Gut Bacteria Promote Phosphine Susceptibility of Tribolium castaneum by Aggravating Oxidative Stress and Fitness Costs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Isolation and Identification of Gut Bacteria
2.3. Antibiotic Treatment and Gnotobiotic Inoculation
2.4. Bioassay of Phosphine Susceptibility
2.5. Determination of Female Fecundity
2.6. Qualification of MDA and H2O2
2.7. Determination of SOD, CAT, and POD Activities
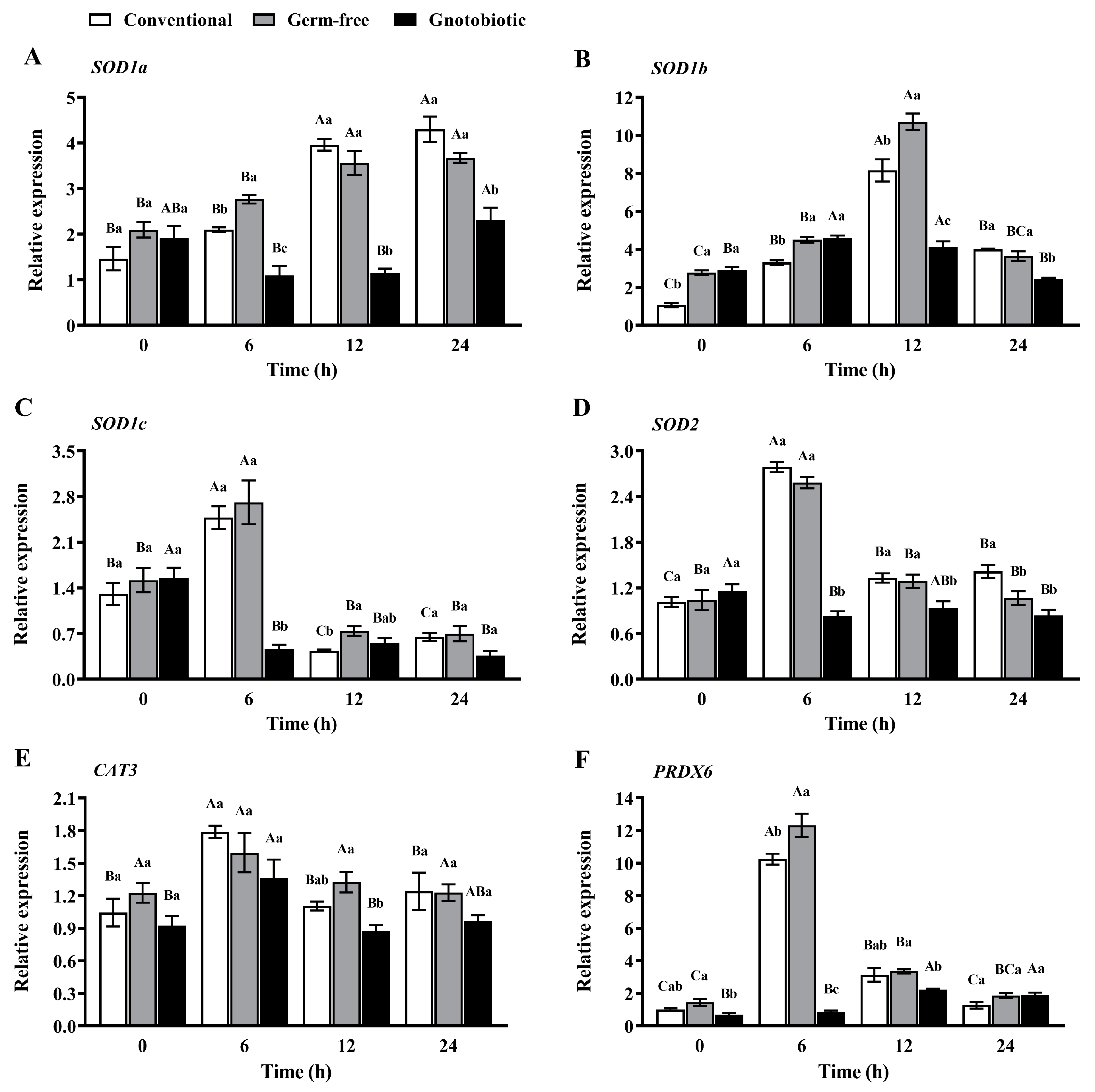
2.8. Determination of SOD, CAT, and POD Gene Expression
2.9. Measurement of Immune Responses
2.10. Statistical Analysis
3. Results
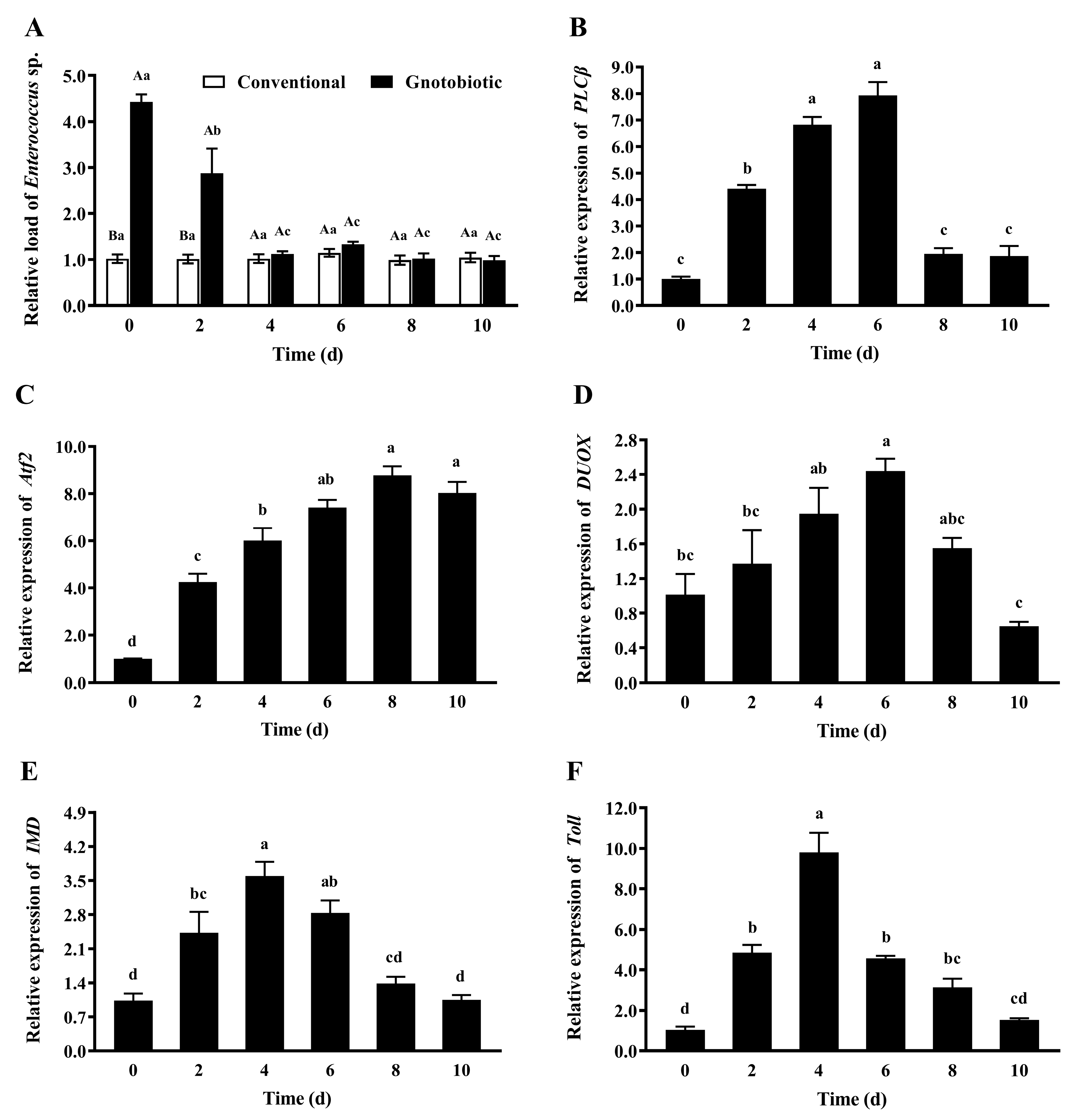
3.1. Impact of Bacterial Treatment on Bacterial Abundance
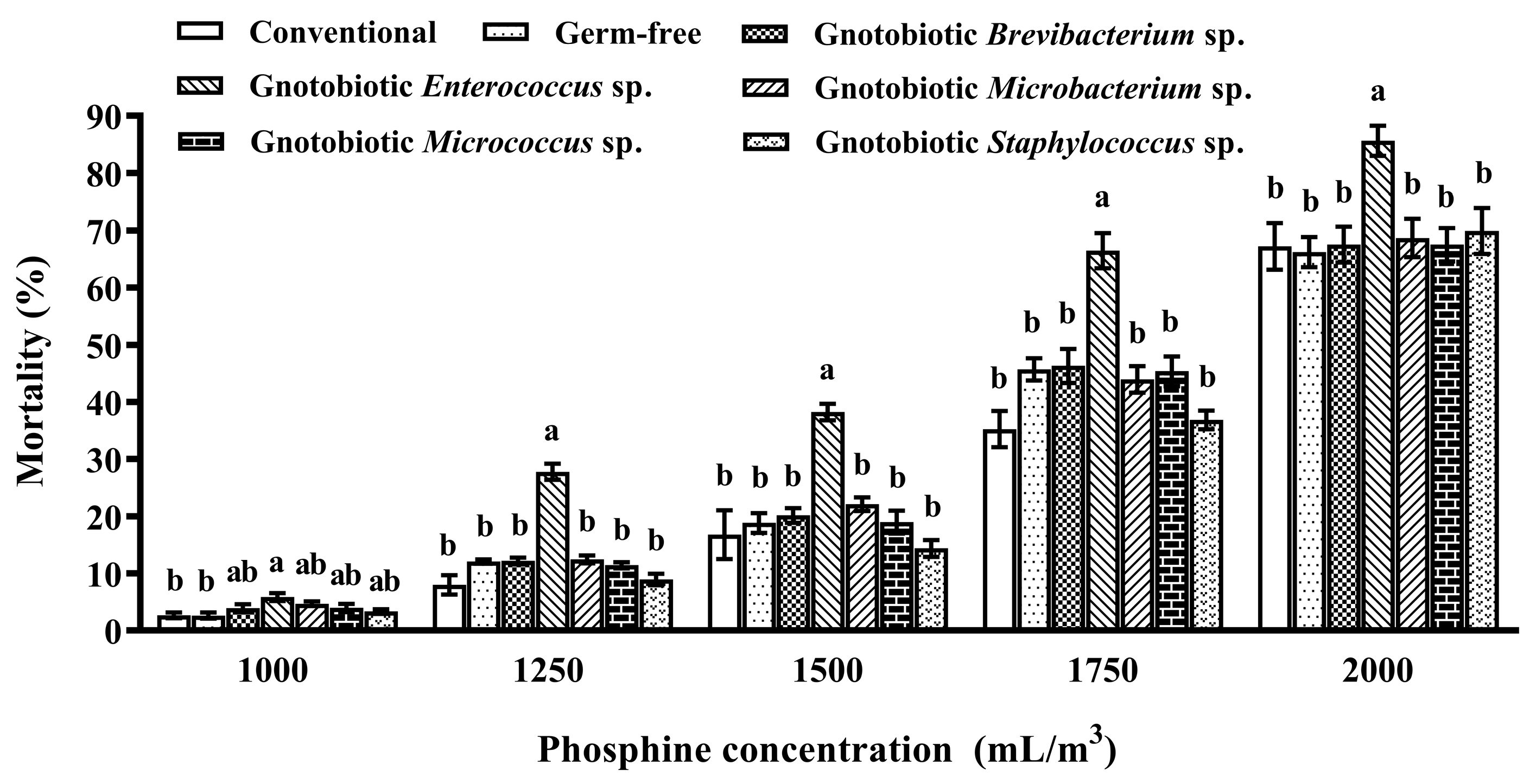
3.2. Impact of Bacteria Inoculation on Host Phosphine Susceptibility
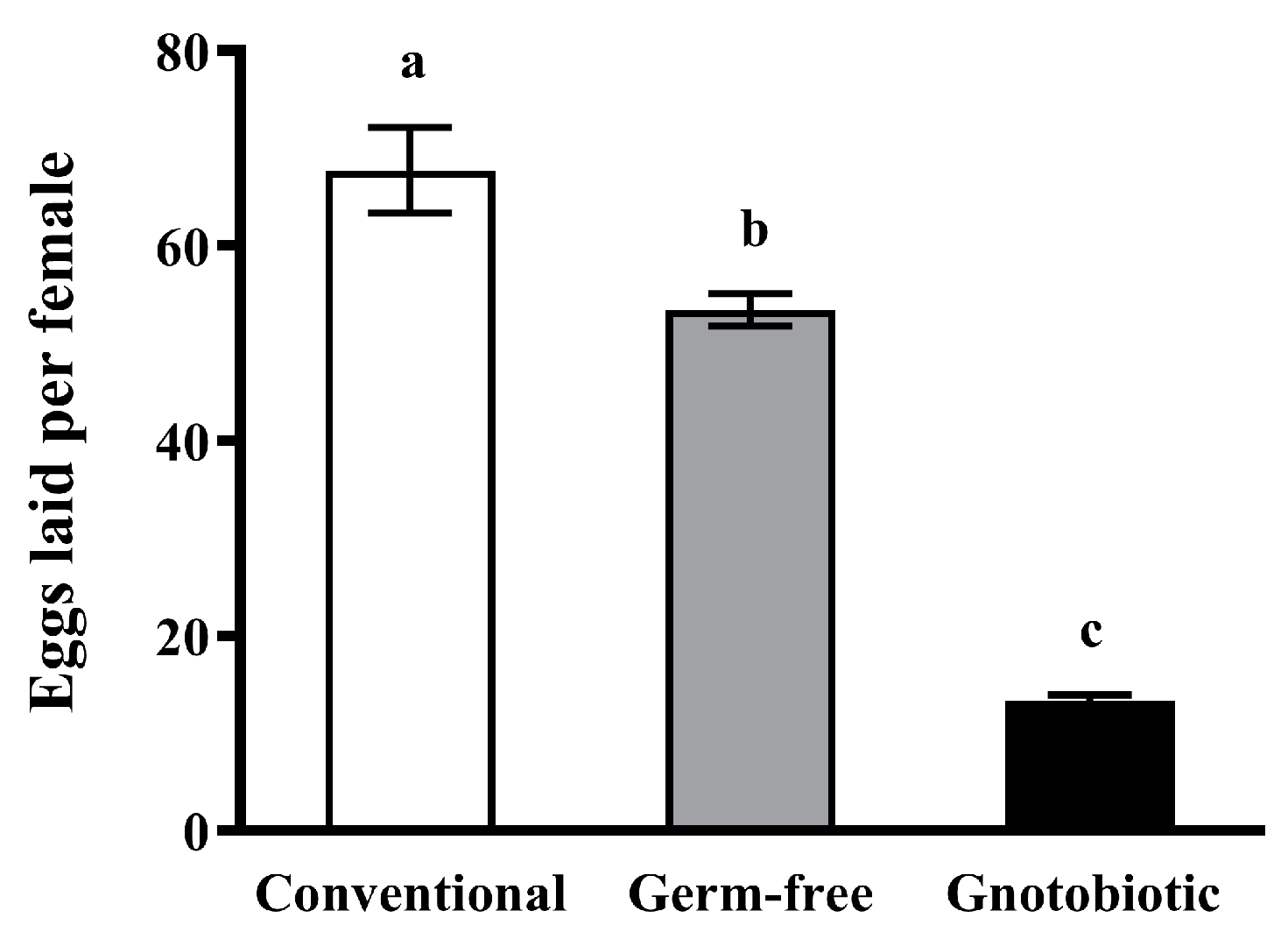
3.3. Impact of Enterococcus sp. Inoculation on Host Fecundity
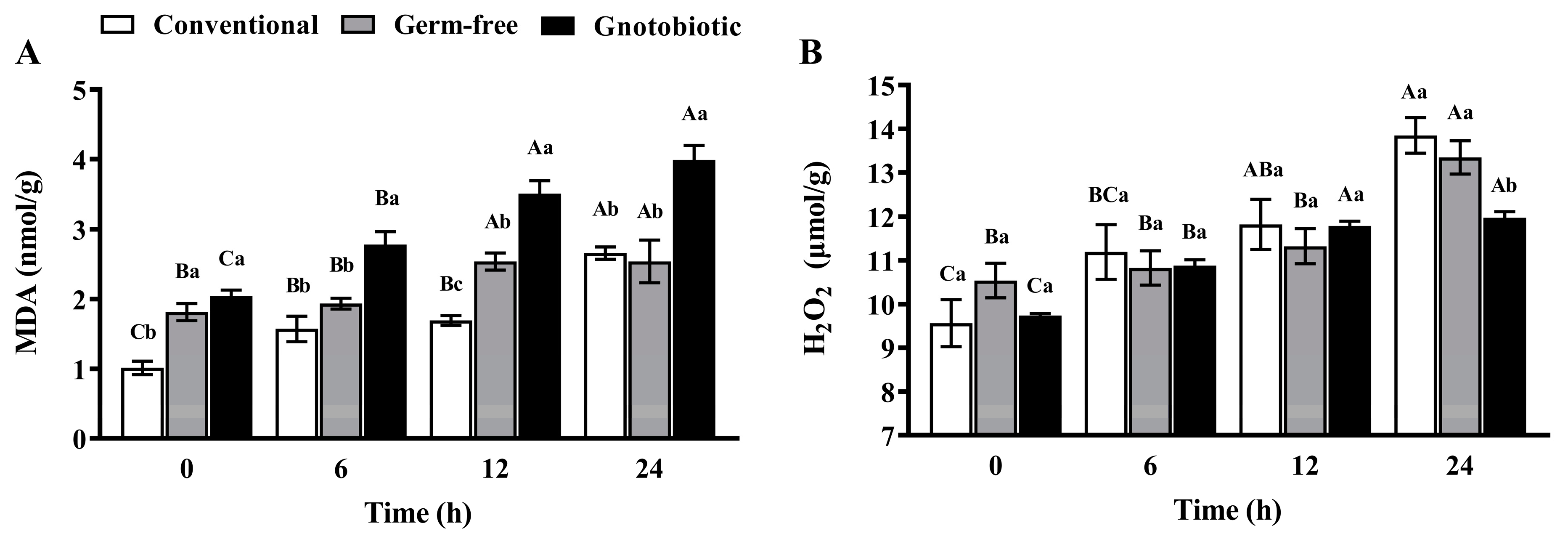
3.4. Impact of Enterococcus sp. Inoculation on Host Oxidative Stress
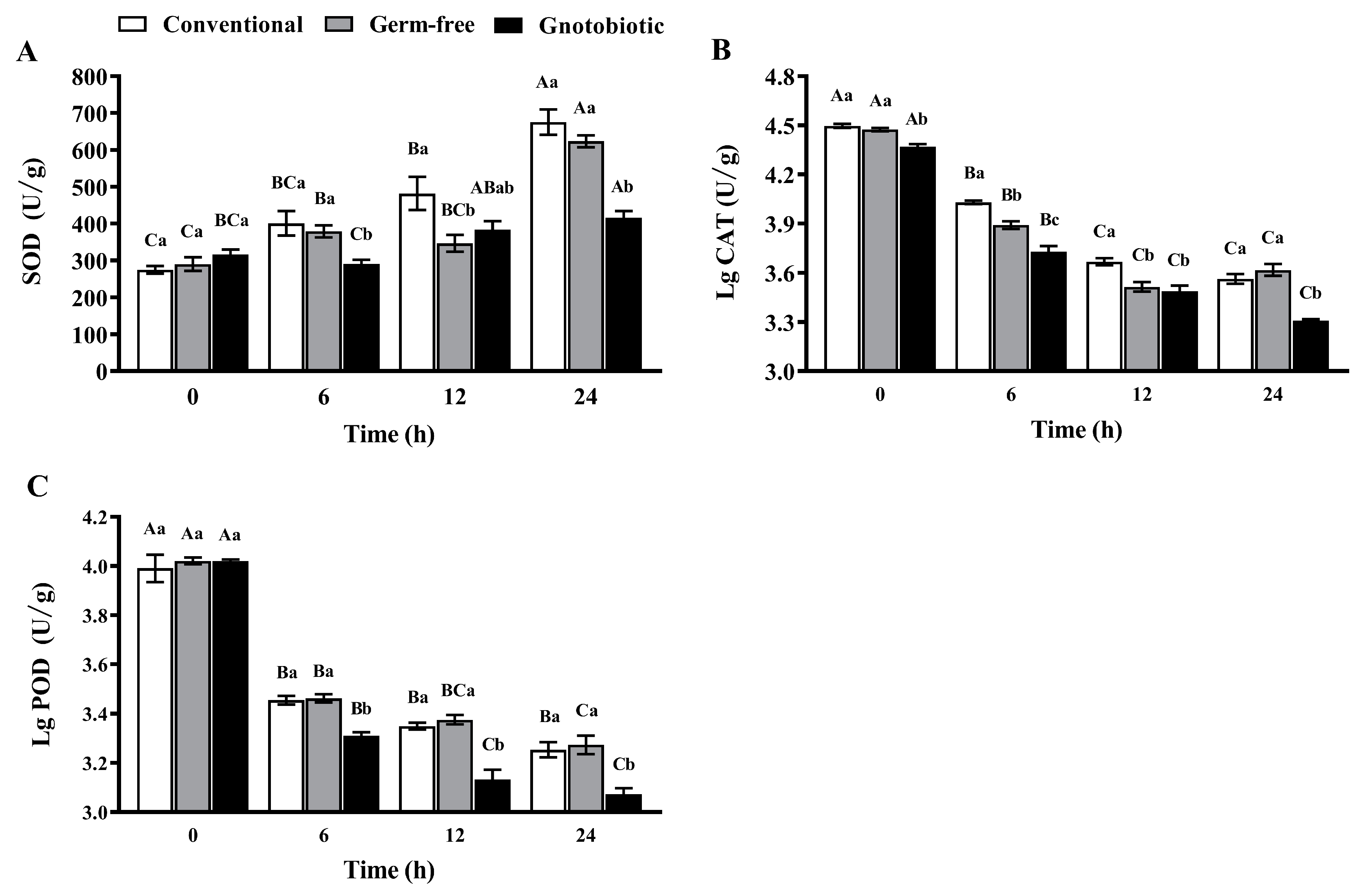
3.5. Impact of Enterococcus sp. Inoculation on the Host Antioxidant System
3.6. Impact of Enterococcus sp. Inoculation on Host Immune Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International Inc.: St. Paul, MN, USA, 2009; pp. 183–184. [Google Scholar]
- Astuti, L.P.; Mutala’liah, M. Host preference of Tribolium castaneum (Herbst) on six kinds of flour. Indones. J. Entomol. 2020, 17, 149–155. [Google Scholar] [CrossRef]
- Rosner, J.; Wellmeyer, B.; Merzendorfer, H. Tribolium castaneum: A model for investigating the mode of action of insecticides and mechanisms of resistance. Curr. Pharm. Des. 2020, 26, 3554–3568. [Google Scholar] [CrossRef] [PubMed]
- Fusetto, R.; Denecke, S.; Perry, T.; O’Hair, R.A.J.; Batterham, P. Partitioning the roles of CYP6G1 and gut microbes in the metabolism of the insecticide imidacloprid in Drosophila melanogaster. Sci. Rep. 2017, 7, 11339. [Google Scholar] [CrossRef]
- Pietri, J.E.; Liang, D. The links between insect symbionts and insecticide resistance: Causal relationships and physiological tradeoffs. Ann. Entomol. Soc. Am. 2018, 111, 92–97. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, A.; Li, Y.; Cai, Z.; Lemaitre, B.; Zhang, H. The dual oxidase gene BdDuox regulates the intestinal bacterial community homeostasis of Bactrocera dorsalis. ISME J. 2016, 10, 1037–1050. [Google Scholar] [CrossRef]
- Wang, Z.; Yong, H.; Zhang, S.; Liu, Z.; Zhao, Y. Colonization resistance of symbionts in their insect hosts. Insects 2023, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction–immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Tiwari, S.; Pelz-Stelinski, K.; Stelinski, L.L. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manag. Sci. 2011, 67, 94–99. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.B.; Patil, N.B.; Sahu, M.; Prabhukarthikeyan, S.R.; Raghu, S.; Pandi, G.P.; Adak, T.; Swain, C.K.; Pokhare, S.; Mohapatra, S.D.; et al. Differential gut bacteria in phosphine resistant and susceptible population of Tribolium castaneum (Herbst) and their biochemical and molecular characterization. Pak. J. Zool. 2022, 54, 1331–1338. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Chang, Z.; Zhang, S.; Lu, Y. Molecular mechanism of Enterococcus faecalis-induced phosphine sensitivity in Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2023, 116, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides: Tentative method for beetles of some major pest species of stored cereals with methyl bromide and phosphine. FAO method no. 16. FAO Plant Prot. Bull. 1975, 23, 12–25. [Google Scholar]
- Wang, Z.; Zhao, Y.; Yong, H.; Liu, Z.; Wang, W.; Lu, Y. The contribution of gut bacteria to pesticide resistance of Tribolium castaneum (Herbst). J. Stored Prod. Res. 2023, 103, e102160. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Lu, Y. Biodegradation of insecticides by gut bacteria isolated from stored grain beetles and its implication in host insecticide resistance. J. Stored Prod. Res. 2022, 96, e101943. [Google Scholar] [CrossRef]
- Douglas, A.E. Mycetocyte symbiosis in insects. Biol. Rev. 1989, 64, 409–434. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Seif, M.M.; Madboli, A.N.; Marrez, D.A.; Aboulthana, W.M.K. Hepato-renal protective effects of Egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Pellavio, G.; Rossino, G.; Gastaldi, G.; Rossi, D.; Linciano, P.; Collina, S.; Laforenza, U. Sigma-1 receptor agonists acting on aquaporin-mediated H2O2 permeability: New tools for counteracting oxidative stress. Int. J. Mol. Sci. 2021, 22, 9790. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, C.; Bao, H.; Li, S.; Huang, Z.; Gu, D.; Xiong, L.; Miao, L. Estimation of gingival crevicular fluid oxidative stress markers in school-aged children and teenagers with insufficient sleep. BMC Oral Health 2022, 22, 616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Lin, K.; Yang, C.; Cai, P. Generation and propagation of yeast prion [URE3] are elevated under electromagnetic field. Cell Stress Chaperones 2018, 23, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, Y.; Wang, Z.; Liu, B.; Zhao, H.; Li, H.; Cai, Q.; Mo, C.; Li, Q. Bioaccumulation and phytotoxicity and human health risk from microcystin-LR under various treatments: A pot study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Liu, J.; Yu, X.; Zhang, W.; You, C. Biological activities and gene expression of detoxifying enzymes in Tribolium castaneum induced by Moutan cortex essential oil. J. Toxicol. Environ. Health A 2022, 85, 591–602. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Dearing, M.D. The woodrat gut microbiota as an experimental system for understanding microbial metabolism of dietary toxins. Front. Microbiol. 2016, 7, 1165. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Jones, A.G.; Felton, G.W. Co-option of microbial associates by insects and their impact on plant–folivore interactions. Plant. Cell Environ. 2019, 42, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA 2006, 103, 15196–15199. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef]
- Tiwari, S.; Pelz-Stelinski, K.; Mann, R.S.; Stelinski, L.L. Glutathione transferase and cytochrome P450 (general oxidase) activity levels in Candidatus Liberibacter asiaticus-infected and uninfected Asian citrus psyllid (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2011, 104, 297–305. [Google Scholar] [CrossRef]
- Daisley, B.A.; Trinder, M.; McDowell, T.W.; Collins, S.L.; Sumarah, M.W.; Reid, G. Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent interactions with Lactobacilli in a Drosophila melanogaster insect model. Appl. Environ. Microbiol. 2018, 84, e02820-17. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Tu, Y.; Guo, P.; He, W.; Jing, T.; Wang, J.; Wei, D. Antioxidant enzymes and heat shock protein genes from Liposcelis bostrychophila are involved in stress defense upon heat shock. Insects 2020, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, L.; Zhang, F.; Wang, Y. Transcriptional inhibition of the catalase gene in phosphine-induced oxidative stress in Drosophila melanogaster. Pestic. Biochem. Physiol. 2015, 124, 1–7. [Google Scholar] [CrossRef]
- Bae, Y.S.; Choi, M.K.; Lee, W.J. Dual oxidase in mucosal immunity and host–microbe homeostasis. Trends Immunol. 2010, 31, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Duron, O.; Labbe, P.; Berticat, C.; Rousset, F.; Guillot, S.; Raymond, M.; Weill, M. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 2006, 60, 303–314. [Google Scholar] [CrossRef]
- Agnew, P.; Berticat, C.; Bedhomme, S.; Sidobre, C.; Michalakis, Y. Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution 2004, 58, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Slos, S.; Stoks, R. Predation risk induces stress proteins and reduces antioxidant defense. Funct. Ecol. 2008, 22, 637–642. [Google Scholar] [CrossRef]
- Yadav, S.K.; Srivastava, C.; Sabtharishi, S. Phosphine resistance and antioxidant enzyme activity in Trogoderma granarium Everts. J. Stored Prod. Res. 2020, 87, 101636. [Google Scholar] [CrossRef]
- Arismendi, N.L.; Reyes, M.; Miller, S.A.; Wijeratne, A.J.; Carrillo, R. Infection of ‘Candidatus Phytoplasma ulmi’ reduces the protein content and alters the activity of detoxifying enzymes in Amplicephalus curtulus. Entomol. Exp. Appl. 2015, 157, 334–345. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, J.; Deng, M.; Jin, Y.; Yang, H.; Liu, Y.; Cao, Q.; Mennigen, J.A.; Tu, W. Acute exposure to environmentally relevant concentrations of Chinese PFOS alternative F-53B induces oxidative stress in early developing zebrafish. Chemosphere 2019, 235, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, X.; Li, Y.; Zhang, J.; Fu, Y. miR-34-5p, encoded by Spodoptera frugiperda, participates in anti-baculovirus by regulating innate immunity in the insect host. Int. J. Biol. Macromol. 2022, 222, 2190–2199. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, S.; Liu, Z.; Chang, Z.; Hu, H. Gut Bacteria Promote Phosphine Susceptibility of Tribolium castaneum by Aggravating Oxidative Stress and Fitness Costs. Insects 2023, 14, 815. https://doi.org/10.3390/insects14100815
Wang Z, Zhang S, Liu Z, Chang Z, Hu H. Gut Bacteria Promote Phosphine Susceptibility of Tribolium castaneum by Aggravating Oxidative Stress and Fitness Costs. Insects. 2023; 14(10):815. https://doi.org/10.3390/insects14100815
Chicago/Turabian StyleWang, Zhengyan, Shan Zhang, Zhiyuan Liu, Zhenzhen Chang, and Haisheng Hu. 2023. "Gut Bacteria Promote Phosphine Susceptibility of Tribolium castaneum by Aggravating Oxidative Stress and Fitness Costs" Insects 14, no. 10: 815. https://doi.org/10.3390/insects14100815



