Effects of Temperature and Host Plant on Hedgehog Grain Aphid, Sipha maydis Demographics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Culture
2.2. Experiment to Determine HGA Development under Different Temperatures
2.3. Supercooling Point Experiment
2.4. Comparison of the Life History of HGA and SA on Different Hosts
2.5. Statistical Analysis
3. Results
3.1. HGA Development at Different Temperatures
3.2. Supercooling Point
3.3. Life History of HGA and SA on Different Host Plants
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrales, C.E.; Castro, A.M.; Ricci, M.; Dixon, A.F.G. Sipha maydis: Distribution and Host Range of a New Aphid Pest of Winter Cereals in Argentina. J. Econ. Entomol. 2007, 100, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Puterka, G.J.; Hammon, R.W.; Franklin, M.; Mornhinweg, D.W.; Springer, T.; Armstrong, S.; Brown, M.J. Distribution of a New Invasive Species, Sipha maydis (Heteroptera: Aphididae), on Cereals and Wild Grasses in the Southern Plains and Rocky Mountain States. J. Econ. Entomol. 2019, 112, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Lampert, S.; Salvadori, J.R.; Lau, D.; Pereira, P.R.V.D.S.; Engel, E.; Savaris, M. Sipha maydis (Hemiptera: Aphididae) in the Humid Subtropical Region of Brazil: Distribution, Seasonality and Biology. Florida Entomol. 2023, 106, 1–9. [Google Scholar] [CrossRef]
- Hammon, B. Sipha maydis: A Potential Threat to Colorado Wheat Production; Tri River Area Extension: Gdand Junction, CO, USA, 2015; p. 1838. Available online: http//wci.colostate.edu/Assets/pdf/Sipha.maydis.pdf (accessed on 29 October 2018).
- Jankielsohn, A.; Prinsloo, G. Sipha maydis (Passerini) (Hemiptera: Aphididae) on Wheat (Triticum aestivum) in South Africa. African Entomol. 2020, 28, 192–194. [Google Scholar] [CrossRef]
- Mahmood, R.; Poswal, M.A.; Shehzad, A. Distribution, Host Range and Seasonal Abundance of Sipha sp. (Homoptera: Aphididae) and Their Natural Enemies in Pakistan. Pakistan J. Biol. Sci. 2001, 5, 47–50. [Google Scholar] [CrossRef]
- Sorensen, J.T.; Center, P.P.D. Sipha maydis Passerini: A New Grass/Cereal Aphid in North America; Plant Pest Diagnostic Center, California Department of Food and Agriculture: Sacramento, CA, USA, 2007. [Google Scholar]
- Wieczorek, K.; Bugaj-Nawrocka, A. Invasive Aphids of the Tribe Siphini: A Model of Potentially Suitable Ecological Niches. Agric. For. Entomol. 2014, 16, 434–443. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd.: London, UK, 2000; ISBN 0471851914. [Google Scholar]
- Yamani, M. El Identification and Importance of Barley Yellow Dwarf Virus in Morocco. Plant Dis. 1990, 74, 291. [Google Scholar] [CrossRef]
- Van Munster, M. Impact of Abiotic Stresses on Plant Virus Transmission by Aphids. Viruses 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, S.; Lognay, G.; Verheggen, F.; Detrain, C. Today and Tomorrow: Impact of Climate Change on Aphid Biology and Potential Consequences on Their Mutualism with Ants. Physiol. Entomol. 2019, 44, 77–86. [Google Scholar] [CrossRef]
- Dampc, J.; Mołoń, M.; Durak, T.; Durak, R. Changes in Aphid—Plant Interactions under Increased Temperature. Biology 2021, 10, 480. [Google Scholar] [CrossRef]
- Sun, J.; Tan, X.; Li, Q.; Francis, F.; Chen, J. Effects of Different Temperatures on the Development and Reproduction of Sitobion miscanthi from Six Different Regions in China. Front. Ecol. Evol. 2022, 10, 794495. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Sun, J.; Shi, W.; Harwood, J.D.; Monticelli, L.S.; Tan, X.; Chen, J. Effects of Field Simulated Warming on Feeding Behavior of Sitobion avenae (Fabricius) and Host Defense Systems. Entomol. Gen. 2021, 41, 567–578. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Lee, R.E.; Peairs, F.B. Application of Ice-Nucleating Active Bacteria Decreases the Supercooling Capacity of the Russian Wheat Aphid (Homoptera: Aphididae). In Response Model for an Introduced Pest: The Russian Wheat Aphid; Entomological Society of America: Baltimore, MD, USA, 1998; pp. 248–257. [Google Scholar]
- van Emden, H.F.; Harrington, R. Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wellingborough, UK, 2017; ISBN 9781780647098. [Google Scholar]
- Lee, R.E. A Primer on Insect Cold-Tolerance. In Low Temperature Biology of Insects; Cambridge University Press: Cambridge, MA, USA, 2010; pp. 3–34. ISBN 9780511675997. [Google Scholar]
- Moghadam, S.G.; Hosseini, M.; Awal, M.M. Does Leaf Pubescence of Wheat Affect Host Selection and Life Table Parameters of Sipha maydis (Hemiptera: Aphididae)? J. Crop Prot. 2013, 2, 81–92. [Google Scholar]
- Saldúa, V.; Castro, A. Expresión de La Antibiosis y de La Antixenosis Contra El Pulgón Negro de Los Cereales (Sipha maydis) En Cultivares Comerciales de Trigo. Rev. Fac. Agron. 2011, 110, 1–11. [Google Scholar]
- Mornhinweg, D.W.; Carver, B.F.; Springer, T. Registration of ‘USDA Fortress’ Winter Feed Barley with Multiple Aphid Resistance. J. Plant Regist. 2022, 16, 495–503. [Google Scholar] [CrossRef]
- Mornhinweg, D.W.; Puterka, G.J.; Armstrong, J.S. Resistance in Barley (Hordeum vulgare L.) to New Invasive Aphid, Hedgehog Grain Aphid (Sipha maydis, Passerini) (Hemiptera: Aphididae). Am. J. Plant Sci. 2020, 11, 869–879. [Google Scholar] [CrossRef]
- Paudyal, S.; Armstrong, J.S.; Giles, K.L.; Payton, M.E.; Opit, G.P.; Limaje, A. Categories of Resistance to Sugarcane Aphid (Hemiptera: Aphididae) among Sorghum Genotypes. J. Econ. Entomol. 2019, 112, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.; Armstrong, J.S.; Hayes, C.; Hoback, W.W.; Zarrabi, A. Evaluation of A3 Cytoplasmic Male Sterile Forage Sorghum Lines for Resistance to Sugarcane Aphid. Planta 2022, 255, 38. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, I.J.; White, P.F. Simple Estimation of Intrinsic Increase Rates for Aphids and Tetranychid Mites. J. Appl. Ecol. 1977, 14, 757. [Google Scholar] [CrossRef]
- Asin, L.; Pons, X. Effect of High Temperature on the Growth and Reproduction of Corn Aphids (Homoptera: Aphididae) and Implications for Their Population Dynamics on the Northeastern Iberian Peninsula. Environ. Entomol. 2001, 30, 1127–1134. [Google Scholar] [CrossRef]
- Jafari, M.; Goldasteh, S.; Aghdam, H.R.; Zamani, A.A.; Soleyman-nejadian, E.; Schausberger, P. Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons. Insects 2023, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature Requirements of Some Aphids and Their Parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Shimizu, G.D.; Marubayashi, R.Y.P.; Goncalves, L.S.A. Experimental Statistics and Graphics for Agricultural Sciences. Available online: https://agronomiar.github.io/AgroR_package/index.html (accessed on 16 June 2022).
- Wickham, H. Programming with Ggplot2. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 241–253. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Burr, I.W.; Foster, L.A. A Test for Equality of Variances; University of Purdue: West Lafayette, IN, USA, 1972. [Google Scholar]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Tazerouni, Z.; Talebi, A.A. Temperature-Dependent Life History of Sipha maydis (Hemiptera: Aphididae) on Wheat. J. Plant Prot. Res. 2014, 54, 374–382. [Google Scholar] [CrossRef]
- Renault, D.; Salin, C.; Vannier, G.; Vernon, P. Survival at Low Temperatures in Insects: What Is the Ecological Significance of the Supercooling Point? Cryo-Letters 2002, 23, 217–228. [Google Scholar]
- Elliott, N.C.; Kieckhefer, R.W.; Walgenbach, D.D. Effects of Constant and Fluctuating Temperatures on Developmental Rates and Demographic Statistics for the Corn Leaf Aphid (Homoptera: Aphididae). J. Econ. Entomol. 1988, 81, 1383–1389. [Google Scholar] [CrossRef]
- Ricci, M.; Kahan, A.E. Aspectos Biológicos y Poblacionales de Sipha maydis (Passerini) y Schizaphis graminum (Rondani) En Cebada Biological and Populational Aspect of Sipha maydis (Passerini) y Schizaphis graminum (Rondani) on Barley. Rev. Fac. Cienc. Agrar. 2005, 37, 25–32. [Google Scholar]
- Chown, S.L.; Chown, S.L.; Nicolson, S.W. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: London, UK, 2004; Volume 42, ISBN 0198515499. [Google Scholar]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in Fluctuating Thermal Environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Bale, J.S. Insect Cold Hardiness: Freezing and Supercooling—An Ecophysiological Perspective. J. Insect Physiol. 1987, 33, 899–908. [Google Scholar] [CrossRef]
- Vrba, P.; Sucháčková Bartoňová, A.; Andres, M.; Nedvěd, O.; Šimek, P.; Konvička, M. Exploring Cold Hardiness within a Butterfly Clade: Supercooling Ability and Polyol Profiles in European Satyrinae. Insects 2022, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Nielsen, D.C. Supercooling Points for Russian Wheat Aphid and Winter Wheat Tissue as Indicators of Cold Acclimation. Southwest. Entomol. 2000, 25, 265–272. [Google Scholar]
- Way, M.J. Mutualism between Ants and Honeydew Producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Halbert, S.E.; Miller, G.L.; Ames, L.M. The Genus Sipha Passerini (Hemiptera: Aphididae) in North America. Insecta Mundi 2013, 0326, 1–6. [Google Scholar]
- Hentz, M.; Nuessly, G. Development, Longevity, and Fecundity of Sipha flava (Homoptera: Aphididae) Feeding on Sorghum bicolor. Environ. Entomol. 2004, 33, 546–553. [Google Scholar] [CrossRef]
- Wieczorek, K. A Monograph of Siphini Mordvilko, 1928 (Hemiptera, Aphidoidea: Chaitophorinae); Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2010. [Google Scholar]
- Royer, T.A.; Giles, K.; Elliott, N.C.; Zarrabi, A.A. Small Grain Aphids in Oklahoma and Their Management; Oklahoma Cooperative Extension Service: Katowice, Poland, 2020. [Google Scholar]
- Barrufaldi, A.P.F.; Hayashida, R.; Hoback, W.W.; Higley, L.G.; De Carvalho, J.R.; Oliveira, R.C. De Trade-Offs between Temperature and Fitness in Euschistus heros (Fabricius) (Hemiptera: Pentatomidae): Implications for Mass Rearing and Field Management. Insects 2023, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.A.D.M.A.; Armstrong, J.S.; Hoback, W.W.; Mulder, P.G.; Paudyal, S.; Foster, J.E.; Mark, E. Temperature Dependent Development of Sugarcane Aphids Melanaphis sacchari, (Hemiptera: Aphididae) on Three Different Host Plants with Estimates of the Lower and Upper Threshold for Fecundity. Curr. Trends Entomol. Zool. Stud. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Sarkar, N.; Murray, M.J.; Stout, M.J.; Davis, J.A. Impact of Host Plant Resistance on Emergence, Body Parameters, and Supercooling Point of Cylas formicarius elegantulus (Coleoptera: Brentidae). Florida Entomol. 2022, 105, 65–70. [Google Scholar] [CrossRef]

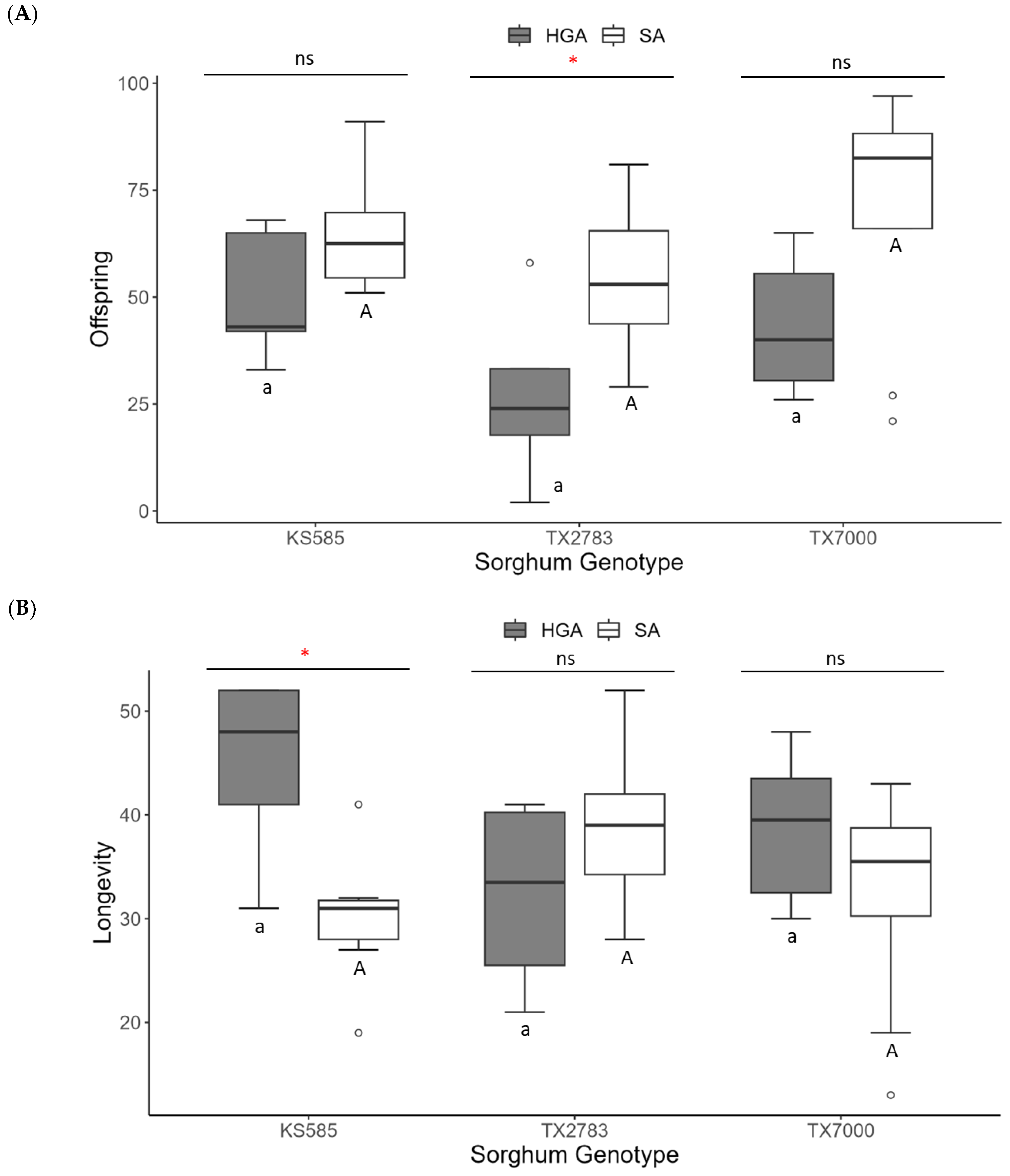
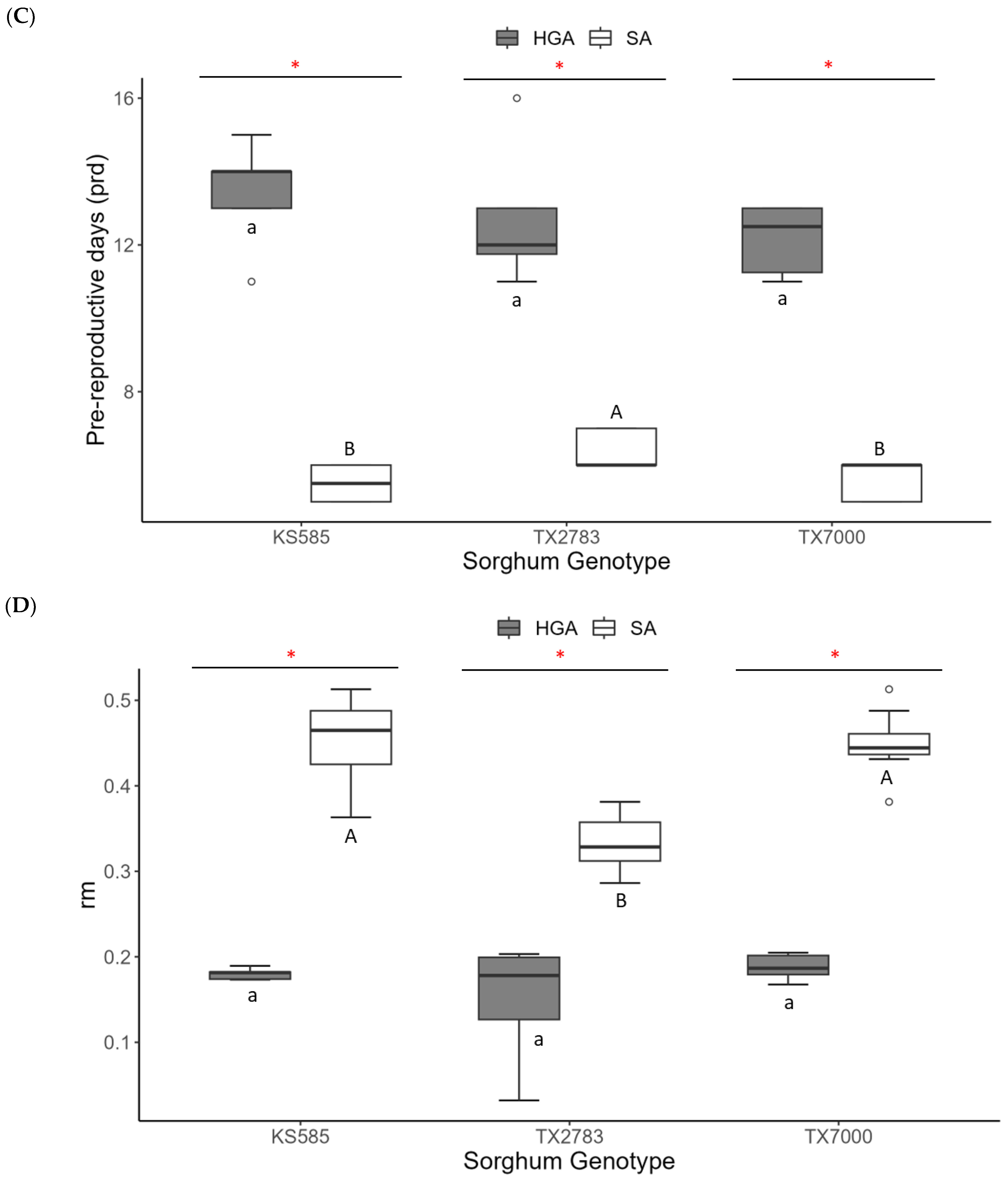
| Temperature (°C) | Offspring | Longevity (Days) | Pre-Reproductive Period (d) 2 | Number of Progenies Produce in d (Md) | Intrinsic Rate of Natural Increase (rm) |
|---|---|---|---|---|---|
| 10 | - | - | - | - | - |
| 15 | 31.46 ± 3.60 b | 63.91 ± 4.71 a | 28.18 ± 1.15 a | 26.09 ± 2.42 a | 0.085 ± 0.005 c |
| 20 | 65.67 ± 4.30 a | 57.58 ± 3.14 a | 14.08 ± 0.43 b | 32.67 ± 2.63 a | 0.183 ± 0.009 b |
| 25 | 63.58 ± 7.39 a | 39.83 ± 3.76 b | 10.50 ± 0.23 c | 33.00 ± 2.83 a | 0.245 ± 0.011 a |
| 30 | 11.00 ± 1.89 c | 18.50 ± 1.70 c | 10.50 ± 0.22 c | 10.20 ± 1.57 b | 0.153 ± 0.018 b |
| 35 | - | - | - | - | - |
| F | 27.40 | 30.59 | 175.64 | 17.45 | 35.72 |
| DFnumdf;demdf | 3; 41 | 3; 41 | 3; 41 | 3; 41 | 3; 41 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Host Plant | Offspring | Longevity (d) | Pre-Reproductive Period (d) | Intrinsic Rate of Natural Increase (rm) |
|---|---|---|---|---|
| TX7000 | 37.00 ± 8.32 | 38.67 ± 2.96 | 12.17 ± 0.40 | 0.19 ± 0.01 |
| TX2783 | 27.00 ± 11.57 | 32.25 ± 4.92 | 12.75 ± 1.11 | 0.15 ± 0.04 |
| KS585 | 50.20 ± 6.90 | 44.80 ± 3.99 | 13.40 ± 0.68 | 0.18 ± 0.00 |
| Custer | 50.71 ± 9.01 | 40.14 ± 3.81 | 11.00 ± 0.58 | 0.21 ± 0.02 |
| Millex32 | 21.71 ± 7.44 | 37.20 ± 6.01 | 11.60 ± 0.51 | 0.17 ± 0.00 |
| F | 1.62 | 0.94 | 2.28 | 1.91 |
| DFnumdf;demdf | 4; 22 | 4; 22 | 4; 22 | 4; 22 |
| p | 0.20 | 0.46 | 0.09 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, M.; Hayashida, R.; Hoback, W.W.; Armstrong, J.S. Effects of Temperature and Host Plant on Hedgehog Grain Aphid, Sipha maydis Demographics. Insects 2023, 14, 862. https://doi.org/10.3390/insects14110862
Taylor M, Hayashida R, Hoback WW, Armstrong JS. Effects of Temperature and Host Plant on Hedgehog Grain Aphid, Sipha maydis Demographics. Insects. 2023; 14(11):862. https://doi.org/10.3390/insects14110862
Chicago/Turabian StyleTaylor, Mason, Rafael Hayashida, William Wyatt Hoback, and John Scott Armstrong. 2023. "Effects of Temperature and Host Plant on Hedgehog Grain Aphid, Sipha maydis Demographics" Insects 14, no. 11: 862. https://doi.org/10.3390/insects14110862






