Weeds Enhance Insect Diversity and Abundance and May Improve Soil Conditions in Mango Cultivation of South Florida
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- How does weed presence under mango trees affect the number and diversity of beneficial and pest arthropod on the mango trees?
- (2)
- How do weeds impact soil conditions?
- (1)
- There will be a higher abundance and diversity of beneficial insect species on the mango trees with weeds than on the trees without weeds present.
- (2)
- The presence of weeds may change properties of soil health beneath mango trees.
2. Materials and Methods
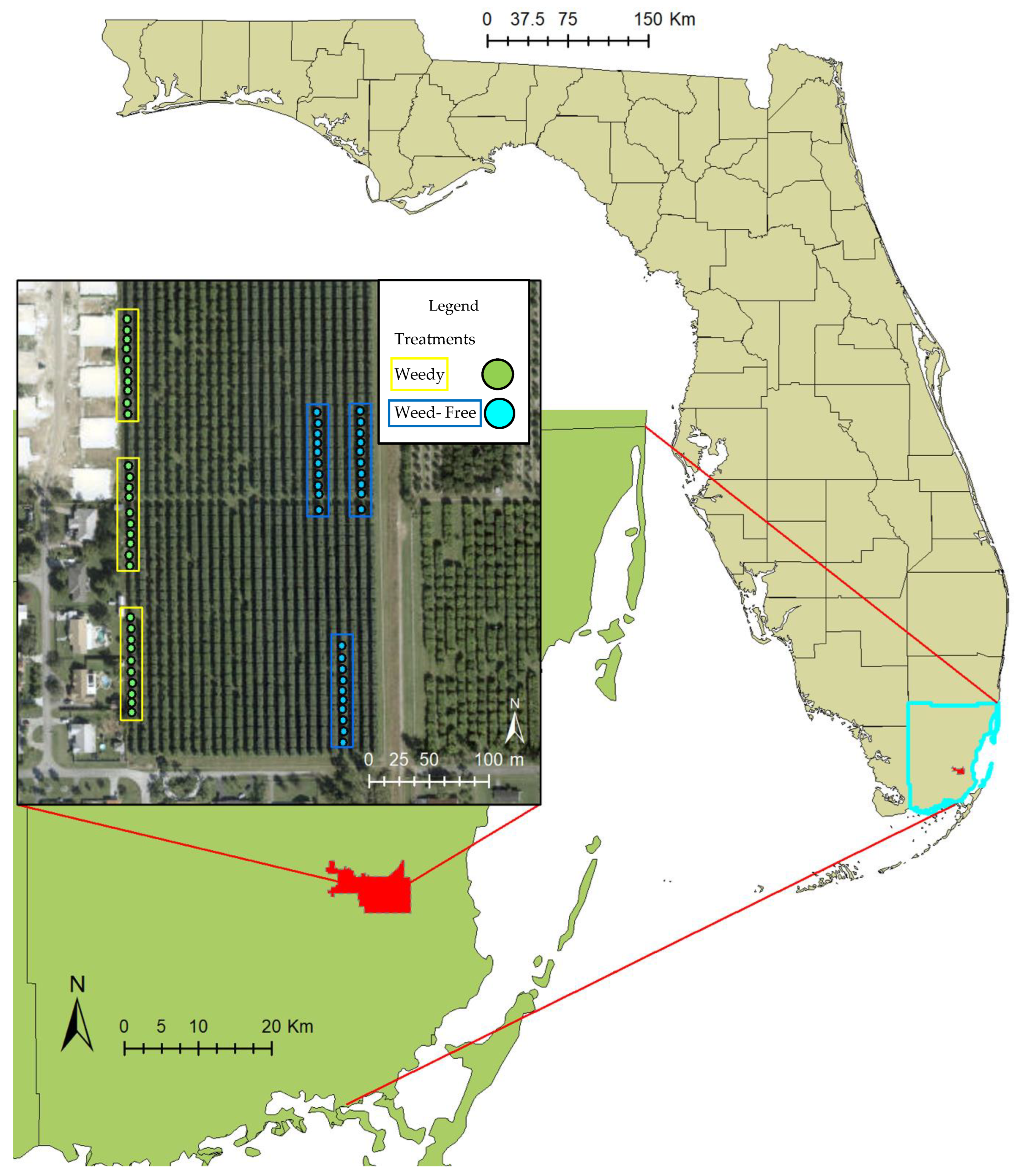
2.1. Site Description
2.2. Field Data Collection
2.3. Soil Analysis
2.4. Soil pH
2.5. Total Carbon and Nitrogen
2.6. Total Phosphorus
2.7. Chlorophyll Analysis
2.8. Statistical Analyses
3. Results
3.1. Insects on Mango
3.1.1. Insect Orders
3.1.2. Lacewings
3.1.3. Insect Families
3.1.4. Insect Interactions
3.1.5. Spider Interactions
3.1.6. Mango Diseases and Insect Damage
3.2. Soil
3.2.1. Phosphorous
3.2.2. Soil pH
3.2.3. Chlorophyll Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nutrient | Weed-Free ( ± SE) | Weedy ( ± SE) | F3,11, p |
|---|---|---|---|
| Nitrogen | 0.59, 0.629 | ||
| Beginning | 0.5 ± 0.1 | 0.4 ± 0.1 | |
| End | 0.6 ± 0.1 | 0.6 ± 0.1 | |
| Carbon | 1.311, 0.297 | ||
| Beginning | 12.3 ± 0.8 | 11.1 ± 0.2 | |
| End | 12.7 ± 0.8 | 12.8 ± 0.5 | |
| Phosphorous | 0.465, 0.511 | ||
| Beginning | 3.4 + 0.9 | 2.7 + 0.6 | |
| End | 4.0 + 0.7 | 3.5 + 0.9 |
References
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R.; Clough, Y. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Parolin, P.; Bresch, C.; Desneux, N.; Brun, R.; Bout, A.; Boll, R.; Poncet, C. Secondary plants used in biological control: A review. Int. J. Pest. Manag. 2012, 58, 91–100. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Pathways for the amplification of agroecology. Agroecol. Sustain. Food Syst. 2018, 42, 1170–1193. [Google Scholar] [CrossRef]
- Hogg, B.N.; Bugg, R.L.; Daane, K.M. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 2011, 56, 76–84. [Google Scholar] [CrossRef]
- Araj, S.; Shields, M.W.; Wratten, S.D. Weed floral resources and commonly used insectary plants to increase the efficacy of a whitefly parasitoid. Biocontrol 2019, 64, 553–561. [Google Scholar] [CrossRef]
- Denis, C.; Riudavets, J.; Gabarra, R.; Molina, P.; Arnó, J. Selection of insectary plants for the conservation of biological control agents of aphids and thrips in fruit orchards–corrigendum. Bull. Entomol. Res. 2021, 111, 768. [Google Scholar] [CrossRef]
- Kleiman, B.; Primoli, A.; Koptur, S.; Jayachandran, K. Weeds, pollinators, and parasitoids-Using weeds for insect manipulation in agriculture. J. Res. Weed Sci. 2020, 3, 382–390. [Google Scholar]
- Norris, R.F.; Kogan, M. Interactions between Weeds, Arthropod Pests, and Their Natural Enemies in Managed Ecosystems. Weed Sci. 2000, 48, 94. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Arnold, S.E.J.; Elisante, F.; Mkenda, P.A.; Tembo, Y.L.B.; Ndakidemi, P.A.; Gurr, G.M.; Darbyshire, I.A.; Belmain, S.R.; Stevenson, P.C. Beneficial insects are associated with botanically rich margins with trees on small farms. Sci. Rep. 2021, 11, 15190. [Google Scholar] [CrossRef] [PubMed]
- Melin, A.; Rouget, M.; Colville, J.F.; Midgley, J.J.; Donaldson, J.S. Assessing the role of dispersed floral resources for managed bees in providing supporting ecosystem services for crop pollination. PeerJ 2018, 6, e5654. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Porto, R.G.; de Almeida, R.F.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. Pollination ecosystem services: A comprehensive review of economic values, research funding and policy actions. Food Sec. 2020, 12, 1425–1442. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Mmond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef] [Green Version]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- Balmer, O.; Géneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower companion plants increase pest parasitation and yield in cabbage fields: Experimental demonstration and call for caution. Biol. Control 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Provost, C.; Pedneault, K. The organic vineyard as a balanced ecosystem: Improved organic grape management and impacts on wine quality. Sci. Hortic. 2016, 208, 43–56. [Google Scholar] [CrossRef]
- Cabrera-Mireles, H.; Murillo-Cuevas, F.D.; Ortega-Zaleta, D.A.; Villanueva-Jimenez, J.A.; Escobar-Dominguez, A.A. Impact of Mango Manila Management Systems on Arthropods in Foliage and Weeds. Trop. Subtrop. Agroecosyst. 2011, 13, 317–326. [Google Scholar]
- Smith, B.M.; Aebischer, N.J.; Ewald, J.; Moreby, S.; Potter, C.; Holland, J.M. The Potential of Arable Weeds to Reverse Invertebrate Declines and Associated Ecosystem Services in Cereal Crops. Front. Sustain. Food Syst. 2020, 3, 118. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Kennedy, C.M.; Kremen, C.; Batáry, P.; Berendse, F.; Bommarco, R.; Bosque-Pérez, N.A.; Carvalheiro, L.G.; Snyder, W.E.; Williams, N.M.; et al. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Change Biol. 2017, 23, 4946–4957. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.S.; Dosdall, L.M.; Spence, J.R.; Willenborg, C.J. Field density and distribution of weeds are associated with spatial dynamics of omnivorous ground beetles (Coleoptera: Carabidae). Agric. Ecosyst. Environ. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Dag, A.; Gazit, S. Mango pollinators in Israel. J. Appl. Hortic. 2000, 2, 39–43. [Google Scholar] [CrossRef]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Seymour, C.L.; Veldtman, R.; Nicolson, S.W. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J. Appl. Ecol. 2010, 47, 810–820. [Google Scholar] [CrossRef]
- Kumar, S.; Joshi, P.C.; Nath, P.; Singh, V.K.; Mansotra, D.K. Role of Insects in Pollination of Mango Trees. Int. Res. J. Biol. Sci. 2016, 5, 64. [Google Scholar]
- Evenhuis, N.L.; Pape, T.; Pont, A.C.; Thompson, F.C. The Catalogue of Life Partnership: Biosystematic Database of World Diptera; Version 10.5; Catalogue of Life: Leiden, The Netherlands, 2008. [Google Scholar]
- Sánchez, M.; Velásquez, Y.; González, M.; Cuevas, J. Activity and foraging behaviour of the hoverfly Eristalinus aeneus (Scopoli, 1763) in protected cultivation of mango (Mangifera indica L.). Bull. Entomol. Res. 2022, 112, 101–109. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Seymour, C.L.; Nicolson, S.W.; Veldtman, R.; Clough, Y. Creating patches of native flowers facilitates crop pollination in large agricultural fields: Mango as a case study. J. Appl. Ecol. 2012, 49, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Carvalheiro, L.G.; Veldtman, R.; Shenkute, A.G.; Tesfay, G.B.; Pirk, C.W.W.; Donaldson, J.S.; Nicolson, S.W. Natural and within- farmland biodiversity enhances crop productivity. Ecol. Lett. 2011, 14, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Freidenreich, A.; Dattamudi, S.; Li, Y.; Jayachandran, K. Influence of Leguminous Cover Crops on Soil Chemical and Biological Properties in a No-Till Tropical Fruit Orchard. Land 2022, 11, 932. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 726, 367. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L. Integrating a complex rotation with no-till improves weed management in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Petit, S.; Boursault, A.; Le Guilloux, M.; Munier-Jolain, N.; Reboud, X. Weeds in agricultural landscapes. A review. Agronomy Sust. Dev. 2010, 31, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, N. In praise of weeds. Saturday Evening Post 2019, 291, 32. [Google Scholar]
- Murthy, R.K.; Basavaraj, B.; Raveendra, H.R. Carbon mineralization in soil amended with weeds and their composts. Karnataka J. Agric. Sci. 2010, 23, 514–516. [Google Scholar]
- U.S. Department of Agriculture. Soil Survey of Dade County, Florida; Natural Resources Conservation Service, 735 Government Printing Office: Washington, DC, USA, 1996.
- NOAA Extreme Weather Information Sheet; NOAA Office for Coastal Management: Charleston, SC, USA, 2006.
- Muhammad, F.; Ibrahim, M.; Pervez, M.A. Effect of fungicides on mango malformation. Pak. J. Biol. Sci. 1999, 2, 772–773. [Google Scholar]
- Kleiman, B.M.; Koptur, S.; Jayachandran, K. Weeds Enhance Pollinator Diversity and Fruit Yield in Mango. Insects 2021, 12, 1114. [Google Scholar] [CrossRef]
- Miller, R.O.; Kissel, D.E. Comparison of Soil pH Methods on Soils of North America. Soil Sci. Soc. Am. J. 2010, 74, 310–316. [Google Scholar] [CrossRef]
- Carter, K. What Soil Is Best for a Mango Tree? 2020. Available online: https://home-guides.sfgate.com/soil-mango-tree-58272.html (accessed on 4 May 2021).
- Microchemical Determination of Carbon, Hydrogen, and Nitrogen, Automated Method, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006; Chapter 12; pp. 5–6.
- Yeomans, J.C.; Bremner, J.M. Carbon and nitrogen analysis of soils by automated combustion techniques. Commun. Soil Sci. Plant Anal. 1991, 22, 843–850. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Method 365.1: Determination of Phosphorus by Semi-Automated Colorimetry; Environmental Monitoring Systems Laboratory, Office of Research and Development: Research Triangle Park, NC, USA, 1993.
- Sanford, J.R.; Larson, R.A. Evaluation of Phosphorus Filter Media for an Inline Subsurface Drainage Treatment System. J. Environ. Qual. 2016, 45, 1919–1925. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynthesis Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, B.; Koptur, S.; Jayachandran, K. Beneficial Interactions of Weeds and Pollinators to Improve Crop Production. J. Res. Weed Sci. 2021, 4, 151–164. [Google Scholar]
- Crochard, L.; Julliard, R.; Gaba, S.; Bretagnolle, V.; Baude, M.; Fontaine, C. Weeds from non-flowering crops as potential contributors to oilseed rape pollination. Agric. Ecosyst. Environ. 2022, 336, 108026. [Google Scholar] [CrossRef]
- Gabriela, R.; Diego, A.; Alejandro, B. Tomato (Solanum lycopersicum) specialized pollination is isolated from neighboring plants and pollinators. J. Pollinat. Ecol. 2022, 30, 29–38. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Gaba, S. Weeds for bees? A review. Agron. Sustain. Dev. 2015, 35, 891–909. [Google Scholar] [CrossRef]
- Kolkman, A.; Dopagne, C.; Piqueray, J. Sown wildflower strips offer promising long term results for butterfly conservation. J. Insect Conserv. 2022, 26, 387–400. [Google Scholar] [CrossRef]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wäckers, F.L. The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control 2006, 39, 179–185. [Google Scholar] [CrossRef]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Lavandero, B.; Wratten, S.; Shishehbor, P.; Worner, S. Enhancing the effectiveness of the parasitoid Diadegma semiclausum (Helen): Movement after use of nectar in the field. Biol. Control 2005, 34, 152–158. [Google Scholar] [CrossRef]
- Atakan, E. Influence of weedy field margins on abundance patterns of the predatory bugs Orius spp. and their prey, the western flower thrips (Frankliniella occidentalis), on faba bean. Phytoparasitica 2010, 38, 313–325. [Google Scholar] [CrossRef]
- Jacobsen, S.K.; Sigsgaard, L.; Johansen, A.B.; Thorup-Kristensen, K.; Jensen, P.M. The impact of reduced tillage and distance to field margin on predator functional diversity. J. Insect Conserv. 2022, 26, 491–501. [Google Scholar] [CrossRef]
- Otieno, N.E.; Jacobs, S.M.; Pryke, J.S. Small-scale traditional maize farming fosters greater arthropod diversity value than conventional maize farming. J. Insect Conserv. 2022, 26, 477–489. [Google Scholar] [CrossRef]
- Russell, E.P. Enemies hypothesis: A review of the effect of vegetational diversity on predatory insects and parasitoids. Environ. Entomol. 1989, 18, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Pompozzi, G.; de Santiago, F.; Blumetto, O.; Simó, M. Livestock systems preserving natural grasslands are biodiversity reservoirs that promote spiders’ conservation. J. Insect Conserv. 2022, 26, 453–462. [Google Scholar] [CrossRef]
- Rischen, T.; Geisbüsch, K.; Ruppert, D.; Fischer, K. Farmland biodiversity: Wildflower-sown islands within arable fields and grassy field margins both promote spider diversity. J. Insect Conserv. 2022, 26, 415–424. [Google Scholar] [CrossRef]
- Brooks, S.J.; Barnard, P.C. The green lacewings of the world: A generic review (Neuroptera: Chrysopidae). Bull. Nat. Hist. Mus. Entomol. Ser. 1990, 59, 117–286. [Google Scholar]
- Blackwell, M. Made for Each Other: Ascomycete Yeasts and Insects. Microbiol. Spectr. 2017, 5, 13. [Google Scholar] [CrossRef]
- Gundappa, A.T.; Shukla, P.K. Seasonal dynamics of mango hoppers and their management under subtropics. GERF Bull. Biosc. 2016, 7, 6–9. [Google Scholar]
- Schlesinger, W.H.; Andrews, J.A. Soil Respiration and the Global Carbon Cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Filippelli, G.M. The global phosphorus cycle; past, present, and future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Wasielewski, J. The Garden: Extension Connection. Let’s Talk Dirty: S. Florida Soils. 2015. Available online: https://ediblesouthflorida.ediblecommunities.com/things-do/lets-talk-dirty-s-florida-soils (accessed on 19 August 2022).
- Swain, S.C.; Dora, D.K.; Sahoo, S.C.; Padhi, S.K.; Sanyal, D. Influence of Mango-Based Intercropping Systems on Improvement of Soil Health under Rainfed Situation. Commun. Soil Sci. Plant Anal. 2012, 43, 2018–2026. [Google Scholar] [CrossRef]
- Shafagh-Kolvanagh, J.; Zehtab-Salmasi, S.; Javanshir, A.; Moghaddam, M.; Nasab, A.D.M. Effects of nitrogen and duration of weed interference on grain yield and SPAD (chlorophyll) value of soybean (Glycine max (L.) Merrill. J. Food Agric. Environ. 2008, 6, 368–373. [Google Scholar]
- Monasterolo, M.; Chacoff, N.P.; Segura, Á.D.; Benavidez, A.; Schliserman, P. Native pollinators increase fruit set while honeybees decrease the quality of mandarins in family farms. Basic Appl. Ecol. 2022, 64, 79–88. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gratton, C.; Diekötter, T. Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. J. Appl. Ecol. 2015, 52, 323–330. [Google Scholar] [CrossRef]
- Forbes, S.J.; Northfield, T.D. Increased pollinator habitat enhances cacao fruit set and predator conservation. Ecol. Appl. 2017, 27, 887–899. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Blare, T.; Ballen, F.; Singh, A.; Haley, N.; Crane, J. Profitability and Cost Estimates for Producing Mango (Mangifera indica L.); Food and Resource Economics Department, UF/IFAS Extension: Homestead, FL, USA, 2022; FE1115. [Google Scholar]
- Needham, J.G. Ecological Notes on the Insect Population of the Flower Heads of Bidens pilosa. Ecol. Monogr. 1948, 18, 431–446. [Google Scholar] [CrossRef]
- Salas, A. Effects of Host-Plant Density on Herbivores and Their Parasitoids: A Field Experiment with a Native Perennial Legume. Master’s Thesis, Florida International University, Miami, FL, USA, 2016. [Google Scholar]
- Stokstad, E. Jury verdicts cloud future of popular herbicide: Fears of health risks and resistant weeds spur search for glyphosate alternatives. Science 2019, 364, 717. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Pryke, J.S.; Settele, J.; Smith, B.; Kratschmer, S.; Maes, D.; León-Cortés, J.L. Journal of Insect Conservation’s special issue on insect diversity in Agriculture. J. Insect Conserv. 2022, 26, 337–338. [Google Scholar] [CrossRef]

| Type of Arthropod | Weed-Free ( ± SE) | Weedy ( ± SE) | F1,57, p |
|---|---|---|---|
| Flower Visitor | 17.47 ± 1.63 | 31.59 ± 1.63 | 37.36, <0.001 |
| Predator | 59.27 ± 1.60 | 62.60 ± 1.60 | 2.16, 0.15 |
| Herbivore | 42.78 ± 0.95 | 45.42 ± 0.95 | 3.82, 0.056 |
| Parasitoid | 7.25 ± 0.74 | 14.01 ± 0.74 | 40.95, <0.0001 |
| Order | Weed-Free ( ± SE) | Weedy ( ± SE) | F1,57, p |
|---|---|---|---|
| Diptera | 58.4 ± 2.5 | 68.5 ± 2.5 | 8.35, 0.005 |
| Hemiptera | 44.0 ± 1.0 | 48.7 ± 1.0 | 11.22, 0.001 |
| Hymenoptera | 9.0 ± 0.9 | 21.2 ± 0.9 | 93.39, 0.000 |
| Lepidoptera | 7.5 ± 0.7 | 9.6 ± 0.7 | 3.97, 0.051 |
| Thysanoptera | 7.8 ± 0.5 | 7.1 ± 0.5 | 1.33, 0.254 |
| Neuroptera | 6.5 ± 0.5 | 4.3 ± 0.5 | 11.33, 0.001 |
| Collembola | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.01, 0.925 |
| Odonata | 2.0 ± 0.3 | 2.2 ± 0.3 | 0.35, 0.55 |
| Coleoptera | 0.7 ± 0.2 | 2.1 ± 0.2 | 16.53, 0.000 |
| Orthoptera | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.12, 0.73 |
| Order/Family | Weed-Free ( ± SE) | Weedy ( ± SE) | F1,57, p |
|---|---|---|---|
| Hemiptera/Coccidae | 37.3 ± 0.8 | 39.8 ± 0.8 | 4.749, 0.034 |
| Aphididae | 2.1 ± 0.4 | 3.3 ± 0.4 | 4.569, 0.037 |
| Cicadellidae | 2.3 ± 0.3 | 1.7 ± 0.3 | 2.469, 0.122 |
| Aleyrodidae | 0.9 ± 0.2 | 1.1 ± 0.2 | 0.613, 0.437 |
| Flatidae | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.003, 0.958 |
| Pseudococcidae | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.214, 0.645 |
| Diptera/Dolichopodidae | 19.7 ± 0.5 | 19.7 ± 0.5 | 0.001, 0.975 |
| Muscidae | 4.8 ± 0.7 | 8.7 ± 0.7 | 17.074, 0.000 |
| Syrphidae | 4.5 ± 0.5 | 7.0 ± 0.4 | 15.277, 0.000 |
| Chironomidae | 6.0 ± 0.4 | 4.6 ± 0.4 | 5.056, 0.028 |
| Tephritidae | 3.6 ± 0.4 | 4.2 ± 0.4 | 1.089, 0.301 |
| Calliphoridae | 2.6 ± 0.4 | 4.0 ± 0.4 | 6.157, 0.016 |
| Sarcophagidae | 3.7 ± 0.4 | 3.6 ± 0.4 | 0.074, 0.786 |
| Ephydridae | 1.8 ± 0.3 | 2.8 ± 0.3 | 6.994, 0.011 |
| Chloropidae | 1.6 ± 0.3 | 2.7 ± 0.3 | 7.345, 0.009 |
| Phoridae | 1.5 ± 0.2 | 1.9 ± 0.2 | 1.600, 0.211 |
| Drosophilidae | 1.3 ± 0.2 | 0.9 ± 0.2 | 1.96, 0.167 |
| Mycetophillidae | 0.8 ± 0.2 | 0.4 ± 0.2 | 2.537, 0.117 |
| Sciaridae | 0.3 ± 0.1 | 0.7 ± 0.1 | 5.150, 0.027 |
| Anisopodidae | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.007, 0.932 |
| Stratiomyidae | 0.1 ± 0.1 | 0.3 ± 0.1 | 5.097, 0.028 |
| Neuroptera/Chrysopidae | 6.6 ± 0.4 | 4.3 ± 0.4 | 15.304, 0.000 |
| Hymenoptera/Apidae | 1.9 ± 0.4 | 4.2 ± 0.4 | 19.075, 0.000 |
| Formicidae | 0.3 ± 0.4 | 2.23 ± 0.4 | 11.723, 0.001 |
| Chalcididae | 0.4 ± 0.2 | 1.4 ± 0.2 | 11.522, 0.001 |
| Vespidae | 0.2 ± 0.2 | 1.1 ± 0.2 | 9.762, 0.003 |
| Ichneumonidae | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.727, 0.194 |
| Braconidae | 0.1 ± 0.1 | 0.8 ± 0.1 | 31.024, 0.000 |
| Odonata/Anisoptera | 1.9 ± 0.3 | 1.9 ± 0.3 | 0.003, 0.958 |
| Lepidoptera/Lycaenidae | 1.4 ± 0.2 | 2.1 ± 0.2 | 4.854, 0.032 |
| Hesperiidae | 0.5 ± 0.2 | 1.1 ± 0.2 | 8.443, 0.005 |
| Geometridae | 0.0 ± 0.1 | 0.6 ± 0.1 | 10.033, 0.002 |
| Nymphalidae | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.066, 0.799 |
| Pieridae | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.009, 0.924 |
| Coleoptera/Coccinellidae | 0.1 ± 0.1 | 1.1 ± 0.1 | 24.782, 0.000 |
| Scarabidae | 0.1 ± 0.1 | 0.3 ± 0.1 | 2.880, 0.095 |
| Leaf Age | Leaf Chlorophyll Concentration on Trees in | |
|---|---|---|
| Weedy Plots (N = 18 All) | Weed-Free Plots (N) | |
| New | 16.9 + 2.4 a | 36.6 + 5.1 b 22 |
| Mature | 54.4 + 1.9 c | 56.4 + 3.1 c 16 |
| Old | 23.0 + 3.1 a | 42.8 + 4.6 b 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleiman, B.; Koptur, S. Weeds Enhance Insect Diversity and Abundance and May Improve Soil Conditions in Mango Cultivation of South Florida. Insects 2023, 14, 65. https://doi.org/10.3390/insects14010065
Kleiman B, Koptur S. Weeds Enhance Insect Diversity and Abundance and May Improve Soil Conditions in Mango Cultivation of South Florida. Insects. 2023; 14(1):65. https://doi.org/10.3390/insects14010065
Chicago/Turabian StyleKleiman, Blaire, and Suzanne Koptur. 2023. "Weeds Enhance Insect Diversity and Abundance and May Improve Soil Conditions in Mango Cultivation of South Florida" Insects 14, no. 1: 65. https://doi.org/10.3390/insects14010065





