Habitat Temperatures of the Red Firebug, Pyrrhocoris apterus: The Value of Small-Scale Climate Data Measurement
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
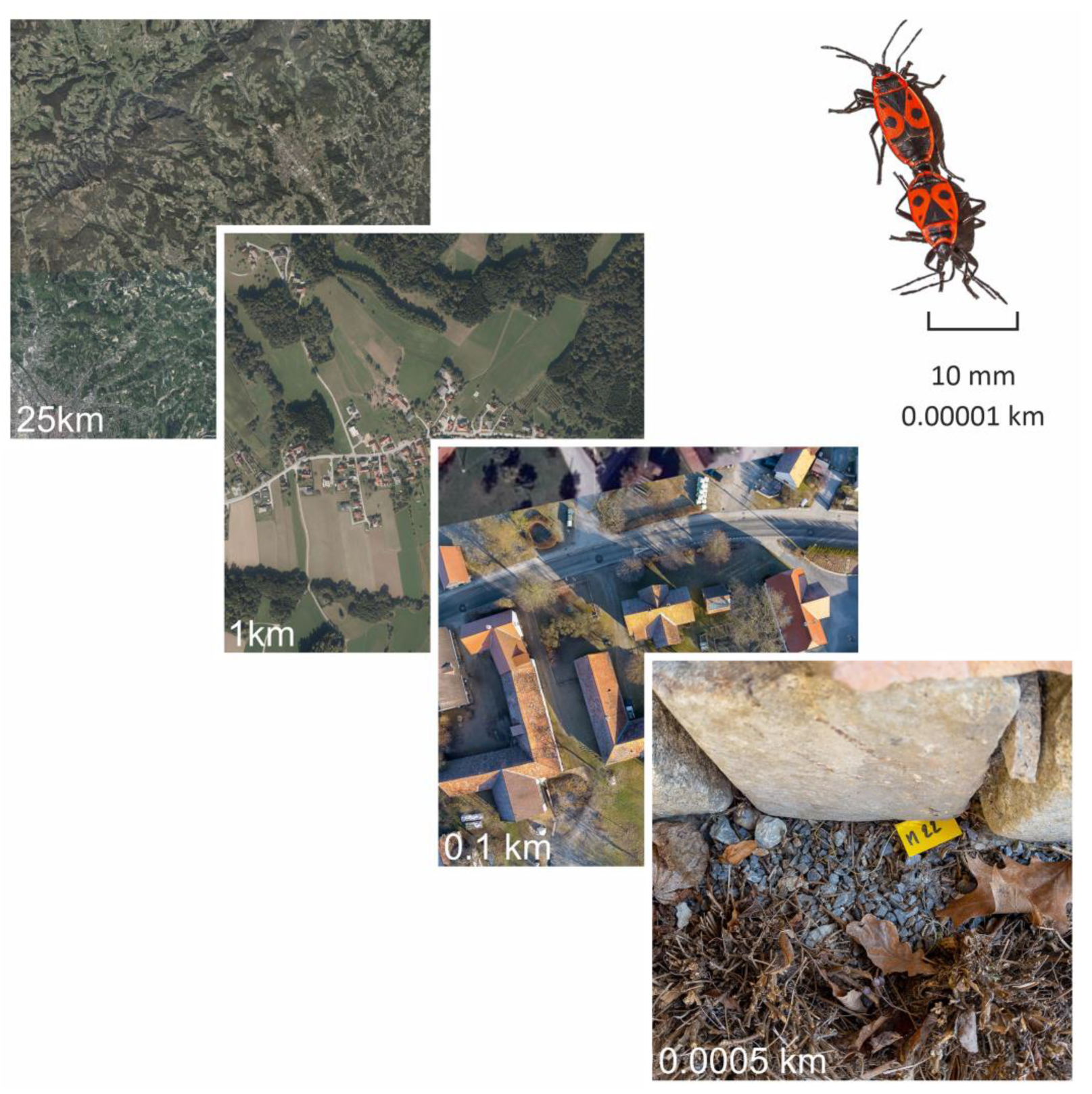
2.1. Animals and Habitat
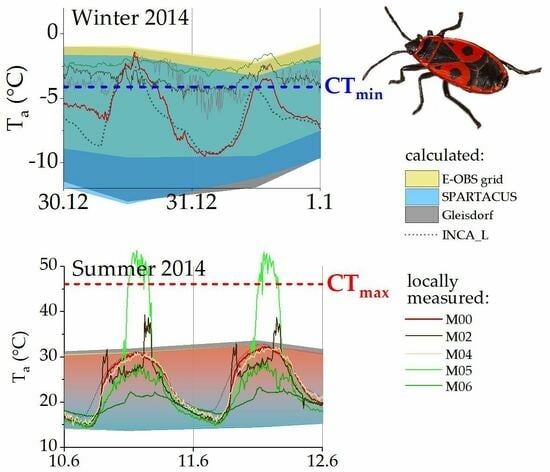
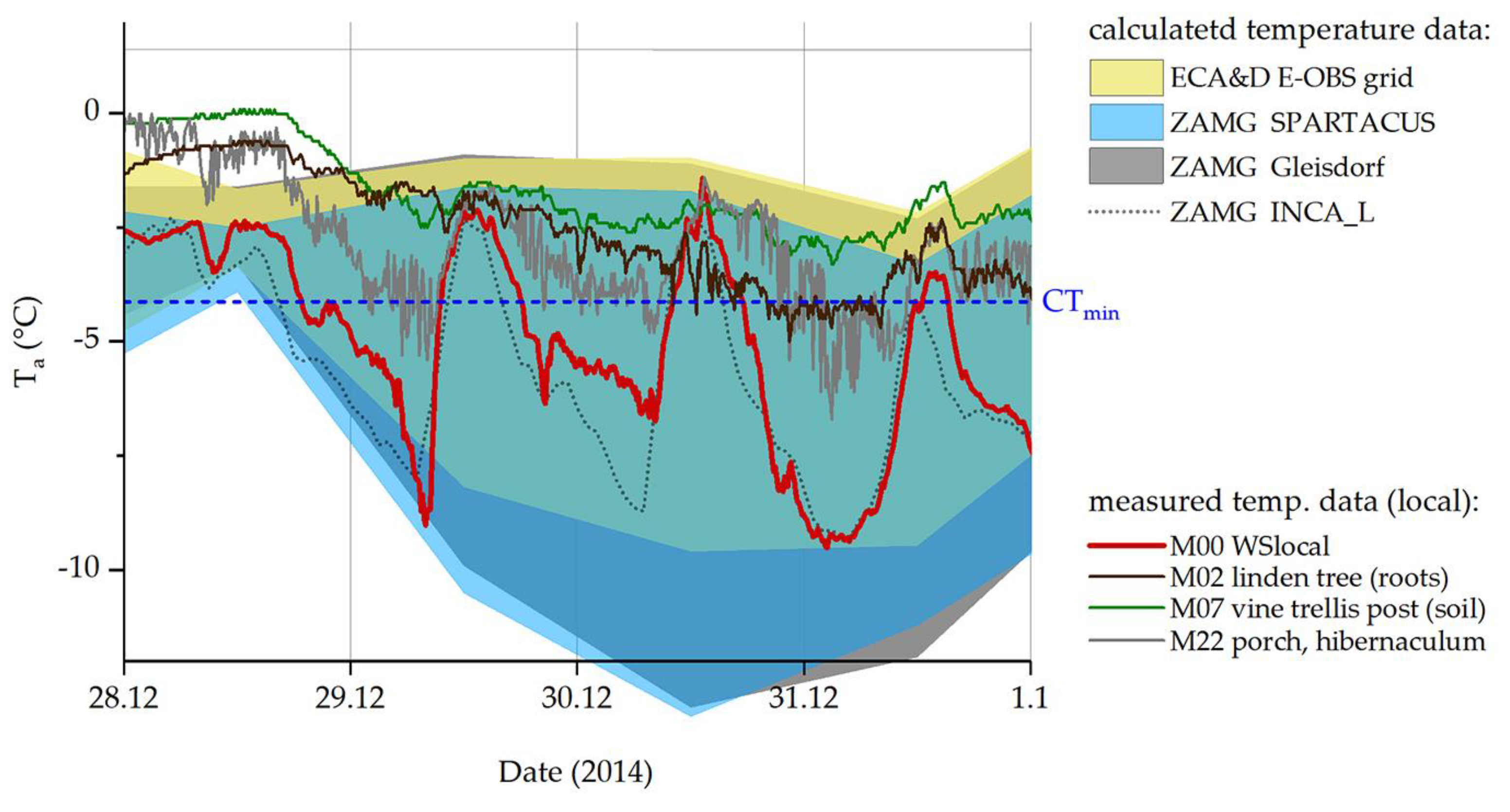
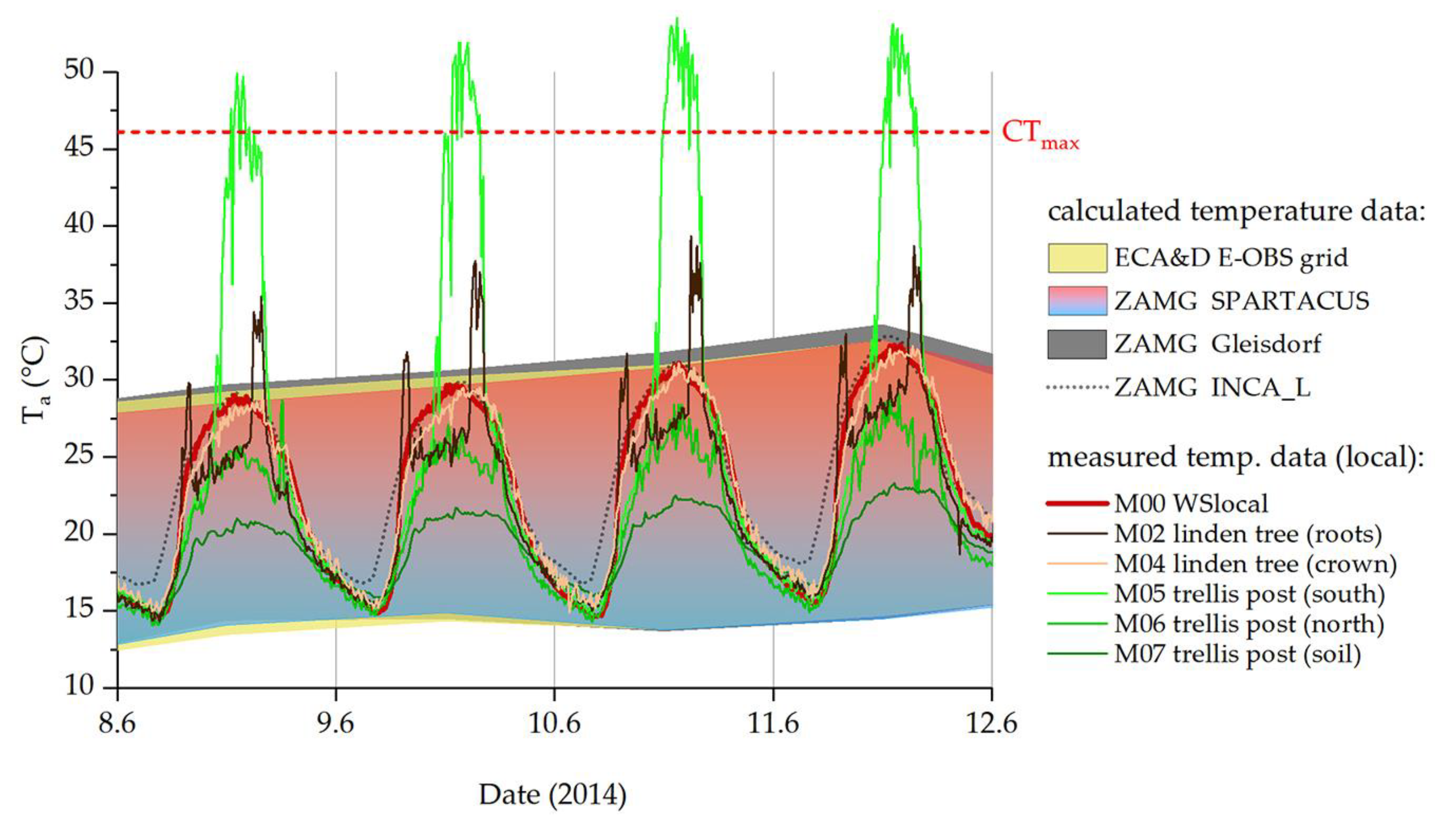
2.2. Temperature Measurement and Climate Data
2.3. Thermal Limits and Safety Margins
2.4. Comparison of Measured and Provided Temperature Data
| Sensor # | Location | Season |
|---|---|---|
| M00 | WSlocal; weather station on site, 2.7 m AGL (above ground level) | s, s, a, w |
| M02 | Tilia sp. near the roots, north (shade), 1 cm AGL; near winter hibernaculum | s, s, a, w |
| M03 | Tilia sp. trunk; north (shade), 100 cm AGL | s, s, a |
| M04 | Tilia sp. crown, 250 cm AGL | s, s, a |
| M05 | Trellis post; south (sun), 1 cm AGL | s, s, a |
| M06 | Trellis post; north (shade), 1 cm AGL | s, s, a |
| M07 | Trellis post; north (shade), soil, under fall foliage; near winter hibernaculum | s, s, a, w |
| M08 | Trellis post; south (sun), 1 cm AGL | s, s, a |
| M22 | Porch, crevices between cobblestones; winter hibernaculum, −10 cm AGL | s, s, a, w |

3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Socha, R. Pyrrhocoris apterus (Heteroptera)—An experimental model species: A review. Eur. J. Entomol. 1993, 90, 241–286. [Google Scholar]
- Stichel, W. Illustrierte Bestimmungstabellen der Wanzen: II. Europa (Hemiptera-Heteroptera Europae); W.Stichel: Berlin-Hermsdorf, Germany, 1955. [Google Scholar]
- Kulik, S.A. Four new species of Miridae (Heteroptera) from the Far East of the USSR. Nauchnye Dokl. Vyss. Shkoly Biol. Nauk. 1973, 16, 19–23. [Google Scholar]
- Marren, P. Bugs Britannica; Chatto & Windus: London, UK, 2010; ISBN 978-0-7011-8180-2. [Google Scholar]
- Endrestøl, A.; Roth, S. The firebug Pyrrhocoris apterus (Linnaeus, 1758) (Hemiptera, Heteroptera) new to the Norwegian fauna—With an explosive expansion in Northern Europe. Nor. J. Entomol. 2020, 67, 81–90. [Google Scholar]
- Mata, L.; Vogel, B.; Palma, E.; Malipatil, M. The Arrival and Spread of the European Firebug (Pyrrhocoris apterus) in Australia as Documented by Citizen Scientists. Urban Nat. Notes 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Taylor, F. Ecology and Evolution of Physiological Time in Insects. Am. Nat. 1981, 117, 283683. [Google Scholar] [CrossRef]
- Chown, S.L.; Nicolson, S.W. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, NY, USA, 2004; ISBN 978-0198515494. [Google Scholar]
- Dixon, A.F.; Honěk, A.; Keil, P.; Kotela, M.A.A.; Šizling, A.L.; Jarošík, V. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 2009, 23, 257–264. [Google Scholar] [CrossRef]
- Bale, J.S. Insects at Low Temperature: A Predictable Relationship? Funct. Ecol. 1991, 5, 291. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. Biol. Sci. 2011, 278, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. Biol. Sci. 2000, 267, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef]
- Winwood-Smith, H.S.; Alton, L.A.; Franklin, C.E.; White, C.R. Does greater thermal plasticity facilitate range expansion of an invasive terrestrial anuran into higher latitudes? Conserv. Physiol. 2015, 3, cov010. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, S.; Baguette, M.; Trochet, A.; Chaput-Bardy, A.; Legrand, D.; Stevens, V.; Fréville, H. Flight endurance and heating rate vary with both latitude and habitat connectivity in a butterfly species. Oikos 2013, 122, 601–611. [Google Scholar] [CrossRef]
- Alford, L.; Blackburn, T.M.; Bale, J.S. Effect of latitude and acclimation on the lethal temperatures of the peach-potato aphid Myzus persicae. Agric. For. Entomol. 2012, 14, 69–79. [Google Scholar] [CrossRef]
- Buckley, L.B.; Huey, R.B. Temperature extremes: Geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Chang. Biol. 2016, 22, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Hoffmann, A.A. Physiological climatic limits in Drosophila: Patterns and implications. J. Exp. Biol. 2010, 213, 870–880. [Google Scholar] [CrossRef]
- Potter, K.A.; Arthur Woods, H.; Pincebourde, S. Microclimatic challenges in global change biology. Glob. Chang. Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef]
- Rebaudo, F.; Faye, E.; Dangles, O. Microclimate Data Improve Predictions of Insect Abundance Models Based on Calibrated Spatiotemporal Temperatures. Front. Physiol. 2016, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Pincebourde, S.; Casas, J. Warming tolerance across insect ontogeny: Influence of joint shifts in microclimates and thermal limits. Ecology 2015, 96, 986–997. [Google Scholar] [CrossRef]
- Kovac, H.; Käfer, H.; Petrocelli, I.; Stabentheiner, A. The respiratory metabolism of overwintering paper wasp gynes (Polistes dominula and Polistes gallicus). Physiol. Entomol. 2022, 47, 62–71. [Google Scholar] [CrossRef]
- Pincebourde, S.; Salle, A. On the importance of getting fine-scale temperature records near any surface. Glob. Chang. Biol. 2020, 26, 6025–6027. [Google Scholar] [CrossRef]
- MacLean, H.J.; Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V.; Beedholm, K.; Kellermann, V.; Overgaard, J. Evolution and plasticity of thermal performance: An analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180548. [Google Scholar] [CrossRef]
- Hadley, N.F. Water Relations of The Desert Scorpion, Hadrurus arizonensis. J. Exp. Biol. 1970, 53, 547–558. [Google Scholar] [CrossRef]
- Hadley, N.F.; Quinlan, M.C.; Kennedy, M.L. Evaporative cooling in the desert cicada. J. Exp. Biol. 1991, 159, 269–283. [Google Scholar] [CrossRef]
- Buckley, L.B.; Miller, E.F.; Kingsolver, J.G. Ectotherm thermal stress and specialization across altitude and latitude. Integr. Comp. Biol. 2013, 53, 571–581. [Google Scholar] [CrossRef]
- Kearney, M.R.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A.; Dillon, M.E.; Pincebourde, S. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol. 2015, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- ZAMG—Zentralanstalt für Meteorologie und Geodynamik. ZAMG Data Hub. Available online: https://data.hub.zamg.ac.at (accessed on 27 July 2023).
- UERRA—Uncertainities in Ensembles of Regional Analyses. E-OBS Dataset from the EU-FP6 Project UERRA. Available online: https://www.uerra.eu/ (accessed on 5 September 2022).
- Copernicus—Europe’s Eyes on Earth. E-OBS Daily Gridded Meteorological Data for Europe from 1950 to Present Derived from In-Situ Observations. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/insitu-gridded-observations-europe?tab=overview (accessed on 5 September 2022).
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An Ensemble Version of the E-OBS Temperature and Precipitation Data Sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Käfer, H.; Kovac, H.; Stabentheiner, A.; Simov, N.; Battisti, A. Thermal traits of true bugs, an insect taxon with high invasive potential. In ECE 2018—XI European Congress of Entomology, Book of Abstracts, Proceedings of the XI European Congress of Entomology, Naples, Italy, 2–6 July 2018; Societá Entomologica Italiana: Naples, Italy, 2018; p. 252. [Google Scholar]
- Käfer, H.; Kovac, H.; Simov, N.; Battisti, A.; Erregger, B.; Schmidt, A.K.D.; Stabentheiner, A. Temperature Tolerance and Thermal Environment of European Seed Bugs. Insects 2020, 11, 197. [Google Scholar] [CrossRef]
- Andersen, J.L.; Manenti, T.; Sørensen, J.G.; MacMillan, H.A.; Loeschcke, V.; Overgaard, J. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 2015, 29, 55–65. [Google Scholar] [CrossRef]
- Chown, S.L.; Jumbam, K.R.; Sørensen, J.G.; Terblanche, J.S. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 2009, 23, 133–140. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Deere, J.A.; Clusella-Trullas, S.; Janion, C.; Chown, S.L. Critical thermal limits depend on methodological context. Proc. R. Soc. Lond. B 2007, 274, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Kovac, H.; Käfer, H.; Stabentheiner, A. The Respiratory Metabolism of Polistes biglumis, a Paper Wasp from Mountainous Regions. Insects 2020, 11, 165. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: Data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997, 75, 1553–1560. [Google Scholar] [CrossRef]
- Hazell, S.P.; Pedersen, B.P.; Worland, M.R.; Blackburn, T.M.; Bale, J.S. A method for the rapid measurement of thermal tolerance traits in studies of small insects. Physiol. Entomol. 2008, 33, 389–394. [Google Scholar] [CrossRef]
- Klok, C.J.; Chown, S.L. Critical thermal limits, temperature tolerance and water balance of a sub-Antarctic caterpillar, Pringleophaga marioni (Lepidoptera: Tineidae). J. Insect Physiol. 1997, 43, 685–694. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 2019, 569, 108–111. [Google Scholar] [CrossRef]
- Kovac, H.; Käfer, H.; Petrocelli, I.; Amstrup, A.B.; Stabentheiner, A. Energetics of Paper Wasps (Polistes sp.) from Differing Climates during the Breeding Season. Insects 2022, 13, 800. [Google Scholar] [CrossRef]
- Pincebourde, S.; Suppo, C. The Vulnerability of Tropical Ectotherms to Warming Is Modulated by the Microclimatic Heterogeneity. Integr. Comp. Biol. 2016, 56, 85–97. [Google Scholar] [CrossRef]
- Pincebourde, S.; Woods, H.A. There is plenty of room at the bottom: Microclimates drive insect vulnerability to climate change. Curr. Opin. Insect Sci. 2020, 41, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.R.; Isaac, A.P.; Porter, W.P. microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci. Data 2014, 1, 140006. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.R.; Shamakhy, A.; Tingley, R.; Karoly, D.J.; Hoffmann, A.A.; Briggs, P.R.; Porter, W.P.; Travis, J. Microclimate modelling at macro scales: A test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods Ecol. Evol. 2014, 5, 273–286. [Google Scholar] [CrossRef]
- Kearney, M.R.; Gillingham, P.K.; Bramer, I.; Duffy, J.P.; Maclean, I.M. A method for computing hourly, historical, terrain-corrected microclimate anywhere on earth. Methods Ecol. Evol. 2020, 11, 38–43. [Google Scholar] [CrossRef]
- Kearney, M.R.; Porter, W.P. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.P.; Ostrowski, S.; Williams, J.B. Modeling Animal Landscapes. PBZ 2010, 83, 705–712. [Google Scholar] [CrossRef]
- Maclean, I.M.D.; Duffy, J.P.; Haesen, S.; Govaert, S.; de Frenne, P.; Vanneste, T.; Lenoir, J.; Lembrechts, J.J.; Rhodes, M.W.; van Meerbeek, K. On the measurement of microclimate. Methods Ecol. Evol. 2021, 12, 1397–1410. [Google Scholar] [CrossRef]
- Maclean, I.M.D. Predicting future climate at high spatial and temporal resolution. Glob. Chang. Biol. 2019, 26, 1003–1011. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Nijs, I.; Lenoir, J. Incorporating microclimate into species distribution models. Ecography 2019, 42, 1267–1279. [Google Scholar] [CrossRef]
- Nadeau, C.P.; Urban, M.C.; Bridle, J.R. Coarse climate change projections for species living in a fine-scaled world. Glob. Chang. Biol. 2017, 23, 12–24. [Google Scholar] [CrossRef]
- Eingrüber, N.; Korres, W.; Schneider, K. Microclimatic field measurements to support microclimatological modelling with ENVI-met for an urban study area in Cologne. Adv. Sci. Res. 2022, 19, 81–90. [Google Scholar] [CrossRef]
- World Meteorological Organisation. Climate Observation Networks. Available online: https://community.wmo.int/climate-observation-networks (accessed on 5 September 2022).
- Copernicus—Europe’s Eyes on Earth. Known Issues in E-OBS. Available online: https://surfobs.climate.copernicus.eu/userguidance/known_issues_eobs.php (accessed on 5 September 2022).
- Honek, A.; Martinkova, Z. Behavioural thermoregulation hastens spring mating activity in Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). J. Therm. Biol. 2019, 84, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V.; Šimek, P. Overwintering strategy in Pyrrhocoris apterus (Heteroptera): The relations between life-cycle, chill tolerance and physiological adjustments. J. Insect Physiol. 2000, 46, 1321–1329. [Google Scholar] [CrossRef]
- Hodkova, M.; Hodek, I. Temperature Regulation of Supercooling and Gut Nucleation in Relation to Diapause of Pyrrhocoris apterus (L.) (Heteroptera). Cryobiology 1997, 34, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ditrich, T. Supercooling point is an individually fixed metric of cold tolerance in Pyrrhocoris apterus. J. Therm. Biol. 2018, 74, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Ditrich, T.; Janda, V.; Vaněčková, H.; Doležel, D. Climatic Variation of Supercooling Point in the Linden Bug Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Insects 2018, 9, 144. [Google Scholar] [CrossRef] [PubMed]


| Dataset | Source | Data Processing |
|---|---|---|
| SPARTACUS | ZAMG | Gridded daily dataset of observed air temperature (°C, min, max) in 1 km resolution over Austria since 1961. Monthly and seasonal (meteorological seasons) aggregates from daily data. |
| INCA_L | ZAMG | Gridded background field-corrected with observational data; uses station observations, remote sensing data, numerical weather forecast models and a high-resolution terrain model; mean temperatures (°C) at 1 km × 1 km and 1 h resolution. |
| WS Gleisdorf | ZAMG | Monthly and daily mean, min and max temperature values (°C) from one of 260 measurement stations in Austria. |
| E-OBS | ECA&D | Ensemble dataset on a 0.25-degree regular grid; daily mean, min, max temperatures (°C). |
| Winter, min. Temp. | Summer, Max. Temp. | ||||||
|---|---|---|---|---|---|---|---|
| Data Source | Data Scale, Type | 2013–14 | 2014–15 | 2015–16 | 2014 | 2015 | 2016 |
| M00, WSlocal | local, measured | −7.4 | −9.5 | −11.4 | 32.3 | 34.2 | 30.4 |
| ZAMG Gleisdorf | macro, measured | −0.1 | −3.5 | −0.1 | 1.3 | 0.3 | 0.9 |
| ZAMG SPARTACUS | large-scale, gridded | −0.6 | −3.8 | 0.4 | 0.3 | 0.0 | −0.1 |
| ECA&D E-OBS | large-scale, gridded | 2.8 | 3.8 | 4.2 | 0.3 | 0.1 | 0.7 |
| M22 Porch, hiber | micro, measured | 1.5 | 2.8 | 4.9 | 5.1 | 2.7 | 2.7 |
| M02 Tilia, roots, hiber | “ | 0.8 | 3.8 | 0.9 | 7.0 | 7.1 | 4.3 |
| M03 Tilia trunk, north | “ | 0.5 | 0.0 | −0.4 | 1.1 | 1.1 | 0.5 |
| M04 Tilia crown | “ | −0.1 | −0.5 | −0.4 | −0.1 | 0.6 | −0.4 |
| M05 Post, south | “ | 4.7 | 7.3 | 1.1 | −6.3 | 0.1 | −1.0 |
| M06 Post, north | “ | 0.7 | 0.4 | 0.0 | −3.0 | −2.6 | 1.7 |
| M07 Post, soil, north, hiber | “ | 2.7 | 6.2 | 3.4 | −9.0 | −3.7 | −6.6 |
| M08 Post, south | “ | 4.7 | 7.3 | 1.1 | −6.3 | 0.1 | −1.0 |
| 2015 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data Source | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec |
| M00, WSlocal | 2.0 | 1.2 | 5.2 | 9.7 | 14.4 | 18.3 | 21.5 | 21.0 | 14.3 | 8.9 | 7.0 | 2.4 |
| ZAMG Gleisdorf | 1.4 | 0.9 | 5.0 | 9.6 | 14.9 | 18.9 | 22.1 | 20.9 | 14.3 | 8.9 | 5.6 | 1.7 |
| ZAMG SPARTACUS | 1.3 | 1.0 | 5.5 | 9.6 | 14.6 | 18.2 | 21.6 | 20.9 | 14.6 | 9.4 | 6.7 | 1.8 |
| ECA&D E-OBS | 2.0 | 1.7 | 6.0 | 10.4 | 15.0 | 18.7 | 21.9 | 21.3 | 14.8 | 9.8 | 7.4 | 2.8 |
| M22 | 2.9 | 2.9 | 7.5 | 12.3 | 16.4 | 20.9 | 23.6 | 23.8 | 16.4 | 10.6 | 8.6 | 3.8 |
| M02 | 1.1 | 0.9 | 4.7 | 9.2 | 13.7 | 18.0 | 21.2 | 20.5 | 14.5 | 9.4 | 5.9 | 1.3 |
| M03 | 1.8 | 1.2 | 5.5 | 10.1 | 14.7 | 18.6 | 21.5 | 21.1 | 14.5 | 9.1 | 7.0 | 2.1 |
| M04 | 2.0 | 1.3 | 5.7 | 10.4 | 14.7 | 18.6 | 21.4 | 20.9 | 14.3 | 8.9 | 7.0 | 2.4 |
| M05 | 1.3 | 1.0 | 5.9 | 11.4 | 16.1 | 21.4 | 22.9 | 21.9 | 15.1 | 9.7 | 5.9 | 1.5 |
| M06 | 1.4 | 1.1 | 5.7 | 9.7 | 14.3 | 17.6 | 20.0 | 19.3 | 13.7 | 8.9 | 5.8 | 1.3 |
| M07 | 1.6 | 1.3 | 4.6 | 9.5 | 14.4 | 17.6 | 19.9 | 19.2 | 13.8 | 9.4 | 6.1 | 2.1 |
| M08 | 1.8 | 1.5 | 5.5 | 10.8 | 14.3 | 18.0 | 20.7 | 19.9 | 14.5 | 9.7 | 6.6 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Käfer, H.; Kovac, H.; Stabentheiner, A. Habitat Temperatures of the Red Firebug, Pyrrhocoris apterus: The Value of Small-Scale Climate Data Measurement. Insects 2023, 14, 843. https://doi.org/10.3390/insects14110843
Käfer H, Kovac H, Stabentheiner A. Habitat Temperatures of the Red Firebug, Pyrrhocoris apterus: The Value of Small-Scale Climate Data Measurement. Insects. 2023; 14(11):843. https://doi.org/10.3390/insects14110843
Chicago/Turabian StyleKäfer, Helmut, Helmut Kovac, and Anton Stabentheiner. 2023. "Habitat Temperatures of the Red Firebug, Pyrrhocoris apterus: The Value of Small-Scale Climate Data Measurement" Insects 14, no. 11: 843. https://doi.org/10.3390/insects14110843