Bacterial Communities of the Internal Reproductive and Digestive Tracts of Virgin and Mated Tuta absoluta
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Breeding
2.2. Sample Preparation and DNA Extraction
2.3. 16S rRNA Gene Amplification and Sequencing
2.4. 16S rRNA-Based Bacterial Community Analysis
3. Results
3.1. Sequencing Data
3.2. Microbiomes of Virgin Tomato Leafminer
3.3. Mating-Induced Changes in Genital Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Skidmore, I.H.; Hansen, A.K. The evolutionary development of plant-feeding insects and their nutritional endosymbionts. Insect Sci. 2017, 24, 910–928. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, J.; Xue, H.; Zhang, W.; Zhang, Y.; Liu, Y.; Fang, L.; Wang, Y.; Wang, H. The Gut Microbiota in Camellia Weevils Are Influenced by Plant Secondary Metabolites and Contribute to Saponin Degradation. mSystems 2020, 5, e00692-19. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Engelstadter, J.; Hurst, G.D.D. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Dion, E.; Polin, S.E.; Simon, J.C.; Outreman, Y. Symbiont infection affects aphid defensive behaviours. Biol. Lett. 2011, 7, 743–746. [Google Scholar] [CrossRef]
- Damodaram, K.J.; Ayyasamy, A.; Kempraj, V. Commensal Bacteria Aid Mate-selection in the Fruit Fly, Bactrocera dorsalis. Microb. Ecol. 2016, 72, 725–729. [Google Scholar] [CrossRef]
- Hadapad, A.B.; Prabhakar, C.S.; Chandekar, S.C.; Tripathi, J.; Hire, R.S. Diversity of bacterial communities in the midgut of Bactrocera cucurbitae (Diptera: Tephritidae) populations and their potential use as attractants. Pest Manag. Sci. 2016, 72, 1222–1230. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Caceres, C.; Bourtzis, K. Medflfly gut microbiota and enhancement of the sterile insect technique: Similarities and differences of klebsiella oxytoca and Enterobacter sp. AA26 probiotics during the larval and adult stages of the VIENNA 8(D53+) genetic sexing strain. Front. Microbiol. 2017, 8, 2064. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Bestion, E.; Jacob, S.; Zinger, L.; Di Gesu, L.; Richard, M.; White, J.; Cote, J. Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat. Ecol. Evol. 2017, 1, 161. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Li, J.Y.; Hu, Z.Q.; Liu, T.X.; Zhang, S.Z. Fall Armyworm Gut Bacterial Diversity Associated with Different Developmental Stages, Environmental Habitats, and Diets. Insects 2022, 13, 762. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, W.X.; Wan, F.H.; Xian, X.Q.; Zhang, Y.B.; Guo, J.Y. Bioecology, damage and management of the tomato leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), a worldwide quarantine pest. J. Biosci. Bioeng. 2018, 3, 155–163. [Google Scholar]
- Desneux, N.; Wajnberg, E.; Tabone, E. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Hai, Y.Q.; Liu, Y. Morphological and biological characteristics of tomato leaf miners. China Plant Prot. 2022, 42, 24–28. [Google Scholar]
- Perlmutter, J.I.; Bordenstein, S.R. Microorganisms in the reproductive tissues of arthropods. Nat. Rev. Microbiol. 2022, 18, 97–111. [Google Scholar] [CrossRef]
- Graber, J.R.; Breznak, J.A. Physiology and nutrition of Treponema primitia, an H2/CO2-acetogenic spirochete from termite hindguts. Appl. Environ. Microbiol. 2004, 70, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Fraune, S.; Bosch, T.C. Why bacteria matter in animal development and evolution. BioEssays News Rev. Mol. Cell. Dev. Biol. 2010, 32, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tokuda, G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, T.Z.; Zhu, H.F.; Pan, H.B.; Yu, X.P. Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. 2019, 27, 883–894. [Google Scholar] [CrossRef]
- Segata, N.; Baldini, F.; Pompon, J.; Garrett, W.S.; Truong, D.T.; Dabiré, R.K.; Diabaté, A.; Levashina, E.A.; Catteruccia, F. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers. Sci. Rep. 2016, 6, 24207. [Google Scholar] [CrossRef]
- Bellinvia, S.; Johnston, P.R.; Mbedi, S.; Otti, O. Mating changes the genital microbiome in both sexes of the common bedbug Cimex lectularius across populations. Proc. Biol. Sci. 2020, 287, 20200302. [Google Scholar] [CrossRef]
- Carayon, J. Traumatic insemination and the paragenital system. In Monograph of Cimicidae (Hemiptera-Heteroptera); Usinger, R., Ed.; Entomological Society of America: Philadelphia, PA, USA, 1966; pp. 81–167. [Google Scholar]
- Pernice, M.; Simpson, S.J.; Ponton, F. Towards an integrated understanding of gut microbiota using insects as model systems. J. Insect Physiol. 2014, 69, 12–18. [Google Scholar] [CrossRef]
- Matos, R.C.; Leulier, F. Lactobacilli-Host mutualism: “Learning on the fly”. Microb. Cell Fact. 2014, 13 (Suppl. 1), S6. [Google Scholar] [CrossRef]
- Brown, J.J.; Jandová, A.; Jeffs, C.T.; Higgie, M.; Nováková, E.; Lewis, O.T.; Hrček, J. Microbiome Structure of a Wild Drosophila Community along Tropical Elevational Gradients and Comparison to Laboratory Lines. Appl. Environ. Microbiol. 2023, 89, e0009923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pang, Z.C.; Yu, X.Q.; Wang, X.Y. Insect gut microbiota research: Progress and applications. China J. Appl. Entomol. 2020, 57, 600–607. [Google Scholar]
- Behar, A.; Jurkevitch, E.; Yuval, B. Bringing back the fruit into fruit fly-bacteria interactions. Mol. Ecol. 2008, 17, 1375–1386. [Google Scholar] [CrossRef]
- Li, G.N.; Xia, X.J.; Tang, W.C.; Zhu, Y. Intestinal microecology associated with fluoride resistance capability of the silkworm (Bombyx mori L.). Appl. Microbiol. Biotechnol. 2016, 100, 6715–6724. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Y.; Jack, C.J.; Diao, Q.; Fu, Z.; Dai, P. Effects of Tropilaelaps mercedesae on midgut bacterial diversity of Apis mellifera. Exp. Appl. Acarol. 2019, 79, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; He, C.; Li, M.L. Analysis of intestinal bacterial community diversity of adult Dastarcus helophoroides. J. Insect Sci. 2014, 14, 114. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, X.; Yi, C.Y.; Chen, X.Y.; Liu, C.H.; Zhang, C.C.; Chen, Q.D.; Chen, S.; Liu, H.L.; Pu, D.Q. The adaptive evolution in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) revealed by the diversity of larval gut bacteria. Genes 2023, 14, 321. [Google Scholar] [CrossRef]
- Li, G.; Zheng, X.; Zhu, Y.; Long, Y.; Xia, X. Bacillus symbiont drives alterations in intestinal microbiota and circulating metabolites of lepidopteran host. Environ. Microbiol. 2022, 24, 4049–4064. [Google Scholar] [CrossRef]
- Lin, C.; Wei, Z.; Yi, Z.; Tingting, T.; Huamao, D.; Lichun, F. Analysis of the effects of nanosilver on bacterial community in the intestinal fluid of silkworms using high-throughput sequencing. Bull. Entomol. Res. 2020, 110, 309–320. [Google Scholar] [CrossRef]
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat. Rev. Genet. 2008, 9, 218–229. [Google Scholar] [CrossRef]
- Brune, A.; Ohkuma, M. Role of the termite gut microbiota in symbiotic digestion. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 439–476. [Google Scholar]
- Zurek, L.; Schal, C.; Watson, D.W. Diversity and contribution of the intestinal bacterial community to the development of Musca domestica (Diptera: Muscidae) larvae. J. Med. Entomol. 2000, 37, 924–928. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Kaltenpoth, M. Actinobacteria as mutualists: General healthcare for insects? Trends Microbiol. 2009, 17, 529–535. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.Y.; Kamagata, Y.; Fukatsu, T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009, 7, 2. [Google Scholar] [CrossRef]
- Ohkuma, M.; Brune, A. Diversity, structure, and evolution of the termite gut microbial community. In Biology of Temites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 413–438. [Google Scholar]
- Zhang, J.; Ding, X.; Guan, R.; Zhu, C.; Xu, C.; Zhu, B.; Zhang, H.; Xiong, Z.; Xue, Y.; Tu, J.; et al. Evaluation of different 16S rRNA gene V regions for exploring bacterial diversity in a eutrophic freshwater lake. Sci. Total Environ. 2018, 618, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhu, Y.H.; Lai, F.X. Research advances in symbiotic microorganisms in insects and their functions. Acta Entomol. Sin. SciEngine 2021, 64, 121–140. [Google Scholar]
- Adlerberth, I.; Ahrne, S.; Johansson, M.L.; Molin, G.; Hanson, L.A.; Wold, A.E. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl. Environ. Microbiol. 1996, 62, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Hoiseth, S.K.; Stocker, B.A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 1981, 291, 238–239. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhu, T.H.; Lai, F.X.; Fu, Q. Diversity and infection frequency of symbiotic bacteria in different populations of the rice brown planthopper in China. J. Entomol. Sci. 2015, 50, 47–66. [Google Scholar] [CrossRef]
- Harish, E.R.; ManiChellappan, K.; MakeshKumar, T.; Mathew, D.; Ranjith, M.T.; Girija, D. Next-generation sequencing reveals endosymbiont variability in cassava whitefly, Bemisia tabaci, across the agro-ecological zones of Kerala, India. Genome 2019, 62, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Gorb, S.N.; Reinhardt, K. Reduction of female copulatory damage by resilin represents evidence for tolerance in sexual conflict. J. R. Soc. Interface 2015, 12, 20141107. [Google Scholar] [CrossRef]
- Landmann, F. The Wolbachia Endosymbionts. Microbiol. Spectr. 2019, 7, BAI-0018-2019. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Slatko, B.E.; Luck, A.N.; Dobson, S.L.; Foster, J.M. Wolbachia endosymbionts and human disease control. Mol. Biochem. Parasitol. 2014, 195, 88–95. [Google Scholar] [CrossRef]
- Rasgon, J.L.; Gamston, C.E.; Ren, X. Survival of Wolbachia pipientis in cell-free medium. Appl. Environ. Microbiol. 2006, 72, 6934–6937. [Google Scholar] [CrossRef]
- Pietri, J.E.; DeBruhl, H.; Sullivan, W. The rich somatic life of Wolbachia. MicrobiologyOpen 2016, 5, 923–936. [Google Scholar] [CrossRef]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Rainey, S.M.; Shah, P.; Kohl, A.; Dietrich, I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: Progress and challenges. J. Gen. Virol. 2014, 95 Pt 3, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Wang, J.J. Reproductive regulation of hosts by Wolbachia and its progress. Insect Knowl. 2006, 288–294. [Google Scholar]
- Zchori-Fein, E.; Borad, C.; Harari, A.R. Oogenesis in the date stone beetle, Coccotrypes dactyliperda, depends on symbiotic bacteria. Physiol. Entomol. 2006, 31, 164–169. [Google Scholar] [CrossRef]
- Chen, S.J.; Lu, F.; Cheng, J.A.; Jiang, M.X.; Way, M.O. Identification and biological role of the endosymbionts Wolbachia in rice water weevil (Coleoptera: Curculionidae). Environ. Entomol. 2012, 41, 469–477. [Google Scholar] [CrossRef]
- Flórez, L.V.; Scherlach, K.; Gaube, P.; Ross, C.; Sitte, E.; Hermes, C.; Rodrigues, A.; Hertweck, C.; Kaltenpoth, M. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat. Commun. 2017, 8, 15172. [Google Scholar] [CrossRef]
- Stammer, H.J. Die Symbiose der Lagriiden (Coleoptera). Zoomorphology 1929, 15, 1–34. [Google Scholar]
- Kosako, Y.; Tamura, K.; Sakazaki, R.; Miki, K. Enterobacter kobei sp. nov., a new species of the family Enterobacteriaceae resembling Enterobacter cloacae. Curr. microbiol. 1996, 33, 261–265. [Google Scholar] [CrossRef]
- Manandhar, S.; Nguyen, Q.; Pham, D.T.; Amatya, P.; Rabaa, M.; Dongol, S.; Basnyat, B.; Dixit, S.M.; Baker, S.; Karkey, A. A fatal outbreak of neonatal sepsis caused by mcr-10-carrying Enterobacter kobei in a tertiary care hospital in Nepal. J. Hosp. Infect. 2022, 125, 60–66. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, M.J.; Jeon, S.J.; Park, S.H.; Han, S.; Park, S.H.; Yu, J.K.; Kang, M.; Jang, J.I.; Lee, J.H.; et al. Identification of a carbapenem-resistant Enterobacter kobei clinical strain co-harbouring mcr-4.3 and mcr-9 in Republic of Korea. J. Glob. Antimicrobe. Resist. 2021, 26, 114–116. [Google Scholar] [CrossRef]
- Reynolds, K.T.; Hoffmann, A.A. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 2002, 80, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bouwma, A.M.; Shoemaker, D. Wolbachia wSinvictaA infections in natural populations of the fire ant Solenopsis invicta: Testing for phenotypic effects. J. Insect Sci. 2011, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Spencer, J.L.; Curzi, M.J.; Zavala, J.A.; Seufferheld, M.J. Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc. Natl. Acad. Sci. USA 2013, 110, 11917–11922.2. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.; Yao, Z.; Awan, U.A.; Zhang, H. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Y.C.; Chen, H.J.; Yuan, J.Z.; Wei, D.Y.; Wang, X.Y. Isolation, identification and analysis of virulence and drug resistance of Klebsiella pneumoniae from companion animals. China Anim. Husb. Vet. Med. 2020, 47, 1583–1592. [Google Scholar]
- Du, H.M.; Lis, W.B.; Liu, R.J. Recent advances in the study of insect symbiotic bacteria. J. Environ. Entomol. 2020, 42, 615–629. [Google Scholar]
- Salem, H.; Bauer, E.; Kirsch, R.; Berasategui, A.; Cripps, M.; Weiss, B.; Koga, R.; Fukumori, K.; Vogel, H.; Fukatsu, T.; et al. Drastic Genome Reduction in an Herbivore’s Pectinolytic Symbiont. Cell 2017, 171, 1520–1531.e13. [Google Scholar] [CrossRef]
- Wilson, A.C.; Ashton, P.D.; Calevro, F.; Charles, H.; Colella, S.; Febvay, G.; Jander, G.; Kushlan, P.F.; Macdonald, S.J.; Schwartz, J.F.; et al. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol. Biol. 2010, 19 (Suppl. 2), 249–258. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P. Endosymbioses of Animals with Plant Microorganisms; John Wiley: Chichester, UK, 1965. [Google Scholar]
- Braendle, C.; Miura, T.; Bickel, R.; Shingleton, A.W.; Kambhampati, S.; Stern, D.L. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 2003, 1, E21. [Google Scholar] [CrossRef] [PubMed]
- Heike, F. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Thierry, M.; Becker, N.; Hajri, A.; Reynaud, B.; Lett, J.M.; Delatte, H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011, 20, 2172–2187. [Google Scholar] [CrossRef]



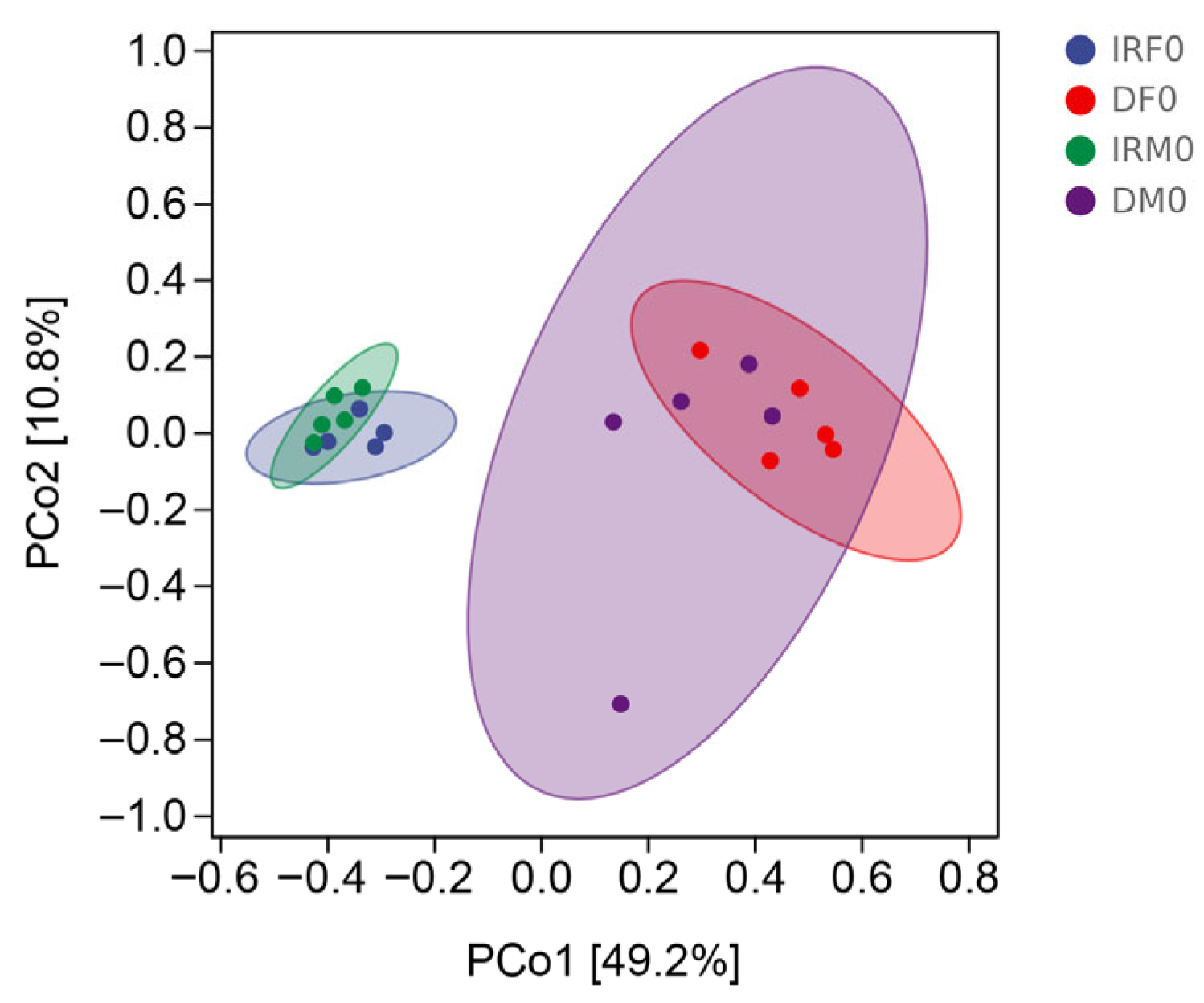
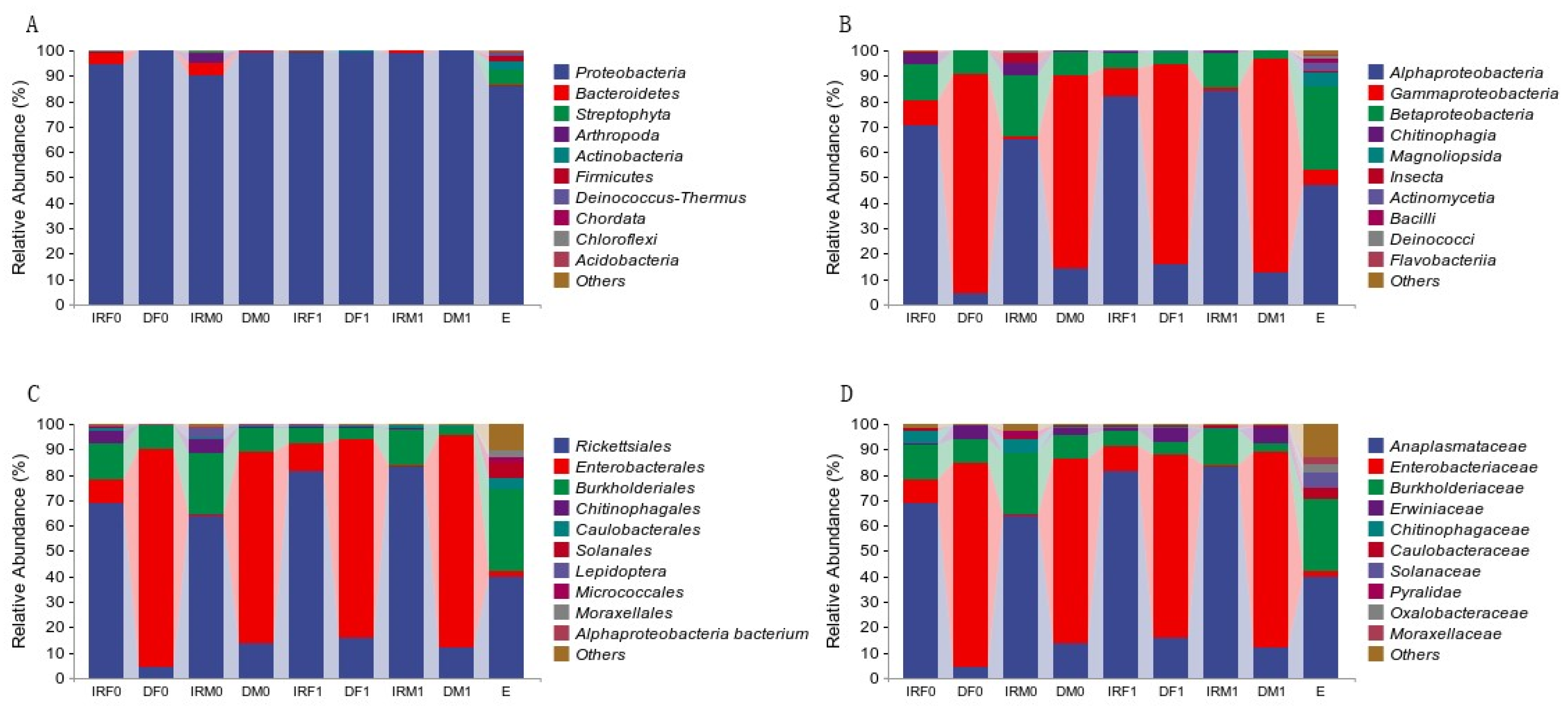
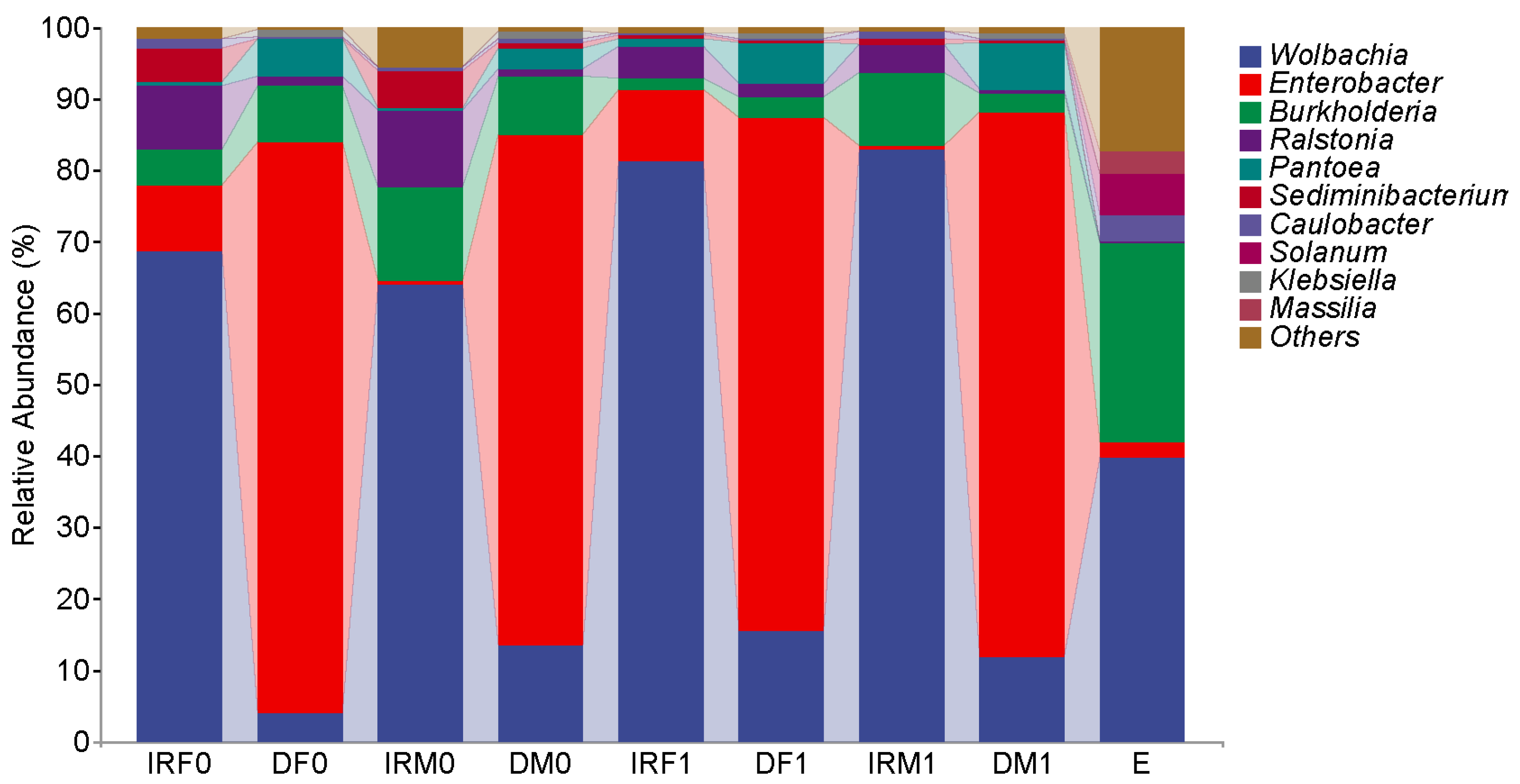
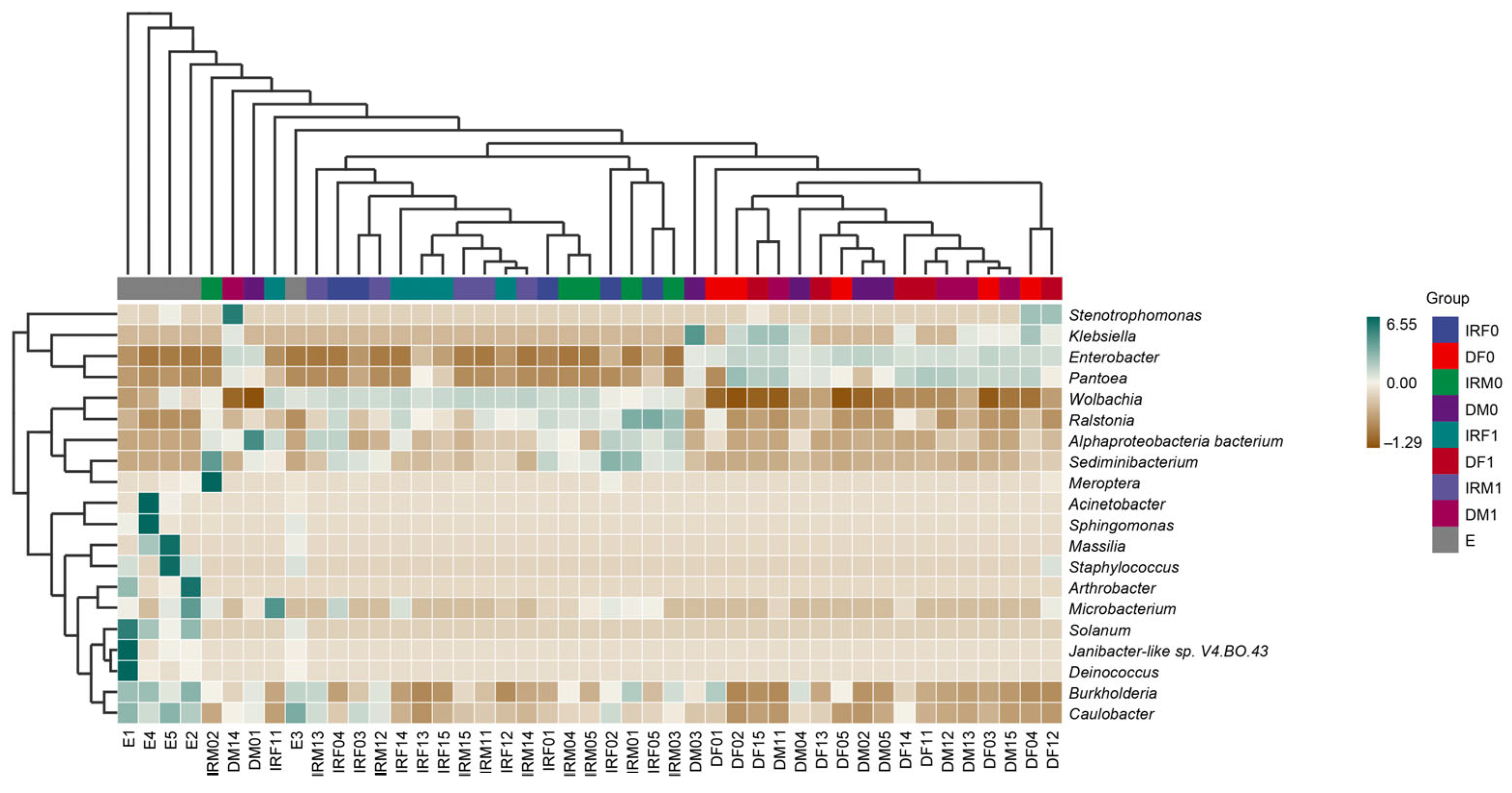

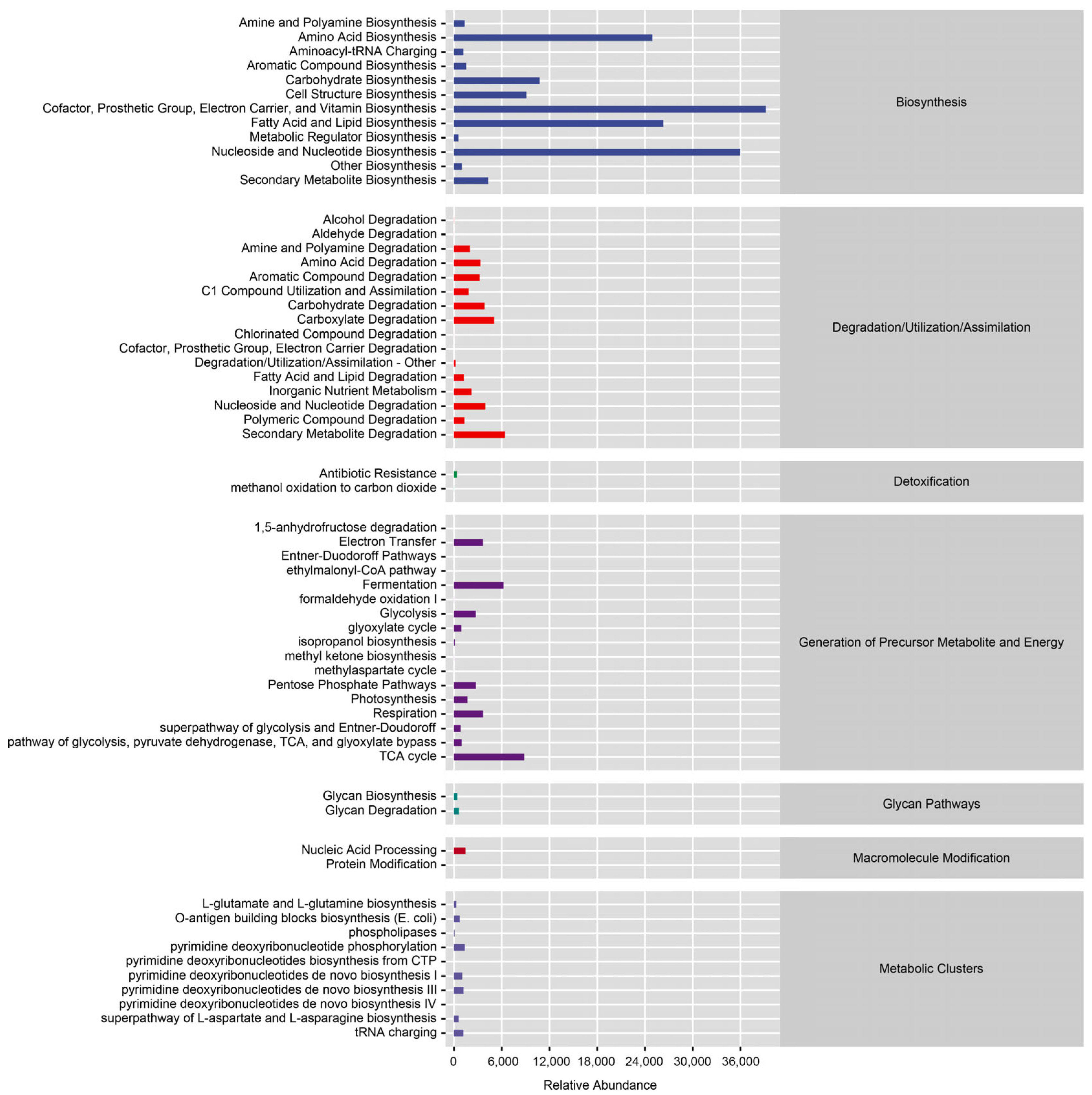
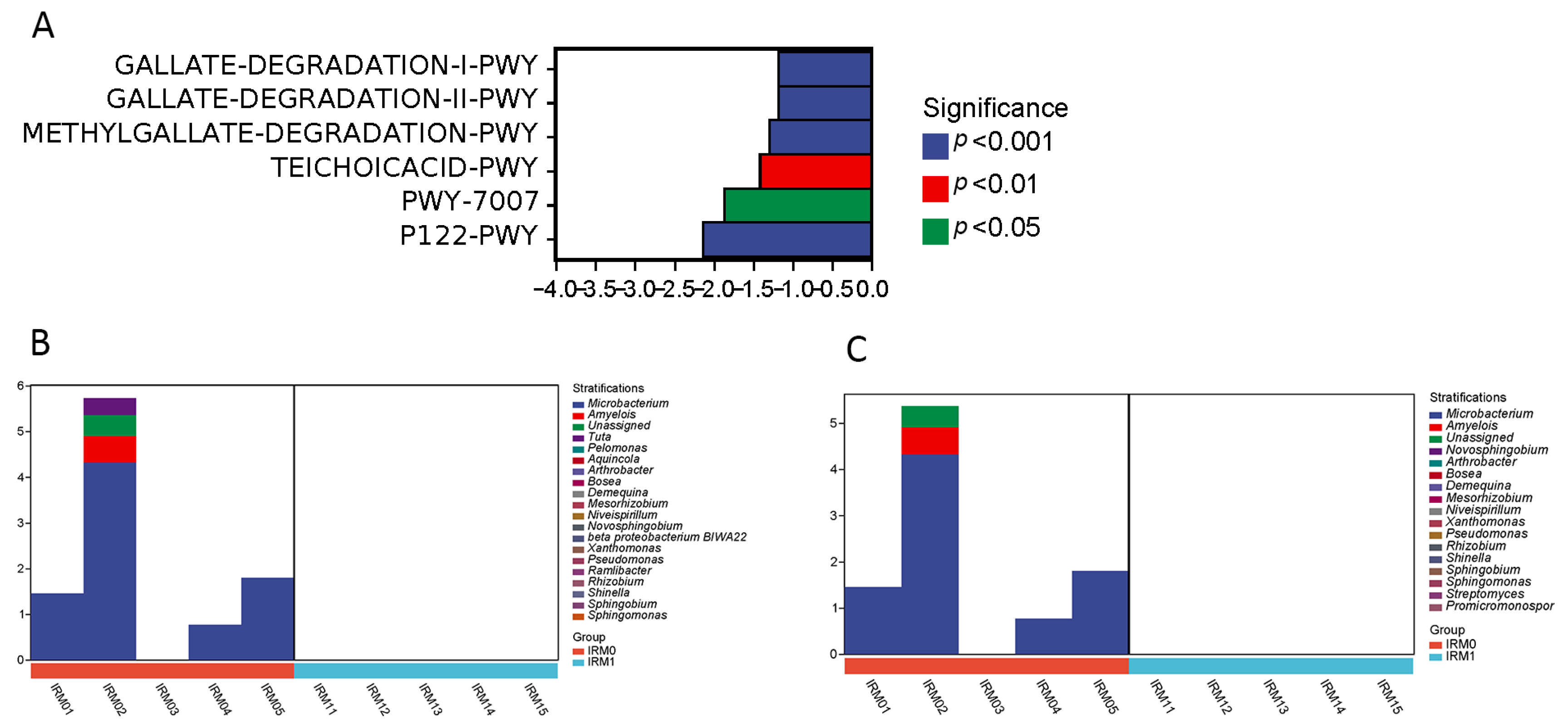
| Sample | Organ | Sex | Mating Status | Total Number |
|---|---|---|---|---|
| IRF0 | Internal Reproductive System | Female | Virgin | 20 × 5 |
| IRM0 | Internal Reproductive System | Male | Virgin | 20 × 5 |
| IRF1 | Internal Reproductive System | Female | Mated | 20 × 5 |
| IRM1 | Internal Reproductive System | Male | Mated | 20 × 5 |
| DF0 | Digestive tract | Female | Virgin | 20 × 5 |
| DM0 | Digestive tract | Male | Virgin | 20 × 5 |
| DF1 | Digestive tract | Female | Mated | 20 × 5 |
| DM1 | Digestive tract | Male | Mated | 20 × 5 |
| Egg | - | - | - | 200 × 5 |
| ID | IRF0 | IRM0 | IRF1 | IRM1 |
|---|---|---|---|---|
| Wolbachia endosymbiont of Bryobia spec. I VIDR-2008 | 68.73 ± 5.66% | 67.34 ± 8.03% | 81.43 ± 2.24% | 82.76 ± 3.25% |
| Burkholderia cenocepacia | 5.05 ± 1.12% | 14.29 ± 4.40% | 1.54 ± 0.39% | 10.19 ± 3.12% |
| Ralstonia sp. 1F2 | 8.93 ± 2.55% | 12.19 ± 2.43% | 4.47 ± 1.19% | 3.92 ± 0.85% |
| Sediminibacterium salmoneum | 4.73 ± 0.91% | 3.90 ± 1.11% | 0.69 ± 0.15% | 0.79 ± 0.31% |
| Enterobacter cancerogenus | 2.02 ± 0.80% | 0.23 ± 0.19% | 4.22 ± 1.25% | 0.36 ± 0.16% |
| Enterobacter asburiae | 1.40 ± 0.32% | 0.08 ± 0.03% | 2.50 ± 0.67% | 0.13 ± 0.07% |
| Enterobacter kobei | 1.04 ± 0.59% | 0.00% | 2.23 ± 1.18% | 0.15 ± 0.06% |
| Caulobacter sp. | 1.23 ± 0.37% | 0.67 ± 0.05% | 0.23 ± 0.07% | 1.02 ± 0.32% |
| Enterobacter hormaechei | 2.60 ± 1.69% | 0.00% | 0.00% | 0.00% |
| Enterobacter cloacae | 1.82 ± 1.15% | 0.00% | 0.00% | 0.00% |
| Pantoea sp. NJ-32 | 0.43 ± 0.25% | 0.00% | 1.10 ± 0.39% | 0.00% |
| Alphaproteobacteria bacterium | 0.51 ± 0.14% | 0.47 ± 0.16% | 0.26 ± 0.10% | 0.22 ± 0.13% |
| Enterobacter sp. CTSP4 | 0.32 ± 0.19% | 0.00% | 0.85 ± 0.45% | 0.00% |
| Microbacterium sp. Sw0106-31(2) | 0.15 ± 0.07% | 0.06 ± 0.02% | 0.28 ± 0.19% | 0.00% |
| Afipia sp. BAC308 | 0.10 ± 0.03% | 0.00% | 0.00% | 0.00% |
| alpha proteobacterium PII-14 | 0.00% | 0.00% | 0.00% | 0.04 ± 0.02% |
| Others | 0.57 ± 0.46% | 0.06 ± 0.04% | 0.12 ± 0.06% | 0.09 ± 0.05% |
| ID | DF0 | DM0 | DF1 | DM1 |
|---|---|---|---|---|
| Enterobacter cancerogenus | 32.79 ± 2.24% | 21.34 ± 1.09% | 30.72 ± 1.95% | 33.50 ± 1.89% |
| Enterobacter asburiae | 26.93 ± 3.44% | 23.94 ± 6.44% | 18.67 ± 2.23% | 19.43 ± 1.76% |
| Enterobacter kobei | 12.61 ± 3.29% | 7.82 ± 1.59% | 16.18 ± 2.63% | 17.40 ± 1.63% |
| Wolbachia endosymbiont of Bryobia spec. I VIDR-2008 | 4.01 ± 1.34% | 13.35 ± 4.67% | 14.34 ± 3.11% | 11.94 ± 3.36% |
| Burkholderia cenocepacia | 7.82 ± 4.39% | 8.22 ± 2.75% | 3.43 ± 0.94% | 2.70 ± 0.87% |
| Pantoea sp. NJ-32 | 5.19 ± 1.55% | 2.84 ± 0.48% | 6.67 ± 0.74% | 6.41 ± 0.61% |
| Enterobacter sp. CTSP4 | 4.83 ± 1.31% | 2.59 ± 0.64% | 5.20 ± 0.68% | 5.16 ± 0.25% |
| Ralstonia sp. 1F2 | 1.47 ± 0.84% | 1.05 ± 0.43% | 2.07 ± 0.50% | 0.64 ± 0.29% |
| Klebsiella aerogenes | 0.64 ± 0.27% | 0.00% | 0.82 ± 0.50% | 0.97 ± 0.36% |
| Enterobacter cloacae | 1.03 ± 0.67% | 0.00% | 0.00% | 0.00% |
| Caulobacter sp. | 0.21 ± 0.08% | 0.55 ± 0.20% | 0.49 ± 0.15% | 0.29 ± 0.14% |
| Sediminibacterium salmoneum | 0.17 ± 0.11% | 0.68 ± 0.36% | 0.18 ± 0.04% | 0.11 ± 0.03% |
| Alphaproteobacteria bacterium | 0.00% | 0.46 ± 0.36% | 0.00% | 0.11 ± 0.05% |
| Others | 0.23 ± 0.10% | 0.04 ± 0.02% | 0.32 ± 0.24% | 0.20 ± 0.12% |
| ID | E |
|---|---|
| Wolbachia endosymbiont of Bryobia spec. I VIDR-2008 | 39.76 ± 7.85% |
| Burkholderia cenocepacia | 27.86 ± 3.78% |
| Solanum violaceimarmoratum | 4.82 ± 1.84% |
| Caulobacter sp. | 2.88 ± 0.48% |
| Enterobacter asburiae | 1.32 ± 1.01% |
| Janibacter-like sp. V4.BO.43 | 0.93 ± 0.75% |
| Solanum pennellii | 0.92 ± 0.27% |
| Caulobacter sp. FWC26 | 0.76 ± 0.48% |
| Asticcacaulis excentricus | 0.67 ± 0.32% |
| Arthrobacter sp. KAR53 | 0.66 ± 0.56% |
| Arthrobacter sp. JSM 101049 | 0.56 ± 0.31% |
| Deinococcus petrolearius | 0.51 ± 0.42% |
| Enterobacter cancerogenus | 0.44 ± 0.11% |
| Staphylococcus warneri | 0.40 ± 0.20% |
| [Empedobacter] haloabium | 0.37 ± 0.16% |
| Others | 13.03 ± 2.48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, S.; Wang, X.; Tang, Y.; Lei, K.; Guo, J.; Yang, N.; Wan, F.; Lü, Z.; Liu, W. Bacterial Communities of the Internal Reproductive and Digestive Tracts of Virgin and Mated Tuta absoluta. Insects 2023, 14, 779. https://doi.org/10.3390/insects14100779
Bi S, Wang X, Tang Y, Lei K, Guo J, Yang N, Wan F, Lü Z, Liu W. Bacterial Communities of the Internal Reproductive and Digestive Tracts of Virgin and Mated Tuta absoluta. Insects. 2023; 14(10):779. https://doi.org/10.3390/insects14100779
Chicago/Turabian StyleBi, Siyan, Xiaodi Wang, Yanhong Tang, Kexin Lei, Jianyang Guo, Nianwan Yang, Fanghao Wan, Zhichuang Lü, and Wanxue Liu. 2023. "Bacterial Communities of the Internal Reproductive and Digestive Tracts of Virgin and Mated Tuta absoluta" Insects 14, no. 10: 779. https://doi.org/10.3390/insects14100779






