RNA Interference of Chitin Synthase 2 Gene in Liriomyza trifolii through Immersion in Double-Stranded RNA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Cloning, Sequence Alignment, and Expression of CHS2 Transcript
2.3. Synthesis and Delivery of dsRNA
2.4. Analysis of Silencing Efficiency
2.5. Statistical analysis
3. Results
3.1. Sequence Characteristics and Phylogenetic Analysis of CHS2 Transcript from L. trifolii
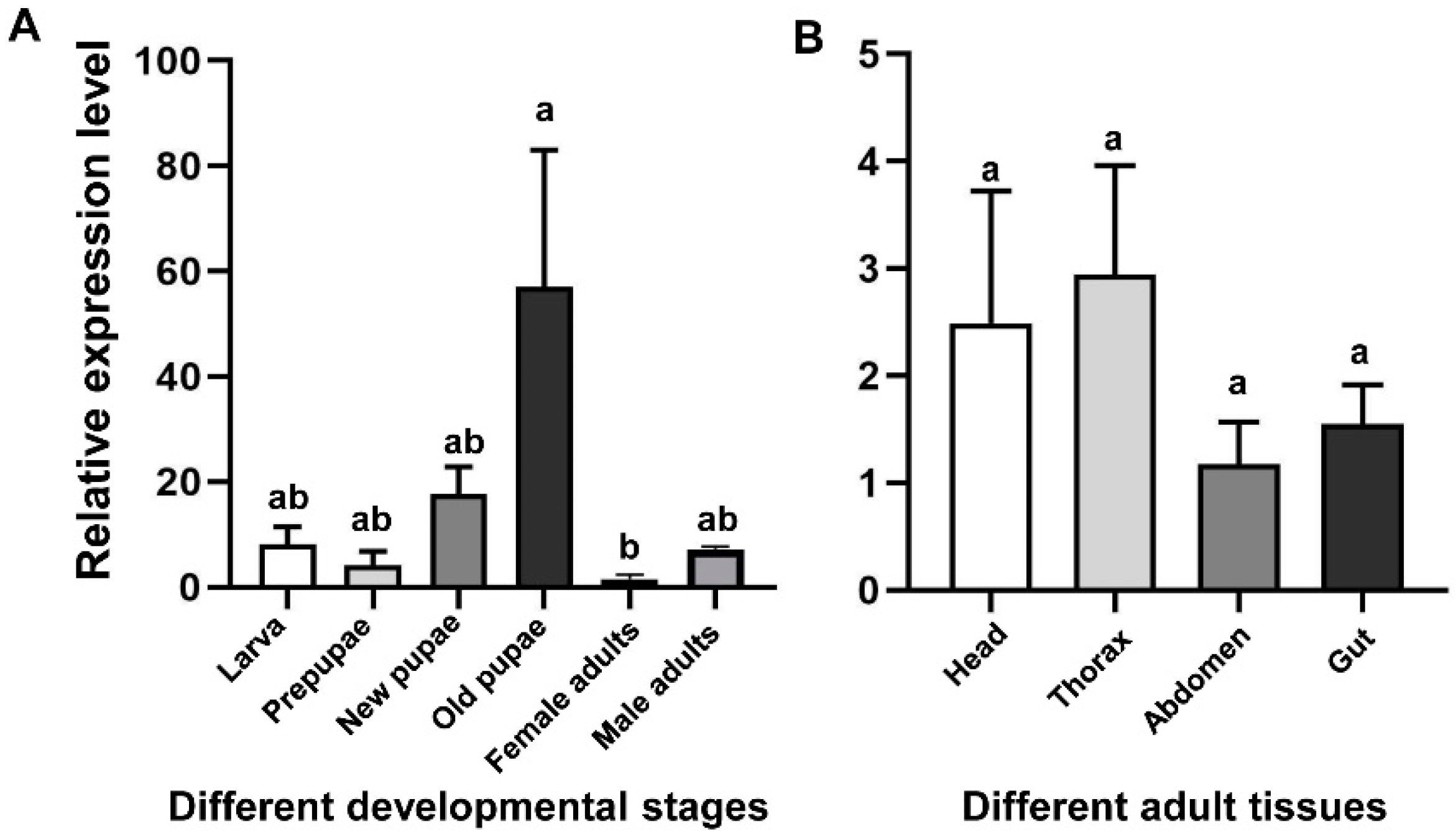
3.2. Expression Pattern of CHS2 Transcript in L. trifolii
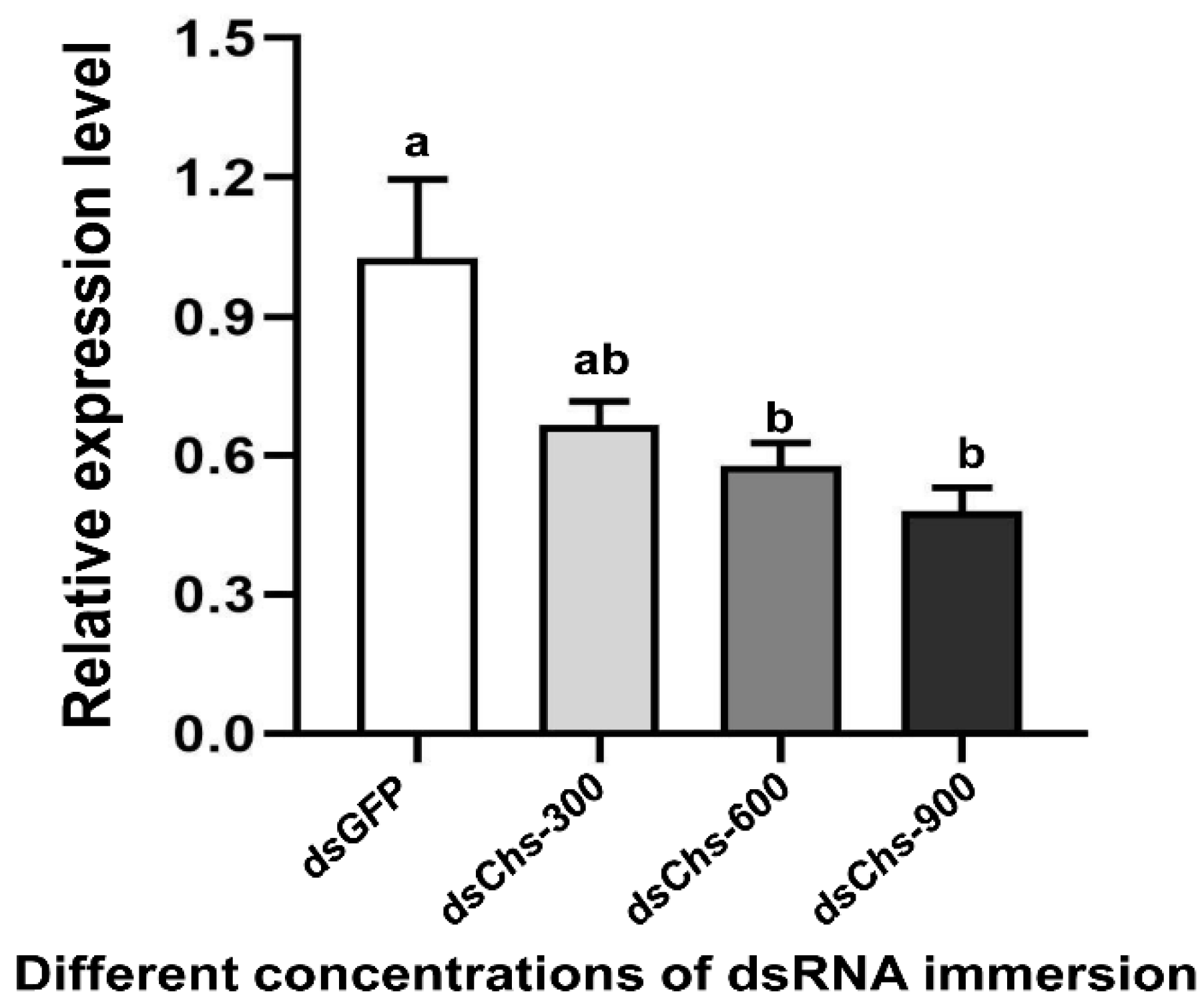
3.3. Functional Verification of CHS2 Transcript of L. trifolii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spencer, K.A. Agromyzidae (Diptera) of Economic Importance. 9: Series Entomologica; The Hague Publishers: Bath, UK, 1973; pp. 19–28. [Google Scholar]
- Wan, F.H.; Yang, N.W. Invasion and management of agricultural alien insects in China. Annu. Rev. Entomol. 2016, 6, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, B.; Wei, J.N.; Liu, T.X. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 2009, 54, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Reitz, S.; Xing, Z.L.; Ferguson, S.; Lei, Z.R. A decade of a leafminer invasion in China: Lessons learned. Pest Manag. Sci. 2017, 73, 1775–1779. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, Y.W.; Tang, X.T.; Zheng, S.Z.; Du, Y.Z. Population genetics of Liriomyza trifolii (Diptera: Agromyzidae) and comparison with four Liriomyza species in China based on COI, EF-1a and microsatellites loci. Sci. Rep. 2019, 9, 17856. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Reitz, S.R.; Wei, Q.B.; Yu, W.Y.; Lei, Z.R. Insecticide-mediated apparent displacement between two invasive species of leafminer fly. PLoS ONE 2012, 7, e36622. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Reitz, S.R.; Xiang, J.C.; Smagghe, G.; Lei, Z.R. Does Temperature-Mediated Reproductive Success Drive the Direction of Species Displacement in Two Invasive Species of Leafminer Fly? PLoS ONE 2014, 9, e98761. [Google Scholar] [CrossRef]
- Chang, Y.W.; Wang, Y.C.; Zhang, X.X.; Iqbal, J.; Lu, M.X.; Gong, H.X.; Du, Y.Z. Comparative transcriptome analysis of three invasive leafminer flies provides insights into interspecific competition. Int. J. Biol. Macromol. 2020, 165, 1664–1674. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Burand, J.P.; Hunter, W.B. RNAi: Future in insect management. J. Invertebr. Pathol. 2013, 112, S68–S74. [Google Scholar] [CrossRef]
- Palli, S.R. RNA interference in Colorado potato beetle: Steps toward development of dsRNA as a commercial insecticide. Curr. Opin. Insect Sci. 2014, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression. Southwest Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Tabara, H.; Grishok, A.; Mello, C.C. RNAi in C. elegans: Soaking in the genome sequence. Science 1998, 282, 430–431. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Guan, R.; Miao, X. Lepidopteran insect species-specific, broad-spectrum, and systemic RNA interference by spraying dsRNA on larvae. Entomol. Exp. Appl. 2015, 155, 218–228. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition, a revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Tellam, R.; Vuocolo, T.; Johnson, S.E.; Jarmey, J.; Pearson, R.D. Insect chitin synthase cDNA sequence, gene organization and expression. Eur. J. Biochem. 2000, 267, 6025–6043. [Google Scholar] [CrossRef]
- Gagou, M.E.; Kapsetaki, M.; Turberg, A.; Kafetzopoulos, D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem. Mol. Biol. 2002, 32, 141–146. [Google Scholar] [CrossRef]
- Arakane, Y.; Zhu, Y.C.; Kramer, K.J.; Charles, A.; Michael, R. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Mol. Biol. 2004, 34, 291–304. [Google Scholar] [CrossRef]
- Hogenkamp, D.G.; Arakane, Y.; Zimoch, L.; Merzendorfer, H.; Kramer, K.J.; Beeman, R.W.; Kanost, M.R.; Specht, C.A.; Muthukrishnan, S. Chitin synthase genes in Manduca sexta, characterization of a gut-specific transcript and differential tissue expression of alternately spliced mRNAs during development. Insect Biochem. Mol. Biol. 2005, 35, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Chen, X.F.; Tang, B.; Tian, H.G.; Chen, J.; Yao, Q. Insect chitin biosynthesis and its regulation. Chinese J. Appl. Entomol. 2011, 48, 475–479. [Google Scholar]
- Yang, X.S.; Xu, Y.; Yin, Q.; Zhang, H.B.; Yin, H.T.; Sun, Y.; Ma, L.; Zhou, D.; Shen, B. Physiological characterization of chitin synthase A responsible for the biosynthesis of cuticle chitin in Culex pipiens pallens (Diptera: Culicidae). Parasit. Vectors 2021, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. RNAi for chitin synthase 1 rather than 2 causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2021, 178, 104934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Xia, L.; Du, J.; Li, S.W.; Zhao, F. Cloning, characterization, and RNAi effect of the chitin synthase B gene in Cnaphalocrocis medinalis. J. Asia Pac. Entomol. 2021, 24, 486–492. [Google Scholar] [CrossRef]
- Chen, B.; Kang, L. Cold hardiness and supercooling capacity in the pea leafminer Liriomyza huidobrensis. Cryo. Lett. 2002, 23, 173–182. [Google Scholar]
- Chang, Y.W.; Zhang, X.X.; Lu, M.X.; Gong, W.R.; Du, Y.Z. Transcriptome analysis of Liriomyza trifolii (Diptera: Agromyzidae) in response to temperature stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100677. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chen, J.Y.; Lu, M.X.; Gao, Y.; Tian, Z.H.; Gong, W.R.; Dong, C.S.; Du, Y.Z. Cloning and expression of genes encoding heat shock proteins in Liriomyza trifolii and comparison with two congener leafminer species. PLoS ONE 2017, 12, e0181355. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal-X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chen, J.Y.; Lu, M.X.; Gao, Y.; Tian, Z.H.; Gong, W.R.; Zhu, W.; Du, Y.Z. Selection and validation of reference genes for quantitative real time PCR analysis under different experimental conditions in the leafminer Liriomyza trifolii (Diptera: Agromyzidae). PLoS ONE 2017, 12, e0181862. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B Biochem. 2006, 176, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, Q.; Gao, Y.; Liu, Z.F.; Shi, G.C.; Zhang, P.J.; Fan, R.J. Molecular cloning, sequence analysis and expression of chitin synthase 2gene in the oriental fruit moth Graphlitha molesta (Busck). Chinese J. Appl. Entomol. 2017, 54, 407–416. [Google Scholar]
- Zhao, W.J.; Zhang, C.L.; Zhai, S.Z.; Sun, Q.; Zhang, J. Expression and Analysis of Chitin Synthase Gene CqCHS1 and CqCHS2 in Culex quinquefasciatus. Genom. Appl. Biol. 2016, 35, 2317–2323. [Google Scholar]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2005, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Wang, Y.C.; Zhang, X.X.; Iqbal, J.; Du, Y.Z. RNA interference of genes encoding the vacuolar-ATPase in Liriomyza trifolii. Insects 2021, 12, 41. [Google Scholar] [CrossRef]
- Baum, J.A.; Roberts, J.K. Progress towards RNAi-mediated insect pest management. Adv. Insect Physiol. 2014, 47, 249–295. [Google Scholar]
- Parrella, M.P. Biology of Liriomyza. Annu. Rev. Entomol. 1987, 32, 201–224. [Google Scholar] [CrossRef]
- Zhuo, W.W.; Chu, F.; Kong, L.F.; Tao, H.; Sima, Y.H.; Xu, S.Q. Chitin synthase B: A midgut-specific gene induced by insect hormones and involved in food intake in Bombyx mori larvae. Arch. Insect Biochem. Physiol. 2014, 85, 36–47. [Google Scholar] [CrossRef]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Tang, B.; Chen, X.F.; Tin, H.G.; Zhang, W.Q. Molecular cloning, expression pattern and comparative analysis of chitin synthase gene B in Spodoptera exigua. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008, 149, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Cui, M.; Li, D.Q.; Zhang, H.H.; Yang, M.L.; Zhang, J.Z. Expression, function and regulation of Chitin synthase 2 gene in Locusta migratoria. Sci. Agric. Sin. 2014, 47, 1330–1340. [Google Scholar]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]


| Gene | Primer Sequences (5′→3′) | Length (bp) | Tm (°C) | ||
| Primers for cDNA cloning and full-length cDNA amplification | |||||
| CHS2 | F | TACACACTGAGGGTTCCCATTT | 999 | 53.9 | |
| R | GAACTCATTGAAACCTTCTGGA | ||||
| 5′ | TTGTCATCGCTCTCACCCGCTCC | 393 | 64.5 | ||
| 3′ | CTGCGAATCTGACAGGGCGTTTG | 1566 | 61.6 | ||
| Primers for dsRNA synthesis | |||||
| dsCHS2 | F | TAATACGACTCACTATAGGGTTAGTTTGGATTTTACCTGGCA | 553 | 63.1 | |
| R | TAATACGACTCACTATAGGGTTCTACACGATAGCCTCTTTGC | ||||
| dsGFP | F | TAATACGACTCACTATAGGGCCTCGTGACCACCCTGACCTAC | 314 | 68.2 | |
| R | TAATACGACTCACTATAGGGCACCTTGATGCCGTTCTTCTGC | ||||
| Primers for qRT-PCR | Length (bp) | Tm (°C) | Efficiency (%) | ||
| qPCR-CHS2 | F | TACACACTGAGGGTTCCCATTTA | 116 | 59.0 | 97.5 |
| R | TCCATCATTTCATCCTTAGTTTC | ||||
| qPCR-Actin | F | TTGTATTGGACTCTGGTGACGG | 73 | 59.2 | 108.6 |
| R | GATAGCGTGAGGCAAAGCATAA | ||||
| qPCR-18S | F | GAAGCAGTTTGGGGGCATTA | 88 | 60.0 | 103.2 |
| R | TTGGCAAATGCTTTCGCTTA | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-W.; Wang, Y.-C.; Yan, Y.-Q.; Xie, H.-F.; Yuan, D.-R.; Du, Y.-Z. RNA Interference of Chitin Synthase 2 Gene in Liriomyza trifolii through Immersion in Double-Stranded RNA. Insects 2022, 13, 832. https://doi.org/10.3390/insects13090832
Chang Y-W, Wang Y-C, Yan Y-Q, Xie H-F, Yuan D-R, Du Y-Z. RNA Interference of Chitin Synthase 2 Gene in Liriomyza trifolii through Immersion in Double-Stranded RNA. Insects. 2022; 13(9):832. https://doi.org/10.3390/insects13090832
Chicago/Turabian StyleChang, Ya-Wen, Yu-Cheng Wang, Yu-Qing Yan, Hong-Fang Xie, Deng-Rong Yuan, and Yu-Zhou Du. 2022. "RNA Interference of Chitin Synthase 2 Gene in Liriomyza trifolii through Immersion in Double-Stranded RNA" Insects 13, no. 9: 832. https://doi.org/10.3390/insects13090832





