Co-Occurrence of Wing Deformity and Impaired Mobility of Alates with Deformed Wing Virus in Solenopsis invicta Buren (Hymenoptera: Formicidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ant Colony Collection and Maintenance
2.2. RNA Extraction, cDNA Synthesis, and Reverse-Transcriptase PCR
2.3. Sanger Sequencing
2.4. Detection of DWV Replication in Solenopsis invicta
2.5. Videos of DW and NW S. invicta Alates
3. Results and Discussion
3.1. Symptom of DW Alates of S. invicta
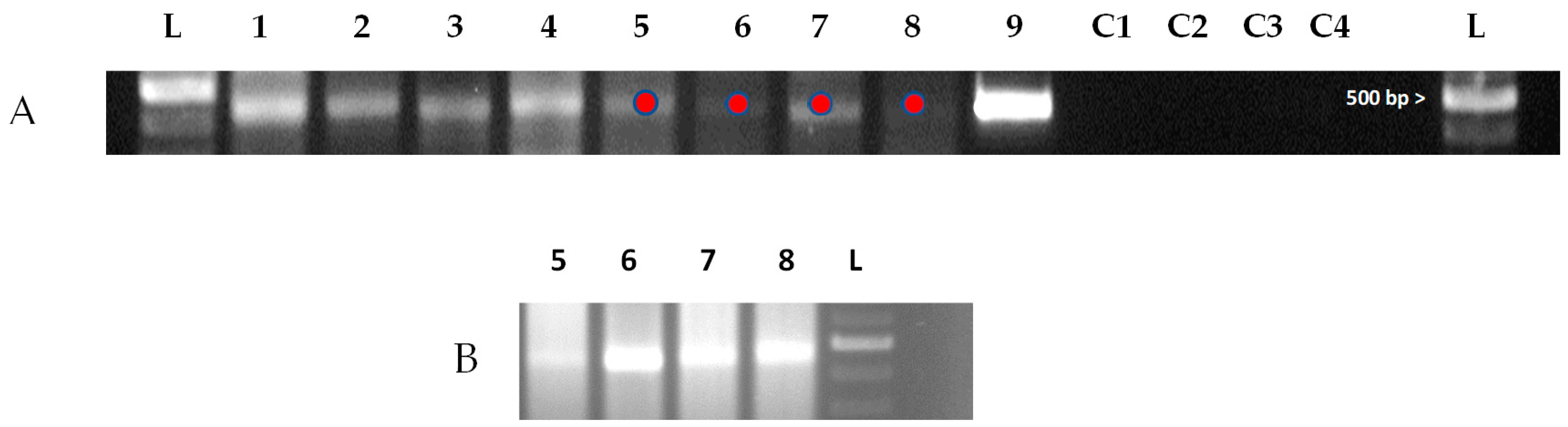
3.2. Identification of DWV in S. invicta DW Alates and Workers
3.3. Identification of the Replication Form of DWV in S. invicta DW Alates and Workers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species. A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2004; 12p. [Google Scholar]
- Ascunce, M.S.; Yang, C.C.; Oakey, J.; Calcaterra, L.; Wu, W.J.; Shih, C.J.; Goudet, J.; Ross, K.G.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B.; Greenberg, L. The biology, physiology, and ecology of imported fire ants. In Economic Impact and Control of Social Insects; Vinson, S.B., Ed.; Praeger: New York, NY, USA, 1986; pp. 193–226. 421p. [Google Scholar]
- Vinson, B.S. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef]
- Payne, A.N.; Shepherd, T.F.; Rangel, J. The detection of honey bee (Apis mellifera)-associated viruses in ants. Sci. Rep. 2020, 10, 2923. [Google Scholar] [CrossRef]
- Oi, D.; Porter, S.D.; Valles, S.M. A review of the biological control of fire ants (Hymenoptera: Formicidae). Myrmecol. News 2015, 21, 101–116. [Google Scholar]
- Martin, S.L.; Brettell, L.E. Deformed wing virus in honeybees and other insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Arévalo, S.; Fernández-Carrión, E.; Goyache, J.; Molero, F.; Puerta, F.; Sánchez-Vizcaíno, J.M. High Load of Deformed Wing Virus and Varroa destructor Infestation Are Related to Weakness of Honey Bee Colonies in Southern Spain. Front. Microbiol. 2019, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Genersch, E. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 2005, 86, 3419–3424. [Google Scholar] [CrossRef]
- Gruber, M.A.; Cooling, M.; Baty, J.W.; Buckley, K.; Friedlander, A.; Quinn, O.; Russell, J.F.; Sébastien, A.; Lester, P.J. Single-stranded RNA viruses infecting the invasive Argentine ant, Linepithema humile. Sci. Rep. 2017, 7, 3304. [Google Scholar] [CrossRef]
- Škubník, K.; Nováček, J.; Füzik, T.; Přidal, A.; Paxton, R.J.; Plevka, P. Structure of deformed wing virus, a major honey bee pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, 3210–3215. [Google Scholar] [CrossRef]
- Kevill, J.L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A Lethal to Honey Bees (Apis mellifera): A Colony Level Survey of DWV Variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 2019, 11, 426. [Google Scholar] [CrossRef]
- Forzan, M.; Felicioli, A.; Sagona, S.; Bandecchi, P.P.; Mazzei, M. Complete Genome Sequence of Deformed Wing Virus Isolated from Vespa crabro in Italy. Genome Announc. 2017, 5, e00961-17. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. Development of a rapid and sensitive RT-PCR method for the detection of deformed wing virus, a pathogen of the honeybee (Apis mellifera). Vet. J. 2005, 169, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Sébastien, A.; Lester, P.J.; Hall, R.J.; Wang, J.; Moore, N.E.; Gruber, M.A.M. Invasive ants carry novel viruses in their new range and form reservoirs for a honeybee pathogen. Biol. Lett. 2015, 11, 20150610. [Google Scholar] [CrossRef] [PubMed]
- Schläppi, D.; Lattrell, P.; Yañez, O.; Chejanovsky, N.; Neumann, P. Foodborne transmission of deformed wing virus to ants (Myrmica rubra). Insects 2019, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Jouvenaz, D.P.; Allen, G.E.; Banks, W.A.; Wojcik, D.P. A survey for pathogens of fire ants, Solenopsis spp. in the southeastern United States. Fla. Entomol. 1977, 60, 275–279. [Google Scholar] [CrossRef]
- Sanger, F.; Nickler, S.; Coulson, A.R. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 1970, 74, 5463–5467. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kevill, J.L.; Highfield, A.; Mordecai, G.J.; Martin, S.M.; Schroeder, S.M. ABC Assay: Method Development and Application to Quantify the Role of Three DWV Master Variants in Overwinter Colony Losses of European Honey Bees. Viruses 2017, 9, 314. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013, 52, 1–55. [Google Scholar] [CrossRef]
- Craggs, J.K.; Ball, J.K.; Thomson, B.J.; Irving, W.L.; Grabowska, A.M. Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. J. Virol. Methods 2001, 94, 111–120. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Espadaler, X.; Wetterer, A.L.; Aguin-Pombo, D.; Franquinho-Aguiar, A.M. Long-term impact of exotic ants on the native ants of Madeira. Ecol. Entomol. 2006, 31, 358–368. [Google Scholar] [CrossRef]
- Cooling, M.; Hartley, S.; Sim, D.A.; Lester, P.J. The widespread collapse of an invasive species: Argentine ants (Linepithema humile) in New Zealand. Biol. Lett. 2012, 8, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar]
- Alburaki, M.; Chen, D.; Skinner, J.A.; Meikle, W.G.; Tarpy, D.R.; Adamczyk, J.; Stewart, S.D. Honey Bee survival and pathogen prevalence: From the perspective of landscape and exposure to pesticides. Insects 2018, 9, 65. [Google Scholar] [CrossRef]
- Baty, J.W.; Bulgarella, M.; Dobelmann, J.; Felden, A.; Lester, P.J. Viruses and their effects in ants (Hymenoptera: Formicidae). Myrmecol. News 2020, 30, 213–228. [Google Scholar]
- Power, K.; Martano, M.; Altamura, G.; Piscopo, N.; Maiolino, P. Histopathological Features of Symptomatic and Asymptomatic Honeybees Naturally Infected by Deformed Wing Virus. Pathogens 2021, 10, 874. [Google Scholar] [CrossRef]
- Zioni, N.; Soroker, V.; Chejanovsky, N. Replication of Varroa destructor virus 1 (VDV-1) and a Varroa destructor virus 1-deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology 2011, 417, 106–112. [Google Scholar] [CrossRef]
- Cilia, G.; Zavatta, L.; Ranalli, R.; Nanetti, A.; Bortolotti, L. Replicative Deformed Wing Virus Found in the Head of Adults from Symptomatic Commercial Bumblebee (Bombus terrestris) Colonies. Vet. Sci. 2021, 8, 117. [Google Scholar] [CrossRef]
- Forzan, M.; Simona, S.; Maurizio, M.; Antonio, F. Detection of deformed wing virus in Vespa crabro. Bull. Insectology 2017, 70, 261–265. [Google Scholar]
- Lester, P.J.; Buick, K.H.; Baty, J.W.; Felden, A.; Haywood, J. Different bacterial and viral pathogens trigger distinct immune responses in a globally invasive ant. Sci. Rep. 2019, 9, 5780. [Google Scholar] [CrossRef]
- Woodford, L.; Steketee, P.C.; Evans, D.J. Doomed drones? Using passage experiments and mathematical modelling to determine Deformed wing virus population dynamics in male honeybees. Proc. Biol. Sci. B 2023, 290, 20231010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, Y. Fire ants feed their nestmates with their own venom. J. Insect Physiol. 2022, 142, 104437. [Google Scholar] [CrossRef] [PubMed]


| Target | Primer Name | Primer Sequence | Product Size (bp) | Ref. |
|---|---|---|---|---|
| DWV | Forward | 5’-GAGATTGAAGCGCATGAACA-3’ | 130 | 25 |
| Reverse | 5’-TGAATTCAGTGTCGCCCATA-3’ | |||
| DWV-A | Forward | 5’-GTCTTGTGGATGAAGGTTATATAACTGG-3’ | 168 | 26 |
| Reverse | 5’-TCCGTAGAAAGCCGAGTTG-3’ | |||
| DWV | DWV-F F6 | 5’-TTTCCAGGTCCATTCCCCTATC-3’ | 393 | 14 |
| DWV-R B8 | 5’-TCATTCGCCTTACGACGGTTAG-3’ | |||
| DWV | DWV-F F15 | 5’-agcctgcgcaccgtggTCCATCAGGTTCTCCAATAACGG-3’ | 451 | 9 |
| DWV-R B23 | 5’-CCACCCAAATGCTAACTCTAACGC-3’ | |||
| Tag | 5’-agcctgcgcaccgtgg-3’ | |||
| DWV-B | DVQ_DWV-B-F | 5’-ACCAACGCGTGTCGTTCCTG-3’ | 108 | 26 |
| DVQ_DWV-B-R | 5’-ACAAGTGGTTGGTCCCGTCG-3’ |
| Colony | Collection Period | Male Alates | DW, (%) | Female Alates | DW, (%) |
|---|---|---|---|---|---|
| 2021 | 28/07/2022–12/09/2022 | 89 | 9, (10) | 0 | 0 |
| 1 | 12/08/2022–26/08/2022 | 122 | 7, (5.7) | 0 | 0 |
| 5 | 24/08/2022–26/09/2022 | 42 | 6, (14) | 398 | 0 |
| 7 | 21/08/2022–27/09/2022 | 657 | 9, (1.4) | 32 | 0 |
| 8 | 21/08/2022–27/09/2022 | 11 | 1, (9.1) | 606 | 6, (1) |
| 8 * | 15/10/2022–29/11/2022 | 118 | 39, (33) * | 0 | 0 |
| 9 | 17/08/2022–13/09/2022 | 55 | 4, (7.5) | 0 | 0 |
| 10 | 22/08/2022–26/08/2022 | 14 | 2, (14.3) | 425 | 0 |
| 12 | 9/08/2022–6/09/2022 | 73 | 11, (15) | 987 | 0 |
| 14 | 9/08/2022–30/08/2022 | 139 | 15, (10) | 4 | 0 |
| 1B | 2/09/2022–27/09/2022 | 325 | 14, (4.3) | 22 | 4, (18) |
| 3B | 4/09/2022–26/09/2022 | 33 | 4, (12) | 385 | 0 |
| 5B | 4/09/2022–14/10/2022 | 36 | 4, (11) | 0 | 0 |
| 1C | 12/04/2023–23/06/2023 | 83 | 11, (13.25) | 0 | 0 |
| 3C | 12/04/2023–22/06/2023 | 113 | 6, (5.3) | 9 | 0 |
| 5C | 12/04/2023–27/06/2023 | 46 | 3, (6.5) | 11 | 0 |
| Colony | Sample Type | Replicates | DWV Detected? | Colony | Sample Type | Replicates | DWV Detected? |
|---|---|---|---|---|---|---|---|
| 1B | DW male alate | 1 | Yes | 9 | DW male alate | 1 | Yes |
| 2 | Yes | 2 | Yes | ||||
| NW male alate | 1 | Yes | Worker | 1 | No | ||
| 2 | Yes | 2 | Yes | ||||
| Worker | 1 | Yes | 10 | DW male alate | 1 | No | |
| 2 | Yes | 2 | Yes | ||||
| 3 | No | Worker | 1 | Yes | |||
| NCBL | DW male alate | 1 | No | 2 | No | ||
| 2 | Yes | 3 | Yes | ||||
| 3 | Yes | 11 | DW male alate | 1 | No | ||
| DW female alate | 1 | No | 2 | No | |||
| 2 | Yes | 3 | No | ||||
| NW male alate | 1 | Yes | Worker | 1 | Yes | ||
| 2 | Yes | 2 | No | ||||
| NW female alate | 1 | Yes | 12 | DW male alate | 1 | No | |
| Worker | 1 | Yes | 2 | No | |||
| 2 | No | 3 | No | ||||
| 3 | Yes | Worker | 1 | No | |||
| 4 | Yes | 2 | No | ||||
| 2021 | Worker | 1 | Yes | 14 | DW male alate | 1 | Yes |
| 2 | Yes | 2 | Yes | ||||
| 3 | Yes | NW male alate | 1 | Yes | |||
| 4 | DW male alate | 1 | No | 2 | Yes | ||
| 2 | No | Worker | 1 | No | |||
| 3 | No | 2 | Yes | ||||
| DW female alate | 1 | No | 3 | Yes | |||
| 2 | No | G1 | Worker | 1 | Yes | ||
| 3 | Yes | 2 | Yes | ||||
| Worker | 1 | No | G2 | Queen | 1 | Yes | |
| 2 | No | Worker | 1 | Yes | |||
| 8 | DW male alate | 1 | No | 2 | No | ||
| 2 | Yes | ||||||
| 3 | Yes | ||||||
| DW female alate | 1 | Yes | |||||
| 2 | No | ||||||
| 3 | Yes | ||||||
| NW male alate | 1 | No | |||||
| 2 | Yes | ||||||
| 3 | Yes | ||||||
| NW female alate | 1 | No | |||||
| 2 | Yes | ||||||
| Worker | 1 | Yes | |||||
| 2 | No | ||||||
| 3 | Yes | ||||||
| 4 | Yes |
| Colony | Caste | Replication Dilution | Is Replication Detected? |
|---|---|---|---|
| 1B | Worker | 2X dilution | Yes |
| 1B | NW male alate | 2X dilution | Yes |
| NBCL | Worker | 2X dilution | Yes |
| 2021 | Worker | Not diluted | No |
| Colony 4 | Deformed wing female alate | 2X dilution | Yes |
| Colony 8 | Deformed wing male alate | 10X dilution | Yes |
| Colony 8 | Deformed wing female alate | 2X dilution | Yes |
| Colony 8 | Normal wing male alate | 10X dilution | Yes |
| Colony 8 | Pupa of male alate | 10X dilution | Yes |
| Colony 8 | Worker | 10X dilution | Yes |
| Colony 9 | Worker | Not diluted | No |
| Colony 10 | Worker | 2X dilution | Yes |
| Colony 10 | Pupa of Workers | 10X dilution | Yes |
| Colony 11 | Worker | Not diluted | No |
| Colony 12 | Worker | Not diluted | No |
| Colony 14 | Normal wing male alate | 2X dilution | Yes |
| Colony 14 | Worker | Not diluted | No |
| Colony G1 | Worker | Not diluted | No |
| Colony G2 | Worker | Not diluted | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miles, G.P.; Liu, X.F.; Amiri, E.; Grodowitz, M.J.; Allen, M.L.; Chen, J. Co-Occurrence of Wing Deformity and Impaired Mobility of Alates with Deformed Wing Virus in Solenopsis invicta Buren (Hymenoptera: Formicidae). Insects 2023, 14, 788. https://doi.org/10.3390/insects14100788
Miles GP, Liu XF, Amiri E, Grodowitz MJ, Allen ML, Chen J. Co-Occurrence of Wing Deformity and Impaired Mobility of Alates with Deformed Wing Virus in Solenopsis invicta Buren (Hymenoptera: Formicidae). Insects. 2023; 14(10):788. https://doi.org/10.3390/insects14100788
Chicago/Turabian StyleMiles, Godfrey P., Xiaofen F. Liu, Esmaeil Amiri, Michael J. Grodowitz, Margaret L. Allen, and Jian Chen. 2023. "Co-Occurrence of Wing Deformity and Impaired Mobility of Alates with Deformed Wing Virus in Solenopsis invicta Buren (Hymenoptera: Formicidae)" Insects 14, no. 10: 788. https://doi.org/10.3390/insects14100788






