Effect of Leaf Removal and Insecticide Applications on Population Densities of Leafhoppers and Mites Associated with Grapevines
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Impact on Grapevine Arthropod Pests
1.2. Effects on Beneficial Arthropods
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design
2.3. Sampling
2.4. Point Quadrat Analysis (PQA)
2.5. Data Analysis
3. Results
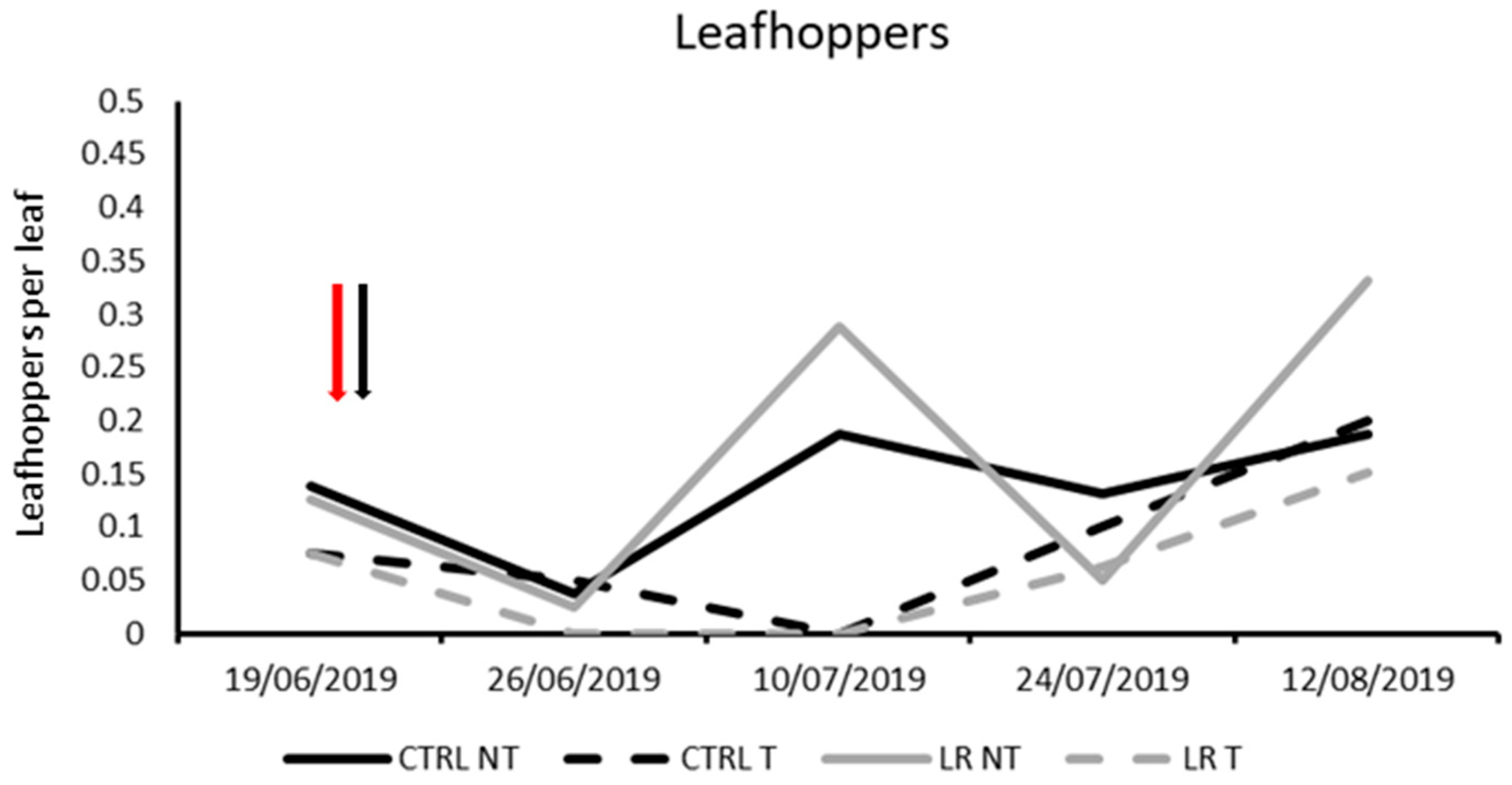
3.1. Farm A—2019
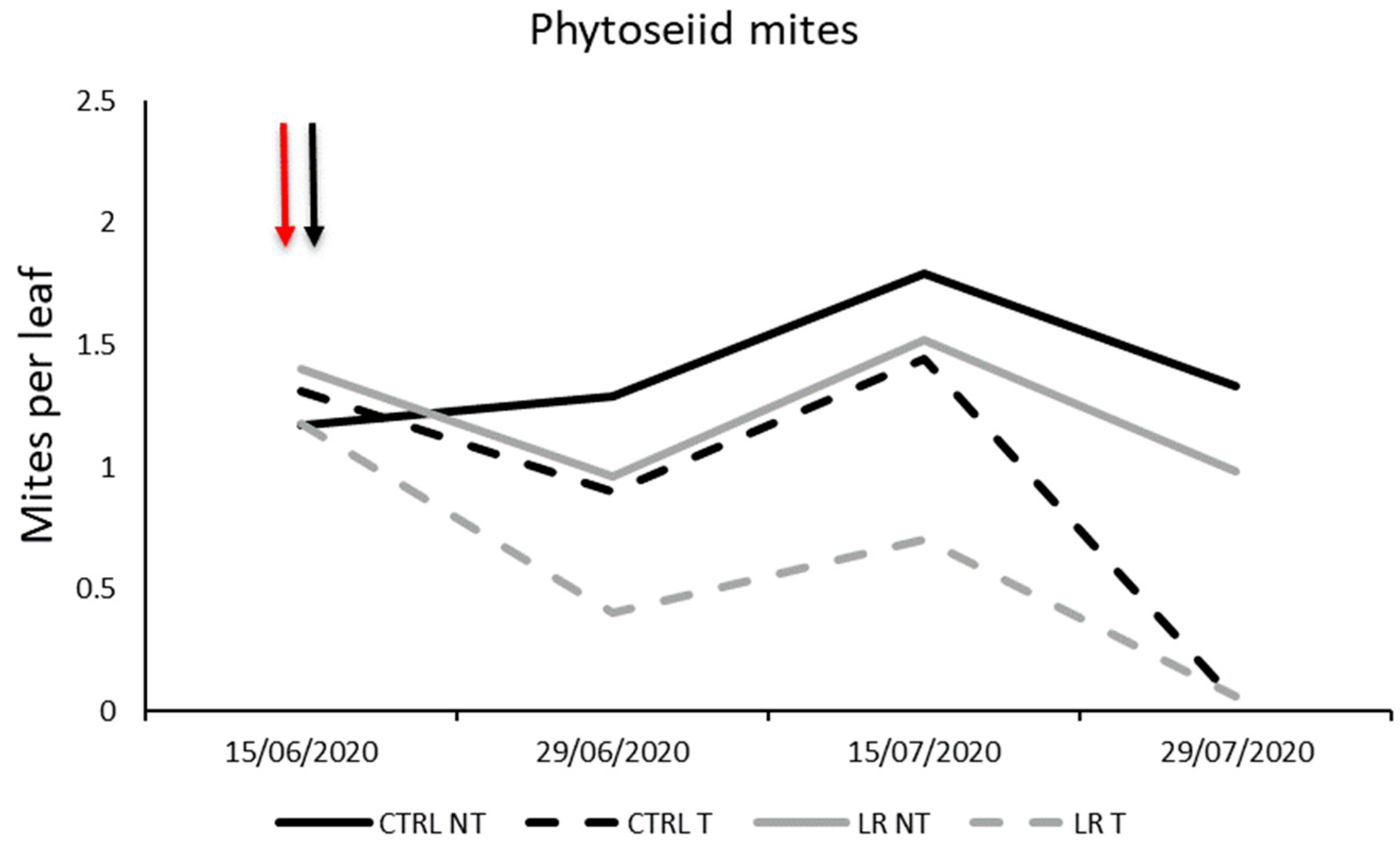
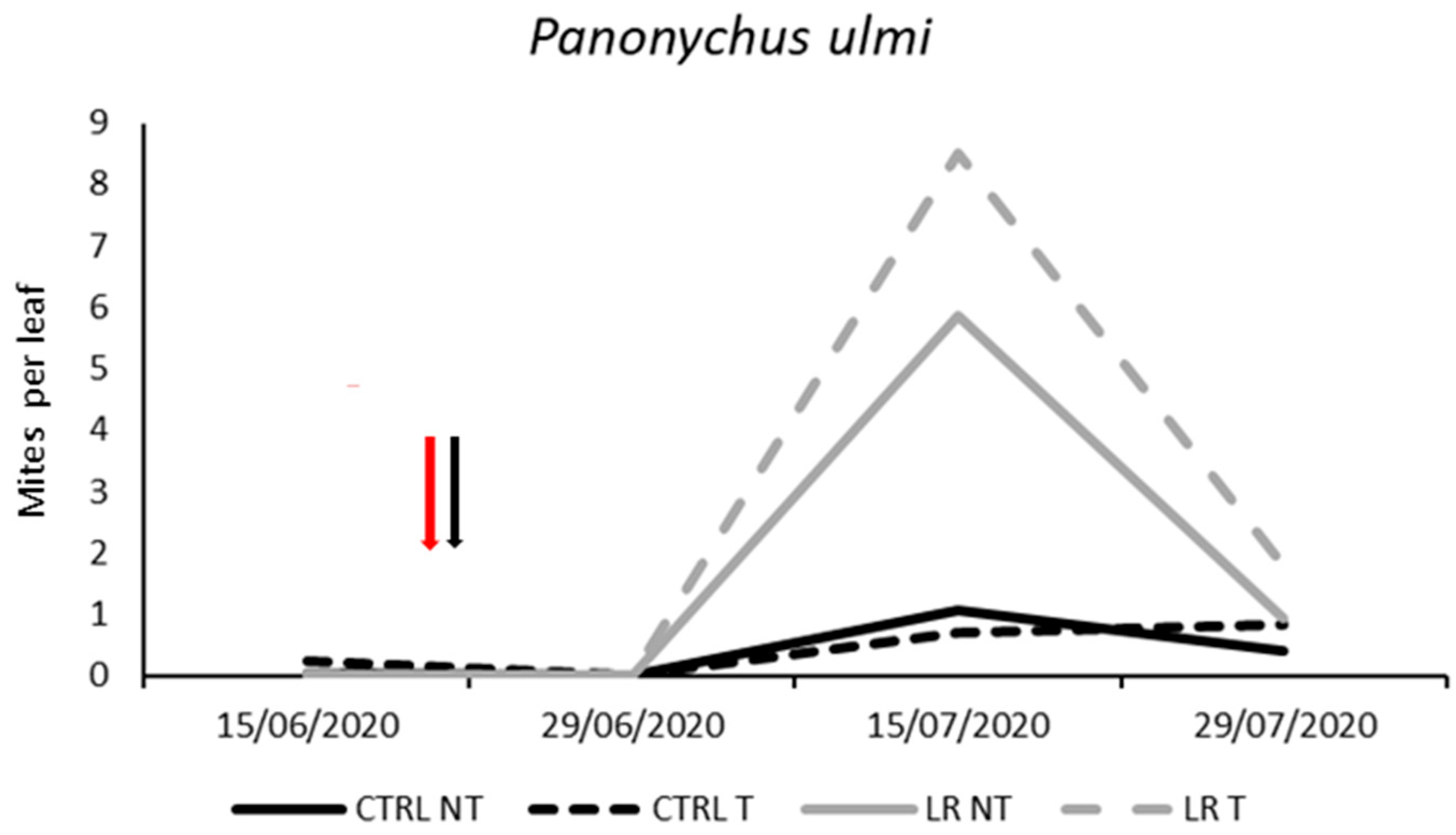
3.2. Farm A—2020
3.3. Farm B—2020
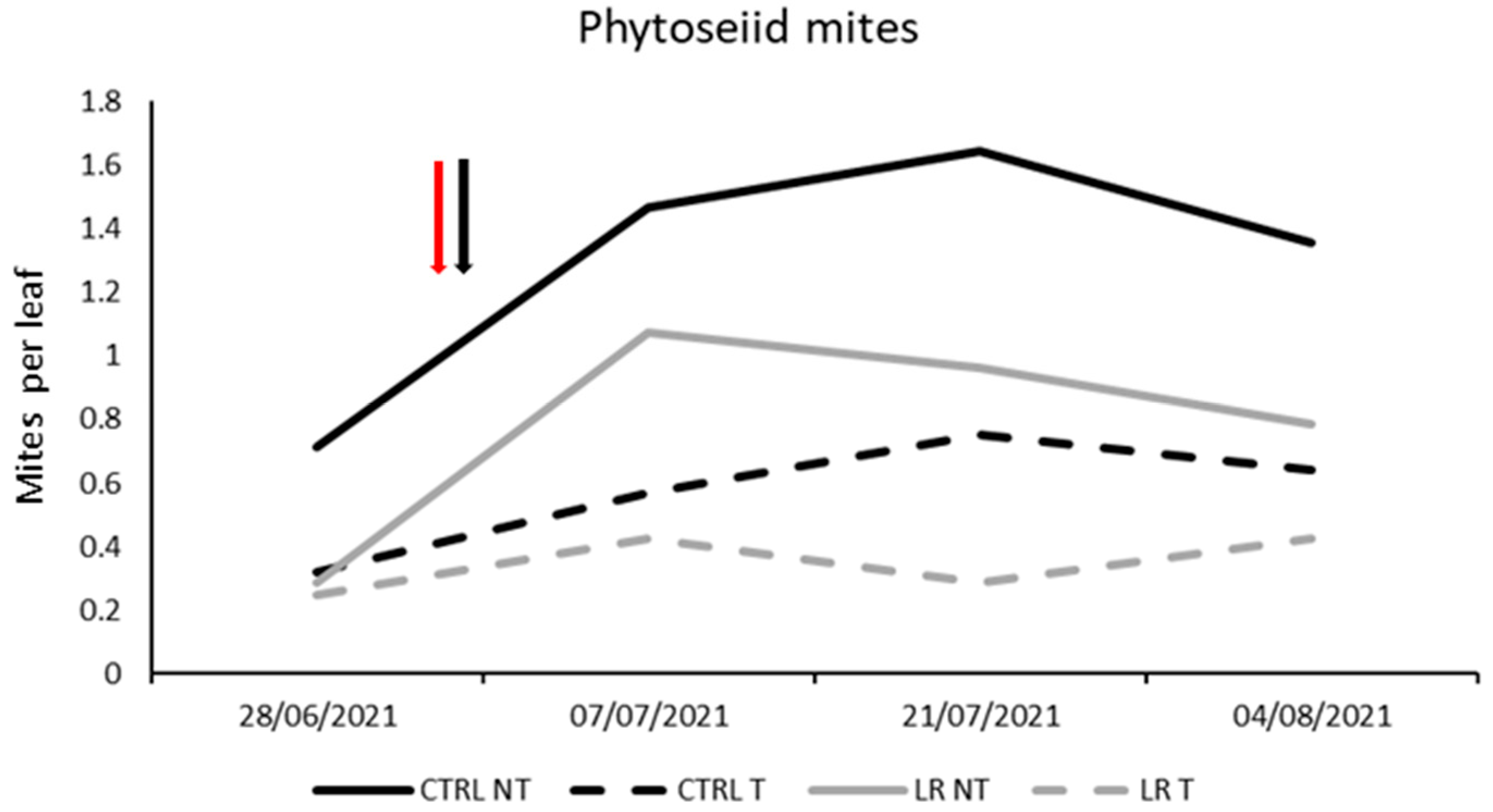
3.4. Farm B—2021
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Statistics, Eurostat: Vineyards in the EU—Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics#Relatively_stable_vine_area_in_EU_between_2015_and_2020_but_sharp_reduction_in_vineyard_holdings (accessed on 2 November 2022).
- Pomarici, E.; Corsi, A.; Mazzarino, S.; Sardone, R. The Italian wine sector: Evolution, structure, competitiveness and future challenges of an enduring leader. Ital. Econ. J. 2021, 7, 259–295. [Google Scholar] [CrossRef]
- Torquati, B.; Giacchè, G.; Venanzi, S. Economic analysis of the traditional cultural vineyard landscapes in Italy. J. Rural Stud. 2015, 39, 122–132. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Fermaud, M.; Giboulot, A. Influence of Lobesia botrana larvae on field severity of Botrytis rot of grape berries. Plant Dis. 1992, 76, 404–409. [Google Scholar] [CrossRef]
- Candolfi, M.P.; Jermini, M.; Carrera, E.; Candolfi-Vasconcelos, M.C. Grapevine leaf gas exchange, plant growth, yield, fruit qualità and carbohydrate reserves influenced by the grape leafhopper, Empoasca vitis. Entomol. Exp. Appl. 1993, 69, 289–296. [Google Scholar] [CrossRef]
- Vidano, C. Le cicaline italiane della vite: (Hemiptera Typhlocibinae). Boll. Zool. Agric. Bachic. 1958, 1, 61–115. [Google Scholar]
- Duso, C.; Moret, R.; Manera, A.; Berto, D.; Fornasiero, D.; Marchegiani, G.; Pozzebon, A. Investigations on the grape leafhopper Erasmoneura vulnerata in North-eastern Italy. Insects 2019, 10, 44. [Google Scholar] [CrossRef]
- Petersen, C.L.; Charles, J.G. Transmission of grapevine leafroll-associated closteroviruses by Planococcus longispinus and P. calceolariae. Plant Pathol. 1997, 46, 509–515. [Google Scholar] [CrossRef]
- Sforza, R.; Boudon-Padieu, E.; Greif, C. New mealybug species vectoring grapevine leafroll-associated viruses-1 and-3 (GLRaV-1 and-3). Eur. J. Plant Pathol. 2003, 109, 975–981. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Temporal analysis of grapevine leafroll associated virus 3 epidemics. Eur. J. Plant Pathol. 2006, 114, 441–446. [Google Scholar] [CrossRef]
- Charles, J.G.; Froud, K.J.; van den Brink, R.; Allan, D.J. Mealybugs and the spread of grapevine leafroll-associated virus 3 (GLRaV-3) in a New Zealand vineyard. Australas. Plant Pathol. 2009, 38, 576–583. [Google Scholar] [CrossRef]
- Daane, K.M.; Almeida, R.P.P.; Bell, V.A.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; Zaveizo, T. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards; Bostanian, N.J., Isaacs, R., Vincent, C., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 271–308. [Google Scholar]
- Candolfi, M.P.; Keller, M.; Boller, E.F. Mite-load function improves precision of feeding damage estimation in Tetranychus urticae. Entomol. Exp. Appl. 1991, 58, 289–293. [Google Scholar] [CrossRef]
- Barba, M.; Ferretti, L.; Pasquini, G. I giallumi della vite: Un problema fitosanitario di rilevanza nazionale. Inf. Fitopatol. 2006, 56, 4–8. [Google Scholar]
- European Commission. Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 15 October 2022).
- Chellemi, D.O.; Marois, J.J. Influence of leaf-removal, fungicide applications, and fruit maturity on incidence and severity of grape powdery mildew. Am. J. Enol. Vitic. 1992, 43, 53–57. [Google Scholar] [CrossRef]
- English, J.T.; Kaps, M.L.; Moore, J.F.; Hill, J.; Nakova, M. leaf-removal for control of Botrytis bunch rot of wine grapes in the midwestern United States. Plant Dis. 1993, 77, 1224–1227. [Google Scholar] [CrossRef]
- Moyer, M.M.; Newhouse, J.M.; Grove, G.G. Efficacy of bioInsecticides and leaf-removal in grapevine powdery mildew management. Plant Health Prog. 2016, 17, 84–91. [Google Scholar] [CrossRef]
- Gubler, W.D.; Marois, J.J.; Bledsoe, A.M.; Bettiga, L.J. Control of Botrytis bunch rot of grape with canopy management. Plant Dis. 1987, 71, 599–601. [Google Scholar] [CrossRef]
- Percival, D.C.; Fisher, K.H.; Sullivan, J.A. Use of fruit zone leaf-removal with vitis vinifera l. Cv. Riesling grapevines. Ii. Effect on fruit composition, yield, and occurrence of bunch rot (botrytis cinerea pers.:Fr.). Am. J. Enol. Vitic. 1994, 45, 133–140. [Google Scholar] [CrossRef]
- R’Houma, A.; Chèif, M.; Boubaker, A. Effect of nitrogen fertilization, green pruning, and fungicide treatments on Botrytis bunch rot of grapes. J. Plant Pathol. 1998, 80, 115–124. [Google Scholar]
- Valdéz-Gómez, H.; Fermaud, M.; Roudet, J.; Calonnec, A.; Gary, C. Grey mould incidence is reduced on grapevines with lower vegetative and reproductive growth. Crop. Prot. 2008, 27, 1174–1186. [Google Scholar] [CrossRef]
- Diago, M.P.; Vilanova, M.; Tardaguila, J. Effects of timing of manual and mechanical early defoliation on the aroma of Vitis vinifera L. Tempranillo wine. Am. J. Enol. Vitic. 2010, 61, 382–391. [Google Scholar] [CrossRef]
- Vartholomaiou, A.N.; Navrozidis, E.I.; Payne, C.C.; Salpiggidis, G.A. Agronomic techniques to control Lobesia botrana. Phytoparasitica 2008, 36, 264–271. [Google Scholar] [CrossRef]
- Pavan, F.; Cargnus, E.; Kiaeianmoosavi, S.; Bigot, G.; Tacoli, F.; Zandigiacomo, P. Bunch-zone leaf-removal of grapevines to prevent damage by Lobesia botrana and grey mould. Bull. Insectol. 2016, 69, 107–115. [Google Scholar]
- Kiaeian Moosavi, F.; Cargnus, E.; Pavan, F.; Zandigiacomo, P. Effects of grapevine bunch exposure to sunlight on berry surface temperature and Lobesia botrana (Lepidoptera: Tortricidae) egg laying, hatching and larval settlement. Agric. For. Entomol. 2018, 20, 420–432. [Google Scholar] [CrossRef]
- Tacoli, F.; Cargnus, E.; Kiaeian Moosavi, F.; Zandigiacomo, P.; Pavan, F. Efficacy and mode of action of kaolin and its interaction with bunch-zone leaf-removal against Lobesia botrana on grapevines. J. Pest. Sci. 2019, 92, 465–475. [Google Scholar] [CrossRef]
- Stapleton, J.J.; Barnett, W.W.; Marois, J.J.; Gubler, W.D. leaf-removal for pest management in wine grapes. Calif. Agric. 1990, 44, 15–17. [Google Scholar] [CrossRef]
- Tacoli, F.; Pavan, F.; Cargnus, E.; Tilatti, E.; Pozzebon, A.; Zandigiacomo, P. Efficacy and mode of action of kaolin in the control of Empoasca vitis and Zygina rhamni (Hemiptera: Cicadellidae) in vineyards. J. Econ. Entomol. 2017, 110, 1164–1178. [Google Scholar] [CrossRef]
- Prishmann, D.A.; James, D.G.; Wright, L.C.; Snyder, W.E. Effects of generalist phytoseiid mites and grapevine canopy structure on spider mite (Acari: Tetranychidae) biocontrol. Environ. Entomol. 2006, 35, 56–67. [Google Scholar] [CrossRef]
- Tacoli, F.; Cargnus, E.; Pozzebon, A.; Duso, C.; Tirello, P.; Pavan, F. Side effects of kaolin and bunch-zone leaf-removal on predatory mite population (Acari: Phytoseiidae) occurring in vineyards. J. Econ. Entomol. 2019, 112, 1292–1298. [Google Scholar] [CrossRef]
- Smart, R.; Robinson, M. Sunlight into the Wine: A Handbook for Winegrape Canopy Management; Winetitles: Adelaide, SA, Australia, 1991; p. 88. [Google Scholar]
- Wei, Q.; Yu, H.-Y.; Niu, C.-D.; Yao, R.; Wu, S.-F.; Chen, Z.; Gao, C.-F. Comparison of insecticide susceptibilities of Empoasca vitis (Hemiptera: Cicadellidae) from three main tea-growing regions in China. J. Econ. Entomol. 2015, 108, 1251–1259. [Google Scholar] [CrossRef]
- Yadav, S.K.; Patel, S.; Agnihotri, M.; Bisht, R.S. Efficacy of insecticides and bio-pesticides against sucking pests in black gram. Ann. Plant Prot. Sci. 2015, 23, 223–226. [Google Scholar]
- Prabhavathi, S.J.; Palaniswamy, S.; Kuttalam, S.; Veeraraghavathatham, D. Studies on persistence toxicity of acetamiprid 20 SP as foliar application against aphids and leafhoppers in cotton. Int. J. Trop. Agric. 2016, 34, 17–22. [Google Scholar]
- Hemadri, T. Management of leafhopper, Amrasca biguttula biguttula (Hemiptera: Cicadellidae) in okra (Abelmoschus esculentus) through new insecticide molecules. Int. J. Chem. Stud. 2018, 6, 687–690. [Google Scholar]
- Fornasiero, D.; Pavan, F.; Pozzebon, A.; Picotti, P.; Duso, C. Relative infestation level and sensitivity of grapevine cultivars to the leafhopper empoasca vitis (Hemiptera: Cicadellidae). J. Econ. Entomol. 2016, 109, 416–425. [Google Scholar] [CrossRef]
- Vidano, C. Alterazioni provocate da insetti in Vitis osservate, sperimentate e comparate. In Annali della Facoltà di Scienze agrarie; Università di Torino: Torino, Italy, 1936; Volume 1, pp. 513–644. [Google Scholar]
- Pavan, F.; Pavanetto, E. Seasonal abundance of Typhlocybinae at different leaf position of vines. In Proceedings of the Meet. EC Experts’ Group “Influence of Environmental Factors on the Control of Grape Pests, Diseases & Weeds”, Thessaloniki, Greece, 6–8 October 1987; Cavalloro, R., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1989; pp. 135–140. [Google Scholar]
- Pavan, F.; Picotti, P. Influence of grapevine cultivars on the leafhopper Empoasca vitis and its egg parasitoids. BioControl 2009, 54, 55–63. [Google Scholar] [CrossRef]
- Decante, D.; Van Leeuwen, C.; Van Helden, M. Influence of plot characteristics and surrounding vegetation on the intra-plot spatial distribution of Empoasca vitis. Agric. For. Entomol. 2009, 11, 377–388. [Google Scholar] [CrossRef]
- Fornasiero, D.; Duso, C.; Pozzebon, A.; Tomasi, D.; Gaiotti, F.; Pavan, F. Effects of irrigation on the seasonal abundance of Empoasca vitis in North-Italian vineyards. J. Econ. Entomol. 2012, 105, 176–185. [Google Scholar] [CrossRef]
- Smart, R.; Dick, J.; Gravett, I.; Fisher, B. Canopy management to improve grape yield and wine quality-principles and practices. S. Afr. J. Enol. Vitic 1990, 11, 3–17. [Google Scholar] [CrossRef]
- Kok, D.; Bal, E.; Celik, S. Influences of various canopy management techniques on wine grape quality of V. vinifera L. cv. Kalecik Karasi. Bulg. J. Agric. Sci. 2013, 19, 1247–1252. [Google Scholar]
- Duso, C.; Ahmad, S.; Tirello, P.; Pozzebon, A.; Klaric, V.; Baldessari, M.; Malagnini, V.; Angeli, G. The impact of insecticides applied in apple orchards on the predatory mite Kampimodromus aberrans (Acari Phytoseiidae). Exp. Appl. Acarol. 2014, 62, 391–414. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A.; Beers, E.H.; Sater, C. Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest Manag. Sci. 2021, 77, 4848–4862. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Khoobdel, M.; Khanjani, M.; Hosseininia, A.; Khederi, S.J. Sublethal effects of acetamiprid on biological aspects of life table of Amblyseius swirskii (Acari: Phytoseiidae) def on Aleuroclava jasmine (Hemiptera: Aleyrodidae). Syst. Applt. Acarol. 2019, 24, 14–824. [Google Scholar]
- English, J.; Thomas, C.; Marois, J.; Gubler, W. Microclimates of grapevine canopies associated with leaf-removal and control of Botrytis bunch rot. Phytopathology 1989, 79, 395–401. [Google Scholar] [CrossRef]
- Aníc, M.; Osrěcak, M.; Andabaka, Ž.; Tomaz, I.; Věcenaj, Ž.; Jelíc, D.; Kozina, B.; Kontíc, J.K.; Karoglan, M. The effect of leaf-removal on canopy microclimate, vine performance and grape phenolic composition of Merlot (Vitis vinifera L.) grapes in the continental part of Croatia. Sci. Hort. 2021, 285, 110161. [Google Scholar] [CrossRef]
- Mori, H.; Chant, D.A. The influence of prey density, relative humidity, and starvation on the predacious behavior of Phytoseiulus persimilis (Acarina: Phytoseiidae). Can. J. Zool. 1966, 44, 483–491. [Google Scholar] [CrossRef]
- Helle, W.; Sabelis, M.W. Spider Mites: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1, p. 458. [Google Scholar]






| Year | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Farm | Leaf Removal | Insecticide Application | Leaf Removal | Insecticide Application | Leaf Removal | Insecticide Application |
| A | 19 June 2019 | 20 June 2019 Tau-fluvalinate (30 mL/hL) | 16 June 2020 | 17 June 2020 Tau-fluvalinate (30 mL/hL) | \ | \ |
| B | \ | \ | 18 June 2020 | 18 June 2020 Acetamiprid (150 mL/hL) | 2 July 2023 | 2 July 2021 Tau-fluvalinate (30 mL/hL) |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 4 | 69 | 18.66 | <0.0001 |
| Insecticide | 1 | 69 | 6.61 | 0.012 |
| Leaf removal | 1 | 69 | 0.99 | 0.323 |
| Insecticide × Leaf removal | 1 | 69 | 1.77 | 0.188 |
| Time × Insecticide | 4 | 69 | 7.10 | <0.0001 |
| Time × Leaf removal | 4 | 69 | 2.32 | 0.052 |
| Time × Insecticide × Leaf removal | 4 | 69 | 1.04 | 0.395 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 5 | 72 | 22.34 | <0.0001 |
| Insecticide | 1 | 72 | 3.35 | 0.071 |
| Leaf removal | 1 | 72 | 2.55 | 0.115 |
| Insecticide × Leaf removal | 1 | 72 | 2.96 | 0.089 |
| Time × Insecticide | 5 | 72 | 1.33 | 0.260 |
| Time × Leaf removal | 5 | 72 | 0.89 | 0.492 |
| Time × Insecticide × Leaf removal | 5 | 72 | 0.53 | 0.755 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 27.10 | <0.0001 |
| Insecticide | 1 | 48 | 76.77 | <0.0001 |
| Leaf removal | 1 | 48 | 11.91 | <0.0001 |
| Insecticide × Leaf removal | 1 | 48 | 1.32 | 0.256 |
| Time × Insecticide | 3 | 48 | 8.55 | <0.001 |
| Time × Leaf removal | 3 | 48 | 1.43 | 0.246 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.45 | 0.721 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 17.20 | <0.0001 |
| Insecticide | 1 | 48 | 28.87 | <0.0001 |
| Leaf removal | 1 | 48 | 5.09 | 0.029 |
| Insecticide × Leaf removal | 1 | 48 | 0.81 | 0.372 |
| Time × Insecticide | 3 | 48 | 2.99 | 0.040 |
| Time × Leaf removal | 3 | 48 | 1.04 | 0.385 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.65 | 0.587 |
| Site | Year | Date | Treatment | LLN | CG (%) | IL (%) |
|---|---|---|---|---|---|---|
| (A) | 2020 | 15-June | CTRL | 5.98 | 0.00 | 67.87 |
| LR | 6.11 | 0.00 | 67.80 | |||
| F-value | 0.047 | - | 0.0005 | |||
| p-value | 0.8332 | - | 0.983 | |||
| 29-June | CTRL | 7.38 | 0.00 | 72.69 | ||
| LR | 5.08 | 0.00 | 66.86 | |||
| F-value | 24.28 | - | 10.97 | |||
| p-value | 0.0026 | - | 0.016 | |||
| 15-July | CTRL | 5.8 | 0.00 | 67.01 | ||
| LR | 4.75 | 0.00 | 58.25 | |||
| F-value | 2.92 | - | 2.43 | |||
| p-value | 0.1383 | - | 0.170 | |||
| 29-July | CTRL | 7.78 | 0.00 | 74.59 | ||
| LR | 4.48 | 0.00 | 57.08 | |||
| F-value | 27.72 | - | 44.30 | |||
| p-value | 0.0019 | - | 0.0006 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 4.62 | 0.006 |
| Insecticide | 1 | 48 | 13.71 | 0.001 |
| Leaf removal | 1 | 48 | 3.80 | 0.057 |
| Insecticide × Leaf removal | 1 | 48 | 0.61 | 0.439 |
| Time × Insecticide | 3 | 48 | 3.25 | 0.029 |
| Time × Leaf removal | 3 | 48 | 0.43 | 0.732 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.23 | 0.877 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 8.68 | 0.0001 |
| Insecticide | 1 | 48 | 5.45 | <0.0001 |
| Leaf removal | 1 | 48 | 3.93 | 0.053 |
| Insecticide × Leaf removal | 1 | 48 | 0.02 | 0.901 |
| Time × Insecticide | 3 | 48 | 2.50 | 0.071 |
| Time × Leaf removal | 3 | 48 | 0.95 | 0.422 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.52 | 0.668 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 30.39 | <0.0001 |
| Insecticide | 1 | 48 | 2.35 | 0.133 |
| Leaf removal | 1 | 48 | 15.50 | <0.001 |
| Insecticide × Leaf removal | 1 | 48 | 0.49 | 0.486 |
| Time × Insecticide | 3 | 48 | 0.50 | 0.683 |
| Time × Leaf removal | 3 | 48 | 10.12 | <0.0001 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.65 | 0.589 |
| Site | Year | Date | Treatment | LLN | CG % | IL % |
|---|---|---|---|---|---|---|
| (B) | 2020 | 15-June | CTRL | 3.35 | 5.00 | 43.81 |
| LR | 3.53 | 0.00 | 45.38 | |||
| F-value | 0.45 | 3 | 0.267 | |||
| p-value | 0.5275 | 0.1340 | 0.624 | |||
| 29-Juny | CTRL | 3.00 | 0.00 | 36.01 | ||
| LR | 2.06 | 2.78 | 22.05 | |||
| F-value | 16.94 | 1.00 | 2.92 | |||
| p-value | 0.0062 | 0.36 | 0.138 | |||
| 15-July | CTRL | 4.03 | 0.00 | 52.14 | ||
| LR | 1.85 | 2.50 | 11.35 | |||
| F-value | 142.81 | 1.00 | 93.95 | |||
| p-value | <0.0001 | 0.36 | 0.0001 | |||
| 29-July | CTRL | 3.38 | 0.00 | 47.42 | ||
| LR | 2.50 | 2.50 | 32.97 | |||
| F-value | 3.30 | 1.00 | 4.37 | |||
| p-value | 0.1194 | 0.3559 | 0.081 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 5.82 | 0.100 |
| Insecticide | 1 | 48 | 8.10 | 0.006 |
| Leaf removal | 1 | 48 | 0.90 | 0.347 |
| Insecticide × Leaf removal | 1 | 48 | 0.40 | 0.530 |
| Time × Insecticide | 3 | 48 | 0.97 | 0.416 |
| Time × Leaf removal | 3 | 48 | 0.70 | 0.557 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.33 | 0.801 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 4.13 | 0.011 |
| Insecticide | 1 | 48 | 23.6 | <0.0001 |
| Leaf removal | 1 | 48 | 9.77 | 0.003 |
| Insecticide × Leaf removal | 1 | 48 | 1.54 | 0.220 |
| Time × Insecticide | 3 | 48 | 1.26 | 0.297 |
| Time × Leaf removal | 3 | 48 | 0.39 | 0.790 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.02 | 0.995 |
| Effect | DF Num | DF Den | F-Value | p-Value |
|---|---|---|---|---|
| Time | 3 | 48 | 6.18 | 0.001 |
| Insecticide | 1 | 48 | 21.19 | <0.0001 |
| Leaf removal | 1 | 48 | 1.63 | 0.207 |
| Insecticide × Leaf removal | 1 | 48 | 1.63 | 0.207 |
| Time × Insecticide | 3 | 48 | 3.48 | 0.023 |
| Time × Leaf removal | 3 | 48 | 0.14 | 0.935 |
| Time × Insecticide × Leaf removal | 3 | 48 | 0.21 | 0.891 |
| Site | Year | Date | Treatment | LLN | CG % | IL % |
|---|---|---|---|---|---|---|
| (B) | 2021 | 28-Jun | CTRL | 3.33 | 0.00 | 45.89 |
| LR | 3.05 | 0.00 | 35.80 | |||
| F-value | 1.42 | - | 3.66 | |||
| p-value | 0.2779 | - | 0.104 | |||
| 7-Jul | CTRL | 3.425 | 0.00 | 45.71 | ||
| LR | 1.88 | 2.50 | 23.62 | |||
| F-value | 40.32 | 1.00 | 28.39 | |||
| p-value | 0.0007 | 0.3559 | 0.002 | |||
| 21-Jul | CTRL | 3.35 | 0.00 | 40.35 | ||
| LR | 1.55 | 0.00 | 16.07 | |||
| F-value | 22.09 | - | 10.07 | |||
| p-value | 0.0033 | - | 0.019 | |||
| 4-Aug | CTRL | 3.45 | 0.00 | 47.62 | ||
| LR | 2.8 | 0.00 | 37.18 | |||
| F-value | 9.57 | - | 11.49 | |||
| p-value | 0.021 | - | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prazaru, S.C.; dal Mas, G.; Padoin, M.; Rizzardo, D.; Meggio, F.; Pitacco, A.; Pozzebon, A.; Duso, C. Effect of Leaf Removal and Insecticide Applications on Population Densities of Leafhoppers and Mites Associated with Grapevines. Insects 2023, 14, 791. https://doi.org/10.3390/insects14100791
Prazaru SC, dal Mas G, Padoin M, Rizzardo D, Meggio F, Pitacco A, Pozzebon A, Duso C. Effect of Leaf Removal and Insecticide Applications on Population Densities of Leafhoppers and Mites Associated with Grapevines. Insects. 2023; 14(10):791. https://doi.org/10.3390/insects14100791
Chicago/Turabian StylePrazaru, Stefan Cristian, Giovanni dal Mas, Matteo Padoin, Denis Rizzardo, Franco Meggio, Andrea Pitacco, Alberto Pozzebon, and Carlo Duso. 2023. "Effect of Leaf Removal and Insecticide Applications on Population Densities of Leafhoppers and Mites Associated with Grapevines" Insects 14, no. 10: 791. https://doi.org/10.3390/insects14100791






