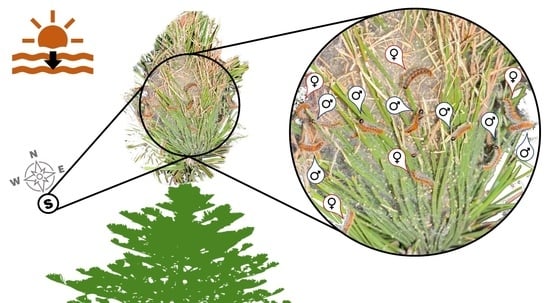
Large Male Caterpillars Are the Primary Builders: Exploring Tent Construction and Foraging Behaviour in Gregarious Pine Processionary Caterpillar
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Daily Caterpillar Activity: Frass Production
2.3. Daily Caterpillar Activity: Tent Construction and Foraging
2.4. Polyethism
2.5. Statistical Analyses
2.5.1. Daily Caterpillar Activity: Frass Production
2.5.2. Daily Caterpillar Activity: Tent Construction and Foraging
2.5.3. Polyethism
3. Results
3.1. Daily Caterpillar Activity: Frass Production
3.2. Daily Caterpillar Activity: Tent Construction and Foraging
3.3. Polyethism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reader, T.; Hochuli, D.F. Understanding Gregariousness in a Larval Lepidopteran: The Roles of Host Plant, Predation, and Microclimate. Ecol. Entomol. 2003, 28, 729–737. [Google Scholar] [CrossRef]
- Theraulaz, G.; Bonabeau, E.; Deneubourg, J.L. The Origin of Nest Complexity in Social Insects. Complexity 1998, 3, 15–25. [Google Scholar] [CrossRef]
- Fitzgerald, T.D.; Costa, J.T. Collective Behavior in Social Caterpillars. In Information Processing in Social Insects; Detrain, C., Deneubourg, J.L., Pasteels, J.M., Eds.; Birkhäuser: Basel, Switzerland, 1999; pp. 379–400. ISBN 9783034887397. [Google Scholar]
- Snodgrass, R.E. The Fall Webworm. Ann. Rep. Smithsonian Inst. 1921, 395–414. [Google Scholar]
- Fitzgerald, T.D.; Willer, D.E. Tent-Building Behavior of the Eastern Tent Caterpillar Malacosoma americanum (Lepidoptera: Lasiocampidae). J. Kansas Entomol. Soc. 1983, 56, 20–31. [Google Scholar]
- Ruf, C.; Freese, A.; Fiedler, K. Larval Sociality in Three Species of Central-Place Foraging Lappet Moths (Lepidoptera: Lasiocampidae): A Comparative Survey. Zool. Anz. 2003, 242, 209–222. [Google Scholar] [CrossRef]
- Dussutour, A.; Nicolis, S.C.; Despland, E.; Simpson, S.J. Individual Differences Influence Collective Behaviour in Social Caterpillars. Anim. Behav. 2008, 76, 5–16. [Google Scholar] [CrossRef]
- Mills, M. Bag Shelter Caterpillars and Their Habits. West. Aust. Nat. 1951, 3, 84–92. [Google Scholar]
- Démolin, G. Grégarisme et Subsocialité Chez Thaumetopoea pityocampa Schiff. Nid d’hiver—Activité de Tissage. In Proceedings of the C. R. Ve Congrès de L’union Internationale Pour L’étude des Insectes Sociaux; 1967; pp. 69–77. [Google Scholar]
- Underwood, D.L.A.; Shapiro, A.M. A Male-Biased Primary Sex Ratio and Larval Mortality in Eucheira socialis (Lepidoptera: Pieridae). Evol. Ecol. Res. 1999, 1, 703–717. [Google Scholar]
- Underwood, D.L.A.; Shapiro, A.M. Evidence for Division of Labor in the Social Caterpillar Eucheira socialis (Lepidoptera: Pieridae). Behav. Ecol. Sociobiol. 1999, 46, 228–236. [Google Scholar] [CrossRef]
- Uemura, M.; Zalucki, M.P.; Battisti, A. Behavioural Plasticity and Tree Architecture Shapes Tent and Foraging Locations of Pine Processionary Larval Colonies. Entomol. Gen. 2020, 41, 121–136. [Google Scholar] [CrossRef]
- Battisti, A.; Avcı, M.; Avtzis, D.N.; Ben Jamaa, M.L.; Berardi, L.; Berretima, W.; Branco, M.; Chakali, G.; El Alaoui El Fels, M.A.; Frérot, B.; et al. Natural History of the Processionary Moths (Thaumetopoea spp.): New Insights in Relation to Climate Change. In Processionary Moths and Climate Change: An Update; Roques, A., Ed.; Springer-Quae: Dordrecht, The Netherlands; Versailles, France, 2015; pp. 15–80. [Google Scholar]
- Battisti, A.; Holm, G.; Fagrell, B.; Larsson, S. Urticating Hairs in Arthropods: Their Nature and Medical Significance. Annu. Rev. Entomol. 2011, 56, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Ferchault de Réaumur, R.-A. Memoires Pour Servir a L’histoire des Insectes; De l’Imprimerie Royale: Paris, France, 1734. [Google Scholar]
- Fabre, J. Souvenirs Entomologiques. Sér 1898, 6, 298–392. [Google Scholar]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of Geographic Range in the Pine Processionary Moth Caused by Increased Winter Temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Poitou, L.; Robinet, C.; Suppo, C.; Rousselet, J.; Laparie, M.; Pincebourde, S. When Insect Pests Build Their Own Thermal Niche: The Hot Nest of the Pine Processionary Moth. J. Therm. Biol. 2021, 98, 102947. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Hódar, J.A.; Hernández, R.; Larsson, S. Aggregative Oviposition Varies with Density in Processionary Moths — Implications for Insect Outbreak Propensity. Ecol. Entomol. 2022, 48, 102–111. [Google Scholar] [CrossRef]
- Hódar, J.A.; Castro, J.; Zamora, R. Pine Processionary Caterpillar Thaumetopoea pityocampa as a New Threat for Relict Mediterranean Scots Pine Forests under Climatic Warming. Biol. Conserv. 2003, 110, 123–129. [Google Scholar] [CrossRef]
- Branco, M.; Santos, M.; Calvão, T.; Telfer, G.; Paiva, M. Arthropod Diversity Sheltered in Thaumetopoea pityocampa (Lepidoptera: Notodontidae) Larval Nests. Insect Conserv. Divers. 2008, 1, 215–221. [Google Scholar] [CrossRef]
- Uemura, M.; Perkins, L.E.; Zalucki, M.P.; Battisti, A. Movement Behaviour of Two Social Urticating Caterpillars in Opposite Hemispheres. Mov. Ecol. 2020, 8, 4. [Google Scholar] [CrossRef]
- Breuer, M.; Devkota, B.; Douma-Petridou, E.; Koutsaftikis, A.; Schmidt, G.H. Studies on the Exposition and Temperature of Nests of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Thaumetopoeidae) in Greece. J. Appl. Entomol. 1989, 107, 370–375. [Google Scholar] [CrossRef]
- Lavenseau, L. Determination of the Sex of Caterpillars without Dissection. Int. J. Insect Morphol. Embryol 1982, 11, 359–362. [Google Scholar] [CrossRef]
- Underwood, D.L.A. Methods for Sexing Lepidoptera Larvae Using External Morphology. J. Lepid. Soc. 1994, 48, 258–263. [Google Scholar]
- Battisti, A. Phytophagous Insects in the Energy Flow of an Artificial Stand of Pinus Nigra Arnold in Northern Italy. Redia 1988, 71, 139–159. [Google Scholar]
- Hoffman, T. SunCalc. Available online: www.suncalc.org/ (accessed on 10 March 2021).
- Grison, P.; Sylvestre, R.d.S.; Galichet, P.F. La Processionnaire Du Pin (Thaumetopoea pityocampa Schiff.). Mœurs, Dégâts, Moyens de Lutte. Rev. Zool. Agric. Appl. Talence 1951, 50, 26–33. [Google Scholar]
- Sebti, S.; Chakali, G. Distribution and Importance of the Pine Processionary Moth Winter Nests Thaumetopoea pityocampa (Denis & Schiffermüller) (Lepidoptera: Notodontidae) in the Forests Cedar of the National Park of Chréa (Algeria). Int. J. Agric. Sci. Res. 2014, 4, 77–84. [Google Scholar]
- Cronin, T.W.; Warrant, E.J.; Greiner, B. Celestial Polarization Patterns during Twilight. Appl. Opt. 2006, 45, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Meglič, A.; Zalucki, M.P.; Battisti, A.; Belušič, G. Spatial Orientation of Social Caterpillars Is Influenced by Polarized Light. Biol. Lett. 2021, 17, 20200736. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Green, E.S.; Zangerl, A.R. Web Costs and Web Defense in the Parsnip Webworm (Lepidoptera: Oecophoridae). Environ. Entomol. 1993, 22, 791–795. [Google Scholar] [CrossRef]
- Yayalar, N.N. The Influence of Sex-Specific Behaviors on Individual Growth and Mortality of a Caterpillar, Eucheira Socialis Westwoodi; California State University: Long Beach, CA, USA, 2009. [Google Scholar]
- Démolin, G. Incidences de Quelques Facteurs Agissant sur le Comportement Social des Chenillesde Thaumetopoea pityocampa Schiff. (Lepidoptera) Pendant la Période des Processions de Nymphose. Répercussion sur l’efficacité des Parasites. Ann. Zool. Ecol. Anim. 1971, 33–56. [Google Scholar]
- Despland, E.; Hamzeh, S. Ontogenetic Changes in Social Behaviour in the Forest Tent Caterpillar, Malacosoma disstria. Behav. Ecol. Sociobiol. 2004, 56, 177–184. [Google Scholar] [CrossRef]
- Nicolis, S.C.; Despland, E.; Dussutour, A. Collective Decision-Making and Behavioral Polymorphism in Group Living Organisms. J. Theor. Biol. 2008, 254, 580–586. [Google Scholar] [CrossRef]
- Ruf, C.; Fiedler, K. Colony Survivorship of Social Caterpillars in the Field: A Case Study of the Small Eggar Moth (Lepidoptera: Lasiocampidae). J. Res. Lepid. 1999, 38, 15–25. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, M.; Zalucki, M.P.; Battisti, A. Large Male Caterpillars Are the Primary Builders: Exploring Tent Construction and Foraging Behaviour in Gregarious Pine Processionary Caterpillar. Insects 2023, 14, 829. https://doi.org/10.3390/insects14100829
Uemura M, Zalucki MP, Battisti A. Large Male Caterpillars Are the Primary Builders: Exploring Tent Construction and Foraging Behaviour in Gregarious Pine Processionary Caterpillar. Insects. 2023; 14(10):829. https://doi.org/10.3390/insects14100829
Chicago/Turabian StyleUemura, Mizuki, Myron P. Zalucki, and Andrea Battisti. 2023. "Large Male Caterpillars Are the Primary Builders: Exploring Tent Construction and Foraging Behaviour in Gregarious Pine Processionary Caterpillar" Insects 14, no. 10: 829. https://doi.org/10.3390/insects14100829