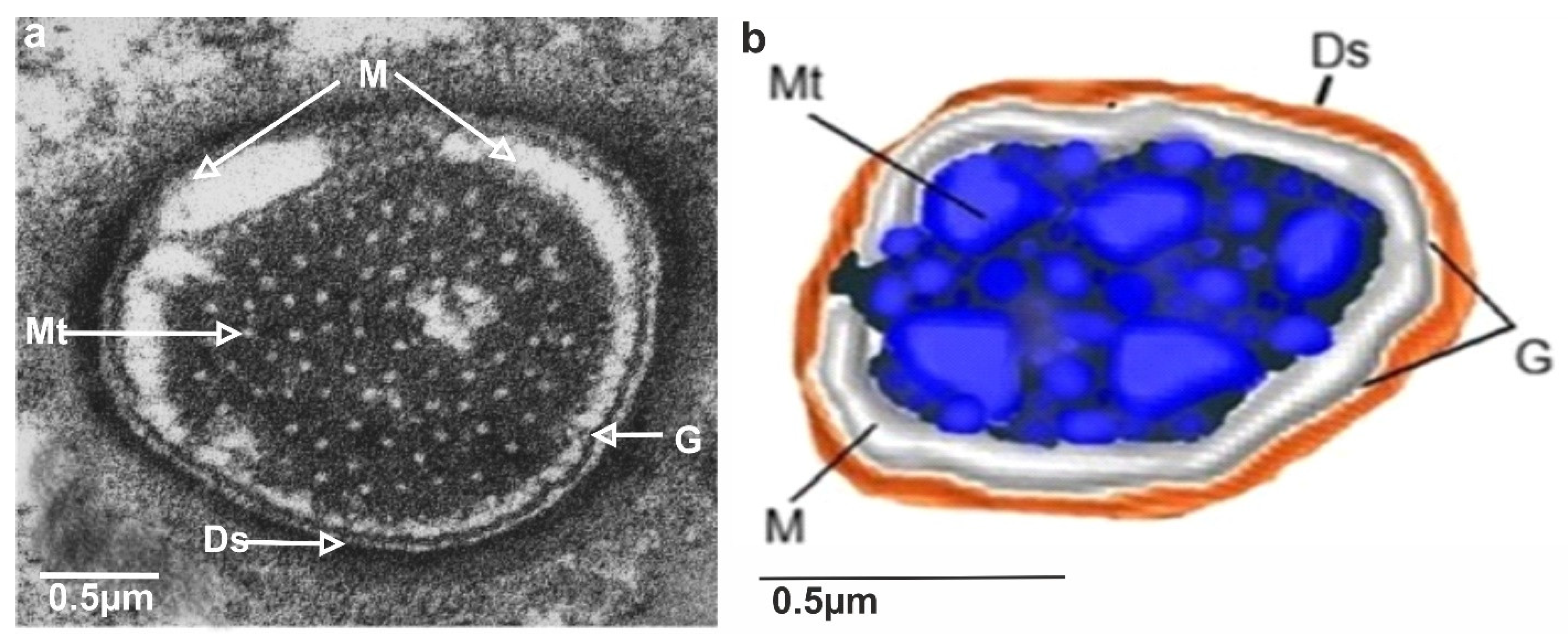
3.3. Ultrastructures of Trichoid Mechanosensillum Using TEM
4. Discussion
4.1. Type of the Antennal Sensilla in N. rugosus
4.2. Structural Components of the Mechanoreceptor Sensilla
4.3. Biological Sensing Mechanism of Trichoid Sensilla
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kerzhner, I.M. Poluzhestkokrylye Semeystva Nabidae [Heteroptera of the Family Zoo Morphology], 2nd ed.; Theodor, O., Ed.; Fauna of the USSR: Leningrad, Russia, 1981; pp. 1–326. [Google Scholar]
- Kerzhner, I.M. Family Nabidae, A. Costa—damsel bugs, 1853. In Catalogue of the Heteroptera of the Palaearctic Region, 2nd ed.; Aukema, B., Rieger, C., Eds.; The Netherlands Entomological Society: Wageningen, The Netherlands, 2004; pp. 84–107. [Google Scholar]
- Brożek, J. Morphology and arrangement of the labial sensilla of the water bugs. Bull. Insectol. 2008, 61, 275–297. [Google Scholar] [CrossRef]
- Brożek, J. Comparative analysis and systematic mapping of the labial sensilla in the Nepomorpha (Heteroptera: Insecta). Sci. World J. 2013, 1, 518034. [Google Scholar] [CrossRef] [PubMed]
- Calderón, L.; Alberto, B. The Function of Mechanosensory SYSTEMS in the Startle Behavior of Planktonic Larvae. Ph.D. Thesis, Eberhard Karls Universität Tübingen, Tübinge, Germany, 2019. [Google Scholar]
- Wiederhold, M.L. Mechanosensory transduction in “sensory” and “motile” cilia. Annu. Rev. Biophys. 1976, 5, 39–62. [Google Scholar] [CrossRef] [PubMed]
- UshaRani, P.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Brożek, J.; Zettel, H. A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha). Eur. J. Entomol. 2014, 111, 275–297. [Google Scholar] [CrossRef]
- Taszakowski, A.; Nowińska, A.; Brożek, J. Morphological study of the labial sensilla in Nabidae (Hemiptera: Heteroptera: Cimicomorpha). Zoomorphology 2019, 138, 483–492. [Google Scholar] [CrossRef]
- Hallberg, E.; Hansson, B.S. Arthropod sensilla: Morphology and phylogenetic considerations. Microsc. Res. Tech. 1999, 47, 428–439. [Google Scholar] [CrossRef]
- Kalogianni, E. Physiological properties of wind-sensitive and tactile trichoid sensilla on the ovipositor and their role during oviposition in the locust. J. Exp. Biol. 1995, 198, 1359–1369. [Google Scholar] [CrossRef]
- Hartenstein, V.; Posakony, J.W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 1989, 107, 389–405. [Google Scholar] [CrossRef]
- Chakilam, S.; Li, D.T.; Xi, Z.C.; Gaidys, R.; Lupeikiene, A. Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors. Eng. Proc. 2020, 2, 70. [Google Scholar] [CrossRef]
- Keil, T.A. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Guo, J.S.; Wang, X.Q.; Li, D.T.; Song, D.D.; Zhang, C.X. Three-dimensional architecture of a mechanoreceptor in the brown planthopper, Nilaparvata lugens, revealed by FIB-SEM. Cell Tissue Res. 2020, 379, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Nuzzaci, G. SEM and TEM techniques. In Eriophyoid Mites Their Biology, Natural Enemies and Control, 1st ed.; World Crop Pests, Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 399–410. [Google Scholar]
- Dey, S.; Hooroo, R.N.; Wankhar, D. Scanning electron microscopic studies of the external morphology of sensilla on the legs of a butterfly, Graphium sarpedon (Lepidoptera—Papillionidae). Micron 1995, 26, 367–376. [Google Scholar] [CrossRef]
- Schneeberg, K.; Bauernfeind, R.; Pohl, H. Comparison of cleaning methods for delicate insect specimens for scanning electron microscopy. Microsc. Res. Tech. 2017, 80, 1199–1204. [Google Scholar] [CrossRef]
- Kanturski, M.; Kajtoch, L.; Wieczorek, K. European species of the aphid genus Eulachnus Del Guercio, 1909 (Hemiptera: Aphididae: Lachninae): Revision and molecular phylogeny. Zootaxa 2017, 4356, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.J.; Brandes, M.C.; Mills, M.J.; DeGraef, M. Diffraction contrast STEM of dislocations: Imaging and simulations. Ultramicroscopy 2011, 111, 1483–1487. [Google Scholar] [CrossRef]
- DeGraef, M. The Transmission electron microscopy. In Introduction to Conventional Transmission Electron Microscopy, 4th ed.; Cambridge University Press: Cambridge, MA, USA, 2003; Volume 1, pp. 136–233. [Google Scholar]
- Slifer, E.H. The structure of arthropod chemoreceptors. Annu. Rev. Entomol. 1970, 15, 121–142. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticle mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo- thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Mendez-Vilas, A., Diaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Nowińska, A.; Brożek, J. Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 2017, 136, 327–347. [Google Scholar] [CrossRef]
- Keil, T.A.; Steinbrecht, R.A. Mechanosensitive and olfactory sensilla of insects. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Plenum: New York, NY, USA; London, UK, 1984; Volume 2, pp. 477–516. [Google Scholar]
- Catalã, S.S. Antennal sensilla of Triatominae (Hemiptera, Reduviidae): A comparative study of five genera. Int. J. Insect Morphol. Embryol. 1997, 26, 67–73. [Google Scholar] [CrossRef]
- Chinta, S.; Dickens, J.C.; Baker, G.T. Morphology and distribution of antennal sensilla of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae). Int. J. Insect Morphol. Embryol. 1997, 26, 21–26. [Google Scholar] [CrossRef]
- Da Rosa, J.A.; Barata, J.M.S.; Cilense, M.; Neto, F.M.B. Head morphology of 1st and 5th instar nymphs of Triatom circummaculata and Triatoma rubrovaria (Hemiptera, Reduviidae). Int. J. Insect Morphol. Embryol. 1999, 28, 363–375. [Google Scholar] [CrossRef]
- Silva, C.C.A.; de Capdeville, G.; Moraes, M.C.B.; Falcão, R.; Solino, L.F.; Laumann, R.A.; Silva, J.P.; Borges, M. Morphology, distribution and abundance of antennal sensilla in three stink bug species (Hemiptera: Pentatomidae). Micron 2010, 41, 289–300. [Google Scholar] [CrossRef]
- Usha Rani, P.; Madhavendra, S.S. External morphology of antennal and rostral sensilla in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Gonzaga, S.; Jorge, V.C.; Víctor, R.C. Sense Organs on the Antennal Flagellum of Leptoglossus zonatus (Heteroptera: Coreidae). Ann. Entomol. Soc. Am. 2013, 106, 510–517. [Google Scholar] [CrossRef]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo-and hygroreceptors. Annu. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Chapman, R.F. Mechanoreception. Chemoreception. In The Insects, Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 610–652. [Google Scholar]
- Slifer, E.H.; Sekhon, S.S. Sense organs on the antennal flagellum of the small milk weed bug, Lygaeus kalmii Stål (Hemiptera: Pentatomidae). J. Insect Morphol. 1963, 112, 165–193. [Google Scholar] [CrossRef]
- Harbach, R.E.; Larsen, J.R. Ultrastructure of sensilla on the distal antennal segment of adult Oncopeltus fasciatus (Dallas) (Hemiptera: Lygaeidae). Int. J. Insect Morphol. Embryol. 1976, 5, 23–33. [Google Scholar] [CrossRef]
- Ventura, M.U.; Panizzi, A.R. Morphology of olfactory sensilla and its role in host plant recognition by Neomegalotomus parvus (Westwood) (Heteroptera: Alydidae). Braz. Arch. Biol. Technol. 2005, 48, 589–597. [Google Scholar] [CrossRef]
- El-Ghany, N.M.A.; Faucheux, M.J. Sensory structures on the larval antennae and mouthparts of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Zool. Anz. 2021, 294, 28–38. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Koteja, J. Campaniform, basiconic, coeloconic and intersegmental sensilla on the antennae in the Coccinea (Homoptera). Acta Biol. Cracoviensia. Ser. Zool. 1980, 22, 73–88. [Google Scholar]
- Steinbrecht, R.A. Pore structures in insect olfactory sensilla: A review of data and concepts. Int. J. Insect Morphol. Embryol. 1997, 26, 229–245. [Google Scholar] [CrossRef]
- Hallberg, E.; Hansson, B.S.; Löfstedt, C. Sensilla and proprioreceptors. In Lepidoptera, Moths and Butterflies: Morphology, Physiology and Development; Kristensen, N.P., Ed.; Walter de Gruyter: Berlin, Germany, 2003; Volume 2, pp. 267–288. [Google Scholar]
- Brézot, P.; Tauban, D.; Renou, M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae) sensillum types and numerical growth during the post-embryonic development. Int. J. Insect Morphol. Embryol. 1997, 25, 427–441. [Google Scholar] [CrossRef]
- Dickens, J.C.; Callahan, F.E.; Wergin, W.P.; Erbe, E.F. Olfaction in a hemimetabolous insect: Antennal-specific protein in adult Lygus lineolaris (Heteroptera: Miridae). J. Insect Physiol. 1995, 41, 857–876. [Google Scholar] [CrossRef]
- Thurm, U.; Erler, G.; Gödde, J.; Kastrup, H.; Keil, T.; Volker, W.; Vohwinkel, B. Cilia specialized for mechanoreception. J. Submicrosc. Cytotol. 1983, 15, 151–155. [Google Scholar]
- Chevalier, R. L The fine structure of campaniform sensilla on the halteres of Drosophila melanogaster. J. Morphol. 1969, 128, 443–463. [Google Scholar] [CrossRef]
- Rice, M.J.; Galun, R.; Margalit, J. Mouthpart sensilla of the tsetse fly and their function. Ann. Trop. Med. Parasitol. 1973, 67, 101–107. [Google Scholar] [CrossRef]
- Mail, M.; Klein, A.; Bleckmann, H.; Schmitz, A.; Scherer, T.; Rühr, P.T.; Lovric, G.; Fröhlingsdorf, R.; Gorb, S.N.; Barthlott, W. A new bioinspired method for pressure and flow sensing based on the underwater air-retaining surface of the backswimmer Notonecta. Beilstein J. Nanotechnol. 2018, 9, 3039–3047. [Google Scholar] [CrossRef]
- Dumpert, K.; Gnatzy, W. Cricket combined mechanoreceptors and kicking response. J. Comp. Physiol. 1977, 122, 9–25. [Google Scholar] [CrossRef]
- Chi, C.; Carlson, S.D. The housefly interfacetal hair, ultrastructure of a presumed mechanoreceptor. Cell Tissue Res. 1976, 166, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.G.; Willingham, A.T.; Zuker, C.S. A Drosophila mechanosensory transduction channel. Science 2000, 287, 2229–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cant, C.; Knowles, B.A.; Mooseker, M.S.; Cooley, L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J. Cell Biol. 1994, 125, 369–380. [Google Scholar] [CrossRef]
- Schroeder, T.B.H.; Houghtaling, J.; Wilts, B.D.; Mayer, M. It’s not a bug, it’s a feature: Functional materials in insects. Adv. Mater. 2018, 30, 1705322–1705370. [Google Scholar] [CrossRef]
- Liang, X.; Madrid, J.; Howard, J. The microtubule-based cytoskeleton is a component of a mechanical signalling pathway in fly campaniform receptors. Biophys J. 2014, 107, 2767–2774. [Google Scholar] [CrossRef]
- Barth, F.G.; Németh, S.S.; Friedrich, O.C. Arthropod touch reception: Structure and mechanics of the basal part of a spider tactile hair. J. Comp. Physiol. 2004, 190, 523–530. [Google Scholar] [CrossRef]
- Liang, X.; Madrid, J.; Saleh, H.S.; Howard, J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton 2011, 68, 1–7. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakilam, S.; Brożek, J.; Chajec, Ł.; Poprawa, I.; Gaidys, R. Ultra-Morphology and Mechanical Function of the Trichoideum Sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha). Insects 2022, 13, 799. https://doi.org/10.3390/insects13090799
Chakilam S, Brożek J, Chajec Ł, Poprawa I, Gaidys R. Ultra-Morphology and Mechanical Function of the Trichoideum Sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha). Insects. 2022; 13(9):799. https://doi.org/10.3390/insects13090799
Chicago/Turabian StyleChakilam, Shashikanth, Jolanta Brożek, Łukasz Chajec, Izabela Poprawa, and Rimvydas Gaidys. 2022. "Ultra-Morphology and Mechanical Function of the Trichoideum Sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha)" Insects 13, no. 9: 799. https://doi.org/10.3390/insects13090799





