The Roles of transformer-2 (tra-2) in the Sex Determination and Fertility of Riptortus pedestris, a Hemimetabolous Agricultural Pest
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Cloning of Rptra-2
2.3. Sequence Analysis of Rptra-2
2.4. RNA Extraction
2.5. Semiquantitative RT-PCR and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Absolute Quantification in Real-Time qPCR
2.7. dsRNA Synthesis and Injection
2.8. Dissection and Fertility Analysis
2.9. Sperm Viability Test
2.10. Maternal RNAi
2.11. Mating Experiment
2.12. Statistical Analysis
3. Results
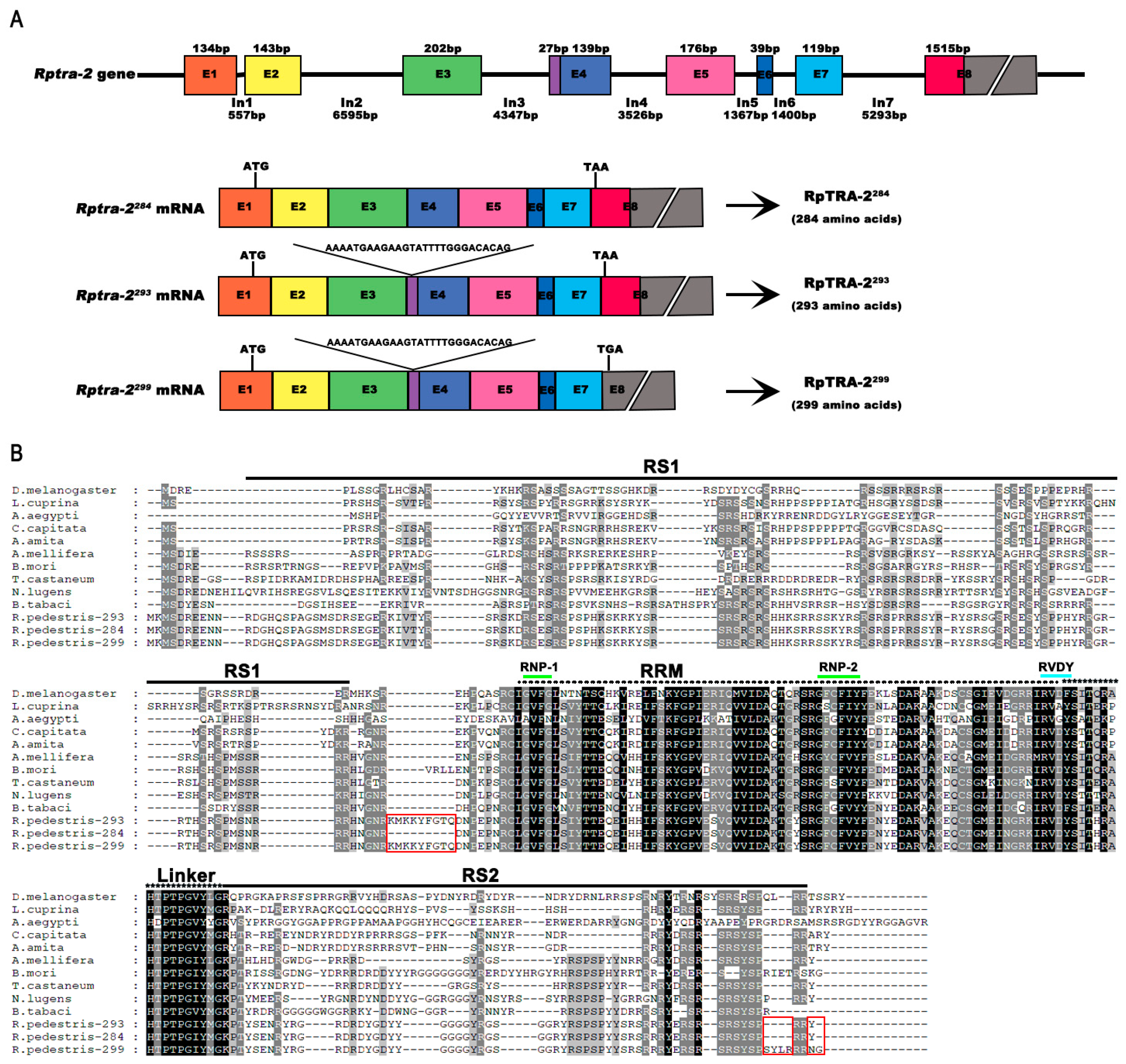
3.1. Structure and Conservation of Rptra-2
3.2. Expression Profile of Rptra-2
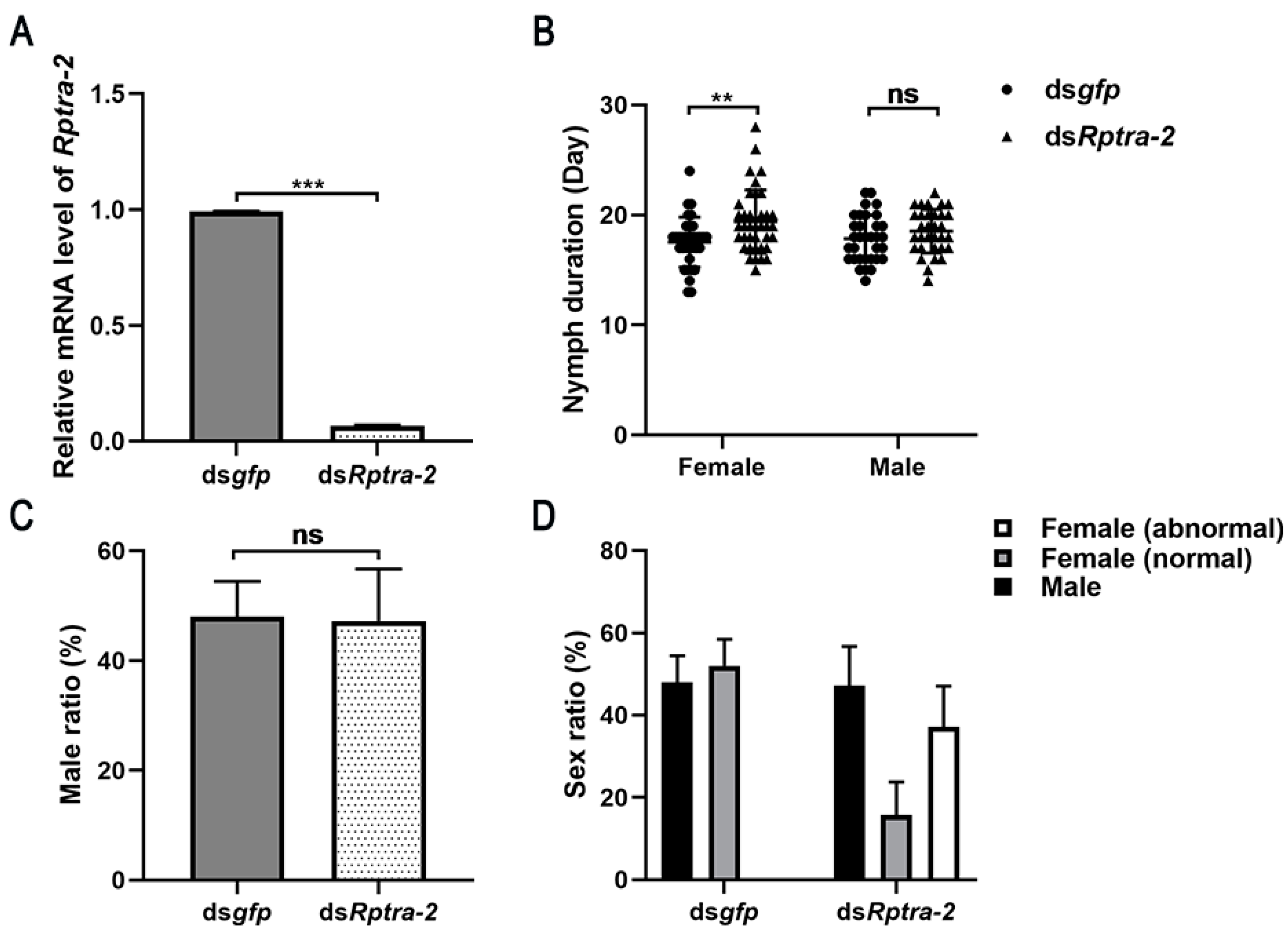
3.3. Nymphs’ RNA Interference Revealed the Function of Rptra-2 in Sex Determination
3.4. Rptra-2 Influenced R. pedestris Fertility in Both Sexes
3.5. Maternal RNAi Knockdown of Rptra-2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marın, I.; Baker, B.S. The evolutionary dynamics of sex determination. Science 1998, 281, 1990–1994. [Google Scholar] [CrossRef] [PubMed]
- Saccone, G.; Pane, A.; Polito, L. Sex determination in flies, fruitflies and butterflies. Genetica 2002, 116, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shearman, D. The evolution of sex determination systems in dipteran insects other than Drosophila. Genetica 2002, 116, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L. Sex-determining mechanisms in insects. Int. J. Dev. Biol. 2008, 52, 837–856. [Google Scholar] [CrossRef]
- Gempe, T.; Beye, M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 2011, 33, 52–60. [Google Scholar] [CrossRef]
- Saccone, G. A history of the genetic and molecular identification of genes and their functions controlling insect sex determination. Insect Biochem. Mol. Biol. 2022, 151, 103873. [Google Scholar] [CrossRef] [PubMed]
- Salz, H.K.; Maine, E.M.; Keyes, L.N.; Samuels, M.E.; Cline, T.W.; Schedl, P. The Drosophila female-specific sex-determination gene, Sex-lethal, has stage-, tissue-, and sex-specific RNAs suggesting multiple modes of regulation. Genes Dev. 1989, 3, 708–719. [Google Scholar] [CrossRef]
- Erickson, J.W.; Quintero, J.J. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol. 2007, 5, e332. [Google Scholar] [CrossRef]
- Amrein, H.; Gorman, M.; Nöthiger, R. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell 1988, 55, 1025–1035. [Google Scholar] [CrossRef]
- Hoshijima, K.; Inoue, K.; Higuchi, I.; Sakamoto, H.; Shimura, Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 1991, 252, 833–836. [Google Scholar] [CrossRef]
- Baker, B.S.; Wolfner, M.F. A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev. 1988, 2, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, M.; Robertson, M.; Aronson, B.; Atkinson, P.; Polito, L.C.; Saccone, G. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 2009, 53, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, M.M.; Lucibelli, F.; Mazzucchiello, S.M.; Fucci, N.; Hay Mele, B.; Giordano, E.; Salvemini, M.; Ruggiero, A.; Vitagliano, L.; Aceto, S.; et al. Female Sex Determination Factors in Ceratitis capitata: Molecular and Structural Basis of TRA and TRA2 Recognition. Insects 2023, 14, 605. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Q.; Li, J.; Zhang, G.; Wan, F. RNAi-Mediated Knock-Down of transformer and transformer 2 to Generate Male-Only Progeny in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). PLoS ONE 2015, 10, e0128892. [Google Scholar] [CrossRef] [PubMed]
- Nissen, I.; Muller, M.; Beye, M. The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. Genetics 2012, 192, 1015–1026. [Google Scholar] [CrossRef]
- Suzuki, M.G.; Suzuki, K.; Aoki, F.; Ajimura, M. Effect of RNAi-mediated knockdown of the Bombyx mori transformer-2 gene on the sex-specific splicing of Bmdsx pre-mRNA. Int. J. Dev. Biol. 2012, 56, 693–699. [Google Scholar] [CrossRef]
- Zhuo, J.C.; Lei, C.; Shi, J.K.; Xu, N.; Xue, W.H.; Zhang, M.Q.; Ren, Z.W.; Zhang, H.H.; Zhang, C.X. Tra-2 Mediates Cross-Talk Between Sex Determination and Wing Polyphenism in Female Nilaparvata lugens. Genetics 2017, 207, 1067–1078. [Google Scholar] [CrossRef]
- Zhuo, J.-C.; Hu, Q.-L.; Zhang, H.-H.; Zhang, M.-Q.; Jo, S.B.; Zhang, C.-X. Identification and functional analysis of the doublesex gene in the sexual development of a hemimetabolous insect, the brown planthopper. Insect Biochem. Mol. Biol. 2018, 102, 31–42. [Google Scholar] [CrossRef]
- Zhuo, J.-C.; Zhang, H.-H.; Hu, Q.-L.; Zhang, J.-L.; Lu, J.-B.; Li, H.-J.; Xie, Y.-C.; Wang, W.-W.; Zhang, Y.; Wang, H.-Q. A feminizing switch in a hemimetabolous insect. Sci. Adv. 2021, 7, eabf9237. [Google Scholar] [CrossRef]
- Son, C.; Park, S.; Hwang, Y.; Choi, B. Field occurrence of stink bug and its damage in soybean. Korean J. Crop Sci. 2000, 45, 405–410. [Google Scholar]
- Lim, U.T. Occurrence and Control Method of Riptortus pedestris (Hemiptera: Alydidae): Korean Perspectives. Korean J. Appl. Entomol. 2013, 52, 437–448. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.; Guo, J.; Penn, H.; Wu, T.; Li, L.; Jiang, H.; Chang, L.; Wu, C.; Han, T. Feeding of Riptortus pedestris on soybean plants, the primary cause of soybean staygreen syndrome in the Huang-Huai-Hai river basin. Crop J. 2019, 7, 360–367. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Hu, Y.; Chen, J.; Zhang, J.; Shi, S. Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China. Insects 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Guo, W.; Jiang, S.; Yan, D.; Shi, Y.; Wu, B.; Xin, X.; Chen, L.; Cai, Y.; Zhang, H.; et al. Transcriptional profiling reveals a critical role of GmFT2a in soybean staygreen syndrome caused by the pest Riptortus pedestris. New Phytol. 2023, 237, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Huang, Y.; Guo, C.; Hu, B.; Zhang, H.; Li, Y.; Chen, J.; Wei, Z.; Sun, Z. A salivary secretory protein from Riptortus pedestris facilitates pest infestation and soybean staygreen syndrome. Mol. Plant Pathol. 2023, 24, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, Y.; Hu, Y.; Li, S.; Bi, R.; Zhang, J.; Shi, S. Effects of the Riptortus pedestris on staygreen syndrome and yield of soybean. Soybean Sci. 2020, 39, 116–122. [Google Scholar]
- Zhang, H.; Wang, Y.; Wang, Z.; Ding, W.; Xu, K.; Li, L.; Wang, Y.; Li, J.; Yang, M.; Liu, X.; et al. Modelling the current and future potential distribution of the bean bug Riptortus pedestris with increasingly serious damage to soybean. Pest. Manag. Sci. 2022, 78, 4340–4352. [Google Scholar] [CrossRef]
- Alim, M.A.; Lim, U.T. Refrigerated eggs of Riptortus pedestris (Hemiptera: Alydidae) added to aggregation pheromone traps increase field parasitism in soybean. J. Econ. Entomol. 2011, 104, 1833–1839. [Google Scholar] [CrossRef]
- Sato, Y.; Jang, S.; Takeshita, K.; Itoh, H.; Koike, H.; Tago, K.; Hayatsu, M.; Hori, T.; Kikuchi, Y. Insecticide resistance by a host-symbiont reciprocal detoxification. Nat. Commun. 2021, 12, 6432. [Google Scholar] [CrossRef]
- Takeuchi, H.; Endo, N. Insecticide susceptibility of Nezara viridula (Heteroptera: Pentatomidae) and three other stink bug species composing a soybean pest complex in Japan. J. Econ. Entomol. 2012, 105, 1024–1033. [Google Scholar] [CrossRef]
- Shepard, M.; Carner, G.; Turnipseed, S. Colonization and resurgence of insect pests of soybean in response to insecticides and field isolation. Environ. Entomol. 1977, 6, 501–506. [Google Scholar] [CrossRef]
- Aumann, R.A.; Hacker, I.; Schetelig, M.F. Female-to-male sex conversion in Ceratitis capitata by CRISPR/Cas9 HDR-induced point mutations in the sex determination gene transformer-2. Sci. Rep. 2020, 10, 18611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Handler, A.M. Temperature-dependent sex-reversal by a transformer-2 gene-edited mutation in the spotted wing drosophila, Drosophila suzukii. Sci. Rep. 2017, 7, 12363. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, R.; Suman, V. C-heterochromatin and its base composition in holokinetic chromosomes of two species of Heteroptera (Insecta: Hemiptera). Nucleus 2012, 55, 163–166. [Google Scholar] [CrossRef]
- Huang, H.J.; Ye, Y.X.; Ye, Z.X.; Yan, X.T.; Wang, X.; Wei, Z.Y.; Chen, J.P.; Li, J.M.; Sun, Z.T.; Zhang, C.X. Chromosome-level genome assembly of the bean bug Riptortus pedestris. Mol. Ecol. Resour. 2021, 21, 2423–2436. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Shin, S.G.; Hwang, S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 2006, 123, 273–280. [Google Scholar] [CrossRef]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef]
- Futahashi, R.; Tanaka, K.; Tanahashi, M.; Nikoh, N.; Kikuchi, Y.; Lee, B.L.; Fukatsu, T. Gene expression in gut symbiotic organ of stinkbug affected by extracellular bacterial symbiont. PLoS ONE 2013, 8, e64557. [Google Scholar] [CrossRef]
- Belote, J.M.; Baker, B.S. Sex determination in Drosophila melanogaster. Analysis of transformer-2, a sex-transforming locus. Proc. Natl. Acad. Sci. USA 1982, 79, 1568–1572. [Google Scholar] [CrossRef]
- Guo, L.; Xie, W.; Liu, Y.; Yang, Z.; Yang, X.; Xia, J.; Wang, S.; Wu, Q.; Zhang, Y. Identification and characterization of doublesex in Bemisia tabaci. Insect Mol. Biol. 2018, 27, 620–632. [Google Scholar] [CrossRef]
- Belote, J.M.; Handler, A.M.; Wolfner, M.F.; Livak, K.J.; Baker, B.S. Sex-specific regulation of yolk protein gene expression in Drosophila. Cell 1985, 40, 339–348. [Google Scholar] [CrossRef]
- Li, X.; Jin, B.; Dong, Y.; Chen, X.; Tu, Z.; Gu, J. Two of the three Transformer-2 genes are required for ovarian development in Aedes albopictus. Insect Biochem. Mol. Biol. 2019, 109, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Belote, J.M.; Baker, B.S. The dual functions of a sex determination gene in Drosophila melanogaster. Dev. Biol. 1983, 95, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.N.; Palli, S.R. Tribolium castaneum Transformer-2 regulates sex determination and development in both males and females. Insect Biochem. Mol. Biol. 2013, 43, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Killiny, N. Effect of parental RNA interference of a transformer-2 homologue on female reproduction and offspring sex determination in Asian citrus psyllid. Physiol. Entomol. 2017, 43, 42–50. [Google Scholar] [CrossRef]
- Pacheco, I.D.; Walling, L.L.; Atkinson, P.W. Gene Editing and Genetic Control of Hemipteran Pests: Progress, Challenges and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 900785. [Google Scholar] [CrossRef]




| Treatment | Eggs | Dead Eggs | Nymph |
|---|---|---|---|
| dsgfp female × dsgfp male (n = 15) | 113 ± 21 | 9 ± 4 | 104 ± 19 |
| dsRptra-2 female × dsgfp male (n = 13) | 0 | 0 | 0 |
| dsgfp female × dsRptra-2 male (n = 12) | 53 ± 13 | 49 ± 13 | 4 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, J.; Wang, H.; Wang, B.; Mao, Z.; Chen, Y.; Li, J.; Zhang, C.; Zhuo, J. The Roles of transformer-2 (tra-2) in the Sex Determination and Fertility of Riptortus pedestris, a Hemimetabolous Agricultural Pest. Insects 2023, 14, 834. https://doi.org/10.3390/insects14110834
Ying J, Wang H, Wang B, Mao Z, Chen Y, Li J, Zhang C, Zhuo J. The Roles of transformer-2 (tra-2) in the Sex Determination and Fertility of Riptortus pedestris, a Hemimetabolous Agricultural Pest. Insects. 2023; 14(11):834. https://doi.org/10.3390/insects14110834
Chicago/Turabian StyleYing, Jinjun, Haiqiang Wang, Biyun Wang, Zeping Mao, Youyuan Chen, Junmin Li, Chuanxi Zhang, and Jichong Zhuo. 2023. "The Roles of transformer-2 (tra-2) in the Sex Determination and Fertility of Riptortus pedestris, a Hemimetabolous Agricultural Pest" Insects 14, no. 11: 834. https://doi.org/10.3390/insects14110834





