Insecticidal Effect of the Entomopathogenic Fungus Lecanicillium araneicola HK-1 in Aphis craccivora (Hemiptera: Aphididae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture Conditions
2.2. Aphid Rearing
2.3. Infection Process of L. araneicola HK-1-GFP
2.4. Scanning Electron Microscopy
2.5. Measurement of Chitinase Activity
2.6. Measurement of Extracellular Protease Activity
2.7. Bioassay to Test the Effects of the Crude Extract in A. craccivora
2.8. Effect of the Crude Extract on Enzyme Activity
2.9. Effect of the Crude Extract on Enzyme Genes
2.10. Effect of the Crude Extract on ATPase
2.11. Isolation of the Crude Extract and GC-MS Analysis
2.12. Data Analysis
3. Results
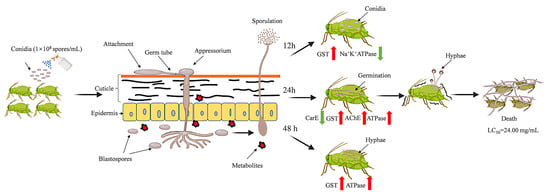
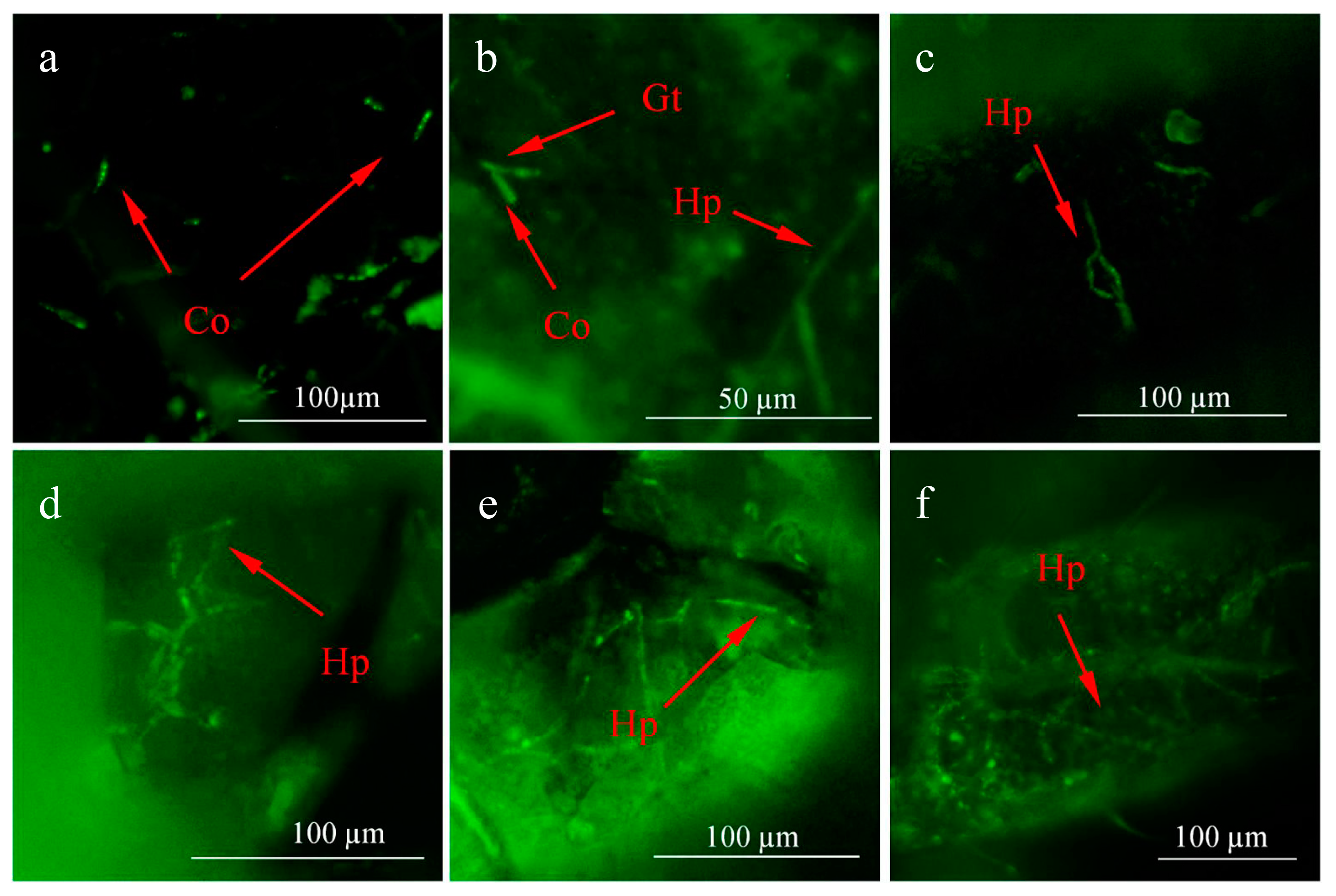
3.1. Infection Process of L. araneicola HK-1-GFP
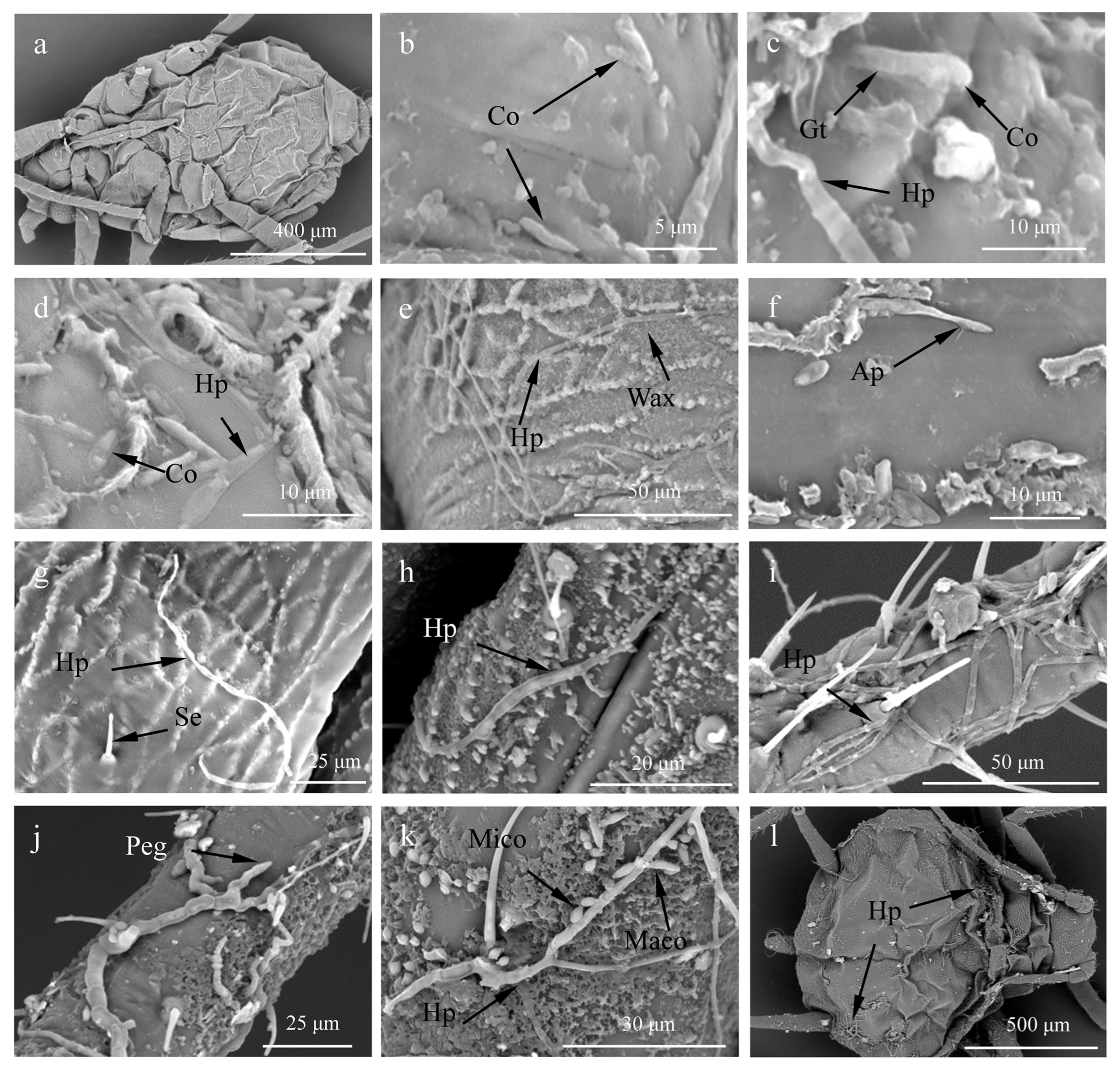
3.2. Scanning Electron Microscopy
3.3. Measurement of Chitinase Activity
3.4. Measurement of Extracellular Protease Activity
3.5. Bioassay on A. craccivora
3.6. Effect of the Crude Extract on Enzyme Activity
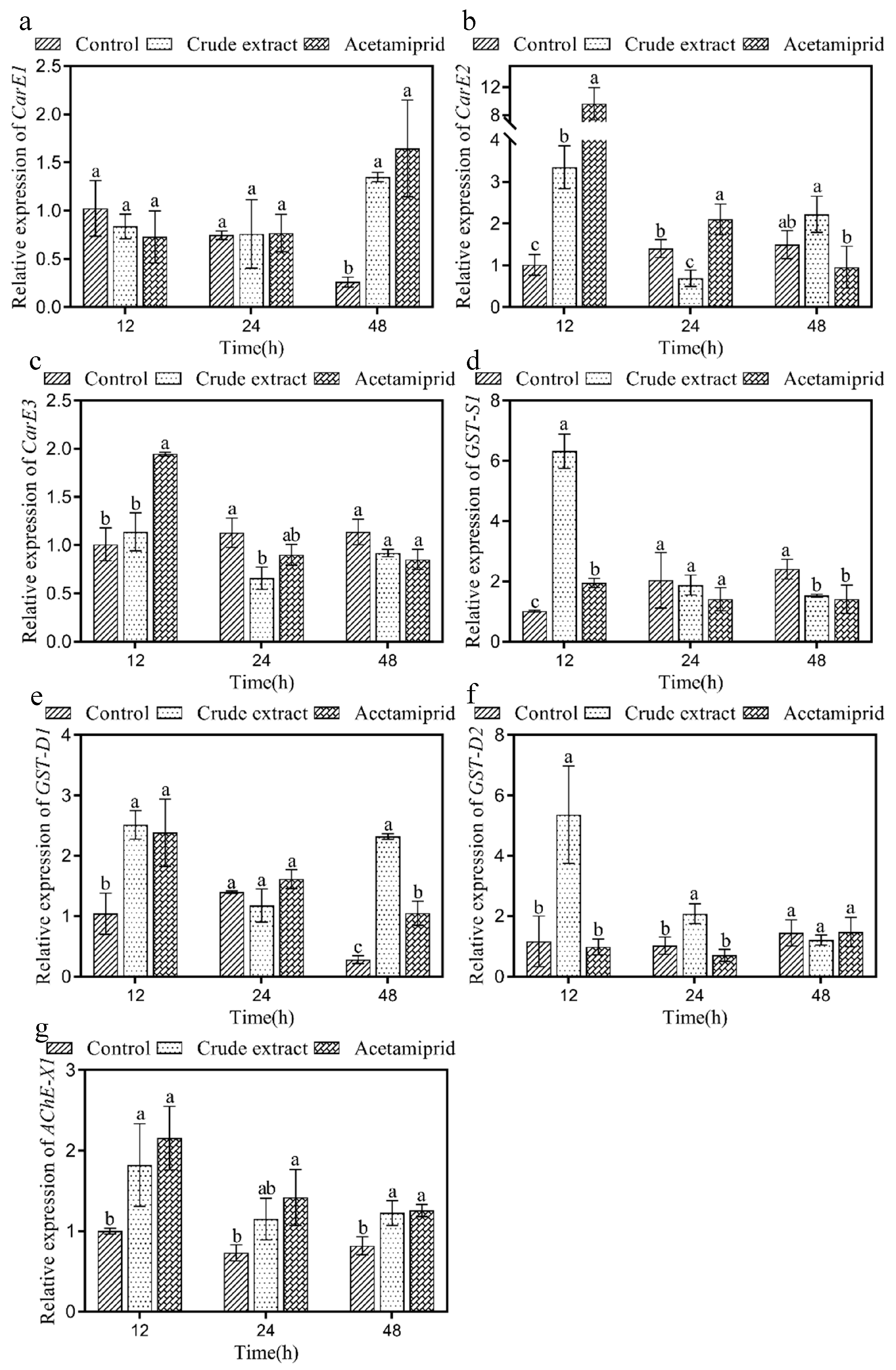
3.7. Effect of the Crude Extract on Enzyme Genes
3.8. Effect of the Crude Extract on ATPase
3.9. Isolation of the Crude Extract and GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, A.F.G. Aphid Ecology: An Optimization Approach, 2nd ed.; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Ahir, K.C.; Saini, A.; Rana, B.S.; Dangi, N.L. Population dynamics of sucking pests in relation to weather parameters in groundnut (Arachis hypogaea L.). Ent. Zool. Stud. 2017, 5, 960–963. [Google Scholar]
- Choudhary, A.L.; Hussain, A.; Choudhary, M.D.; Samota, R.; Jat, S. Bioefficacy of newer insecticides against aphid, Aphis craccivora Koch on cowpea. J. Pharmacogn. Phytochem. 2017, 6, 1788–1792. [Google Scholar]
- Marimuthu, T.; Suganthy, M.; Nakkeeran, S. Common pests and diseases of medicinal plants and strategies to manage them. In New Age Herb; Springer: Singapore, 2018; pp. 289–312. [Google Scholar] [CrossRef]
- Bowling, R.D.; Brewer, M.J.; Kerns, D.L.; Gordy, J.; Seiter, N.; Elliott, N.E.; Buntin, G.D.; Way, M.O.; Royer, T.A.; Biles, S.; et al. Sugarcane Aphid (Hemiptera: Aphididae): A New Pest on Sorghum in North America. J. Integr. Pest. Manag. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons: Chichister, UK, 2000. [Google Scholar]
- Castle, S.J.; Perring, T.M.; Farrar, C.A.; Kishaba, A.N. Field and laboratory transmission of watermelon mosaic virus 2 and zucchini yellow mosaic virus by various aphid species. Phytopathology 1992, 82, 235–240. [Google Scholar] [CrossRef]
- Gray, S.M.; Power, A.G.; Smith, D.M.; Seaman, A.J.; Altman, N.S. Aphid transmission of barley yellow dwarf virus: Acquisition access periods and virus concentration requirements. Phytopathology 1991, 81, 197–214. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Verghese, A.; Ranganath, H.R.; Krishnakumar, N.K.; Venugopalan, R. Role of transient aphid vectors on the temporal spread of Papaya ringspot virus in south India. Acta Hortic. 2007, 740, 251–258. [Google Scholar] [CrossRef]
- Kundoo, A.A.; Dar, S.A.; Mushtaq, M.; Bashir, Z.; Dar, M.S.; Gul, S.; Ali, M.T.; Gulzar, S. Role of neonicotinoids in insect pest management: A review. J. Entomol. Zool. Stud 2018, 6, 333–339. [Google Scholar]
- Kumar, S. Use of pesticides in agriculture and livestock animals and its impact on environment of India. Asian J. Environ. Sci. 2013, 8, 51–57. [Google Scholar]
- Donkor, A.; Osei-Fosu, P.; Dubey, B.; Kingsford-Adaboh, R.; Ziwu, C.; Asante, I. Pesticide residues in fruits and vegetables in Ghana: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 18966–18987. [Google Scholar] [CrossRef]
- Augustyniuk-Kram, A.; Kram, K.J. Entomopathogenic fungi as an important natural regulator of insect outbreaks in forests. For. Ecosyst. 2012, 265–294. [Google Scholar] [CrossRef]
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: A SEM study. Microsc. Res. Tech. 2010, 73, 714–725. [Google Scholar] [CrossRef]
- Gul, H.T.; Saeed, S.; Khan, F.Z. Entomopathogenic fungi as effective insect pest management tactic: A review. Appl. Sci. Bus. Econ. 2014, 1, 10–18. [Google Scholar]
- Luo, S.; He, M.; Cao, Y.Q.; Xia, Y.X. The tetraspanin gene MaPls1 contributes to virulence by affecting germination, appressorial function and enzymes for cuticle degradation in the entomopathogenic fungus, Metarhizium acridum. Environ. Microbiol. 2013, 15, 2966–2979. [Google Scholar] [CrossRef]
- Baek, S.; Noh, M.Y.; Mun, S.; Lee, S.J.; Arakane, Y.; Kim, J.S. Ultrastructural analysis of beetle larva cuticles during infection with the entomopathogenic fungus, Beauveria bassiana. Pest. Manag. Sci. 2022, 78, 3356–3364. [Google Scholar] [CrossRef]
- Roustaee, A.; Dechamp-Guillaume, G.; Gélie, B.; Savy, C.; Dargent, R.; Barrault, G. Ultrastructural Studies of the Mode of Penetration by Phoma macdonaldii in Sunflower Seedlings. Phytopathology 2000, 90, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Umer, M.J.; Wang, Y.Z.; He, K.L.; Zhang, T.T.; Bai, S.X.; Zhi, Y.; Chen, J.; Wang, Z.Y. Synergistic effect of Beauveria bassiana and Trichoderma asperellum to induce Maize (Zea mays L.) defense against the asian corn borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and larval immune response. Int. J. Mol. Sci. 2020, 21, 8215. [Google Scholar] [CrossRef]
- Liu, W.M.; Xie, Y.P.; Xue, J.L.; Gao, Y.; Zhang, Y.F.; Zhang, X.M.; Tan, J.S. Histopathological changes of Ceroplastes japonicus infected by Lecanicillium lecanii. J. Invertebr. Pathol. 2009, 101, 96–105. [Google Scholar] [CrossRef]
- Ganassi, S.; Grazioso, P.; Moretti, A.; Sabatin, M.A. Effects of the fungus Lecanicillium lecanii on survival and reproduction of the aphid Schizaphis graminum. Biocontrol 2010, 55, 299–312. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wang, R.; Liu, C.Z. Infection process and pathogenicity of entomoathogenic fungus Lecanicillium longisporum strain TF-2 to Acyrthosiphon pisum (Hemiptera: Aphididae). Acta Entomol. Sin. 2020, 63, 744–750. [Google Scholar] [CrossRef]
- Pedrini, N. The entomopathogenic fungus Beauveria bassiana shows its toxic side within insects: Expression of genes encoding secondary metabolites during pathogenesis. J. Fungi 2022, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.P.; Bailey, A.M.; Cobb, B.; Vilcinskas, A. Fungi as elicitors of insect immune responses. Arch. Insect Biochem. 2000, 44, 49–68. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Xie, J.; Rantala, M.J. Depressed performance and detoxification enzyme activities of Helicoverpa armigera fed with conventional cotton foliage subjected to methyl jasmonate exposure. Entomol. Exp. Appl. 2013, 147, 186–195. [Google Scholar] [CrossRef]
- Ross, M.K.; Streit, T.M.; Herring, K.L. Carboxylesterases: Dual roles in lipid and pesticide metabolism. J. Pestic. Sci. 2010, 35, 257–264. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Lu, C.; Li, W.L.; Xiang, Z.H.; Zhang, Z. Annotation and expression of carboxylesterases in the silkworm, Bombyx mori. BMC Genom. 2009, 10, 553. [Google Scholar] [CrossRef]
- Sanil, D.; Shetty, V.; Shetty, N.J. Differential expression of glutathione s-transferase enzyme in different life stages of various insecticide-resistant strains of Anopheles stephensi: A malaria vector. J. Vector Dis. 2014, 51, 97–105. [Google Scholar]
- Türkan, F.; Atalar, M.N. The effects of amoxicillin and vancomycin hydrochloride hydrate on glutathione S-transferase enzyme activity: An in vitro study. Iğdır Univ. J. Inst. Sci. Technol. 2018, 8, 141–148. [Google Scholar]
- Roepcke, C.B.; Muench, S.B.; Schulze, H.; Bachmann, T.T.; Schmid, R.D.; Hauer, B. Analysis of Phosphorothionate Pesticides Using a Chloroperoxidase Pretreatment and Acetylcholinesterase Biosensor Detection. J. Agric. Food Chem. 2010, 58, 8748–8756. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Zhu, H.M.; Li, C.Y.; Qian, H.; Yao, W.R.; Guo, Y.H. The present situation of pesticide residues in China and their removal and transformation during food processing. Food Chem. 2021, 354, 129552. [Google Scholar] [CrossRef]
- Liu, S.K.; Chen, C.L.; Shen, Y.Y.; Li, J.H.; Tan, Z.Q.; Jin, P.F. Identification of Lecanicillium araneicola HK-1 and insecticidal potential against Aphis craccivora (Hemiptera: Aphididae). Acta Ent. Sin. 2023, 66, 486–500. [Google Scholar]
- Zhang, Y.J.; Xie, M.; Zhang, X.L.; Peng, D.L.; Yu, W.B.; Li, Q.; Li, Q.; Zhao, J.J.; Zhang, Z.R. Establishment of polyethylene-glycol-mediated protoplast transformation for Lecanicillium lecanii and development of virulence-enhanced strains against Aphis gossypii. Pest. Manag. Sci. 2016, 72, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Sezen, K.; Demirbağ, Z.; Nalcacioğlu, R. The relationship between insecticidal effects and chitinase activities of Coleopteran-originated entomopathogens and their chitinolytic profile. Ann. Microbiol. 2012, 62, 647–653. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, M.C.; Chattha, S.A.; Zhu, Y.W.; Peng, B.Y.; Ye, Y.B. Application of acidic protease in the pickling to simplify the pelt bating process. J. Leather Sci. Eng. 2021, 3, 27. [Google Scholar] [CrossRef]
- Mohamad, S.F.S.; Mohamad, S.; Aziz, A.A. The Susceptibility of Aphids, Aphis gossypii Glover to Lauric Acid based Natural Pesticide. Procedia Eng. 2013, 53, 20–28. [Google Scholar] [CrossRef]
- Yang, C.X.; Pan, H.P.; Liu, Y.; Zhou, X.G. Temperature and Development Impacts on Housekeeping Gene Expression in Cowpea Aphid, Aphis craccivora (Hemiptera: Aphidiae). PLoS ONE 2015, 10, e0130593. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kope, H.H.; Alfaro, R.I.; Lavallée, R. Effects of temperature and water activity on Lecanicillium spp. conidia germination and growth, and mycosis of Pissodes strobi. Biol. Control 2008, 53, 489–500. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, Y.P.; Xiong, Q.; Liu, W.M.; Xue, J.L. Ultrastructural Exploration on the Histopathological Change in Phenacoccus fraxinus Infected with Lecanicillium lecanii. PLoS ONE 2015, 10, e0117428. [Google Scholar] [CrossRef] [PubMed]
- Askary, H.; Benhamou, N.; Brodeur, J. Ultrastructural and cytochemical characterization of aphid invasion by the hyphomycete verticillium lecanii. J. Invertebr. Pathol. 1999, 74, 1–13. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Nemat, O.K. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Czygier, M.; Bogus, M.I. The role of enzymes produced by entomopathogenic fungi in pathogenesis of insects. Pestycydy 2000, 37–45. Available online: http://bwmeta1.element.agro-article-da03e3ed-b396-4638-943b-b791531ca00b (accessed on 2 November 2023).
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Chiu, M.C.; Lin, C.C.; Chung, Y.K.; Chou, J.Y. Efficacy of Entomopathogenic fungus Aspergillus nomius against Dolichoderus thoracicus. Biol. Control 2021, 66, 463–473. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R.; Talaei-Hassanlouei, R.; Malagoli, D. Cellular immune reactions of the sunn pest, Eurygaster integriceps, to the entomopathogenic fungus, Beauveria bassiana and its secondary metabolites. J. Insect Sci. 2011, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Zin, W.W. Toxicity and ovicidal activity of different entomopathogenic fungi, Hirsutella extracts on Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2022, 11, 133–143. [Google Scholar]
- Woo, R.M.; Park, M.G.; Choi, J.Y.; Park, D.H.; Kim, J.Y.; Wang, M.; Kim, H.J.; Woo, S.D.; Kim, J.S.; Je, Y.H. Insecticidal and insect growth regulatory activities of secondary metabolites from entomopathogenic fungi, Lecanicillium attenuatum. J. Appl. Entomol. 2020, 144, 655–663. [Google Scholar] [CrossRef]
- Prapanthadara, L.A.; Hemingway, J.; Ketterman, A.J. Partial Purification and Characterization of Glutathione S-Transferases Involved in DDT Resistance from the Mosquito Anopheles gambiae. Pestic. Biochem. Phys. 1993, 47, 119–133. [Google Scholar] [CrossRef]
- Lin, L.L.; Chen, H.L.; Xie, M.H.; Su, W.H. Research progress in insect acetylcholinesterase. J. Anhui Agric. Sci. 2014, 42, 2053–2055. [Google Scholar] [CrossRef]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.; Hafez, Y. Toxicity of essential oils nanoemulsion against Aphis craccivora and their inhibitory activity on insect enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Dolma, S.K.; Singh, P.P.; Reddy, S.G. Insecticidal and enzyme inhibition activities of leaf/bark extracts, fractions, seed oil and isolated compounds from Triadica sebifera (L.) Small against Aphis craccivora Koch. Molecules 2022, 27, 1967. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.X.; Wang, J.J.; Ding, W.; Zhao, Z.M. Inhibition kinetics on carboxylesterase and acetylcholinesterase of Liposcelis bostrychophila and Liposcelis entomophila (Psocop., Liposcelididae) of two insecticides. J. Appl. Entomol. 2004, 128, 292–297. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Li, M.X.; Fang, Y.L.; Qu, J.W.; Mao, T.T.; Chen, J.; Li, F.C.; Sun, H.N.; Li, B. Responses of detoxification enzymes in the midgut of Bombyx mori after exposure to low-dose of acetamiprid. Chemosphere 2020, 251, 126438. [Google Scholar] [CrossRef]
- Lees, G.J. Inhibition of sodium-potassium-ATPase: A potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res. Rev. 1991, 16, 283–300. [Google Scholar] [CrossRef]
- Ma, Z.Q. Studies on the Relationship between Symptoms and Mechan of Different Kinds of Insecticides; Northwest Sci-Tech University of Agriculture and Forestry: Yangling, China, 2002. [Google Scholar]
- Leng, X.F.; Xiao, D.Q. Effect of deltamethrin on protein phosphorylation of houesfly brain synaptosome. Pestic. Sci. 1995, 44, 88–89. [Google Scholar] [CrossRef]
- Lingrel, J.B. The Physiological Significance of the Cardiotonic Steroid/Ouabain-Binding Site of the Na, K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shi, X.L.; Ma, S.J.; Ma, Z.Q.; Zhang, X. Evaluation of physiological and biochemical effects of two Sophora alopecuroides alkaloids on pea aphids Acyrthosiphon pisum. Pest. Manag. Sci. 2020, 76, 4000–4008. [Google Scholar] [CrossRef]
- Yeom, H.J.; Kang, J.S.; Kim, G.H.; Park, I.K. Insecticidal and Acetylcholine Esterase Inhibition Activity of Apiaceae Plant Essential Oils and Their Constituents against Adults of German Cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Cavalcanti, S.C.H.; Niculau, E.D.; Blank, A.F.; Câmara, C.A.; Araújo, I.N.; Alves, P.B. Composition and acaricidal activity of Lippia sidoides essential oil against two-spotted spider mite (Tetranychus urticae Koch). Bioresour. Technol. 2010, 101, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Choi, W.S.; Lee, S.E.; Park, B.S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, S.E.; Annis, P.C.; Pratt, S.J.; Park, B.C.; Tumaalii, F. Fumigant Toxicity of Essential Oils and Monoterpenes against the Red Flour Beetle, Tribolium castaneum Herbst. J. Asia-Pac. Entomol. 2002, 5, 237–240. [Google Scholar] [CrossRef]
- Kulangiappar, K.; Anbukulandainathan, M.; Raju, T. Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry. Synth. Commun. 2014, 1, 2494–2502. [Google Scholar] [CrossRef]
- Fu, M.Z.; Song, X.P.; Li, G.; Li, C.A.; Wu, M.M. Clinical evaluation on killing psoroptes cuniculi with hymecromone microemulsion in rabbits. Acta Agric. Boreali. Occident. Sin. 2012, 21, 17–21. [Google Scholar]
- Zhuang, L.k.; Zhu, W. GC-MS analysis of chemical compounds in essential oil from cerbera manghas fruit. Agrochemicals 2009, 48, 504–505+514. [Google Scholar] [CrossRef]
- Abdullah, R.R. Insecticidal activity of secondary metabolites of locally isolated fungal strains against some cotton insect pests. J. Plant Prot. Pathol. 2019, 10, 647–653. [Google Scholar] [CrossRef]
- Zhou, L.J.; Huang, J.G.; Xu, H.H.; Wu, R.H. Insecticidal activities of two active components from a chinese indigenous plant, Sinacalia tangutica (Maxim.) B. Nord against Musca domestica bicina Macquart adults. Acta Entomol. Sin. 2006, 49, 74–79. [Google Scholar] [CrossRef]


| Colloidal Chitin (%) | Chitinase Activity Mean ± SE (U mL−1) | ||||
|---|---|---|---|---|---|
| 3 d | 4 d | 5 d | 6 d | 7 d | |
| 0 | 2.13 ± 0.05 Da | 2.22 ± 0.05 Cc | 2.31 ± 0.04 ABb | 2.37 ± 0.05 Ab | 2.27 ± 0.04 BCa |
| 0.5 | 2.15 ± 0.06 Ca | 2.37 ± 0.05 Ba | 2.32 ± 0.04 Bb | 2.48 ± 0.06 Aa | 2.31 ± 0.01 Ba |
| 1 | 2.17 ± 0.06 Ca | 2.32 ± 0.08 ABab | 2.41 ± 0.11 Aab | 2.25 ± 0.09 Cc | 2.24 ± 0.06 Ca |
| 2 | 2.12 ± 0.03 Ca | 2.26 ± 0.03 Bbc | 2.5 ± 0.05 Aa | 2.17 ± 0.04 Ccd | 2.14 ± 0.04 Cb |
| 4 | 2.08 ± 0.06 Ca | 2.23 ± 0.03 Bc | 2.47 ± 0.02 Aa | 2.14 ± 0.03 Cd | 2.06 ± 0.05 Cc |
| Gelatin (%) | Extracellular Protease Activity Mean ± SE (U mL−1) | ||||
|---|---|---|---|---|---|
| 3 d | 4 d | 5 d | 6 d | 7 d | |
| 0 | 0.15 ± 0.13 Bd | 0.21 ± 0.02 Be | 0.10 ± 0.10 Be | 0.12 ± 0.13 Be | 4.33 ± 0.81 Ae |
| 0.5 | 7.05 ± 0.49 Cc | 5.90 ± 0.45 Cd | 4.40 ± 0.70 Dd | 9.69 ± 0.99 Bd | 11.30 ± 0.76 Ad |
| 1 | 11.78 ± 0.94 Cb | 11.06 ± 0.66 Cc | 11.11 ± 0.17 Cc | 13.94 ± 0.45 Bc | 15.65 ± 0.17 Ac |
| 2 | 13.88 ± 2.76 Cb | 16.12 ± 0.53 BCb | 15.85 ± 0.86 BCb | 17.97 ± 0.17 ABb | 19.48 ± 0.91 Ab |
| 4 | 18.03 ± 0.23 Da | 18.52 ± 0.89 CDa | 20.46 ± 1.90 BCa | 21.48 ± 1.77 Ba | 24.72 ± 0.56 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, J.; Feng, Q.; Chu, L.; Tan, Z.; Ji, X.; Jin, P. Insecticidal Effect of the Entomopathogenic Fungus Lecanicillium araneicola HK-1 in Aphis craccivora (Hemiptera: Aphididae). Insects 2023, 14, 860. https://doi.org/10.3390/insects14110860
Liu S, Li J, Feng Q, Chu L, Tan Z, Ji X, Jin P. Insecticidal Effect of the Entomopathogenic Fungus Lecanicillium araneicola HK-1 in Aphis craccivora (Hemiptera: Aphididae). Insects. 2023; 14(11):860. https://doi.org/10.3390/insects14110860
Chicago/Turabian StyleLiu, Shengke, Jinhua Li, Qing Feng, Linglong Chu, Zhiqiong Tan, Xuncong Ji, and Pengfei Jin. 2023. "Insecticidal Effect of the Entomopathogenic Fungus Lecanicillium araneicola HK-1 in Aphis craccivora (Hemiptera: Aphididae)" Insects 14, no. 11: 860. https://doi.org/10.3390/insects14110860