Accumulation of Fungal Pathogens Infecting the Invasive Spotted Lanternfly, Lycorma delicatula
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Fungal Isolation and Identification
2.3. Testing Pathogenicity
2.4. Photographing Fungal Forms
2.5. Analyses
3. Results
3.1. Pathogen Biodiversity
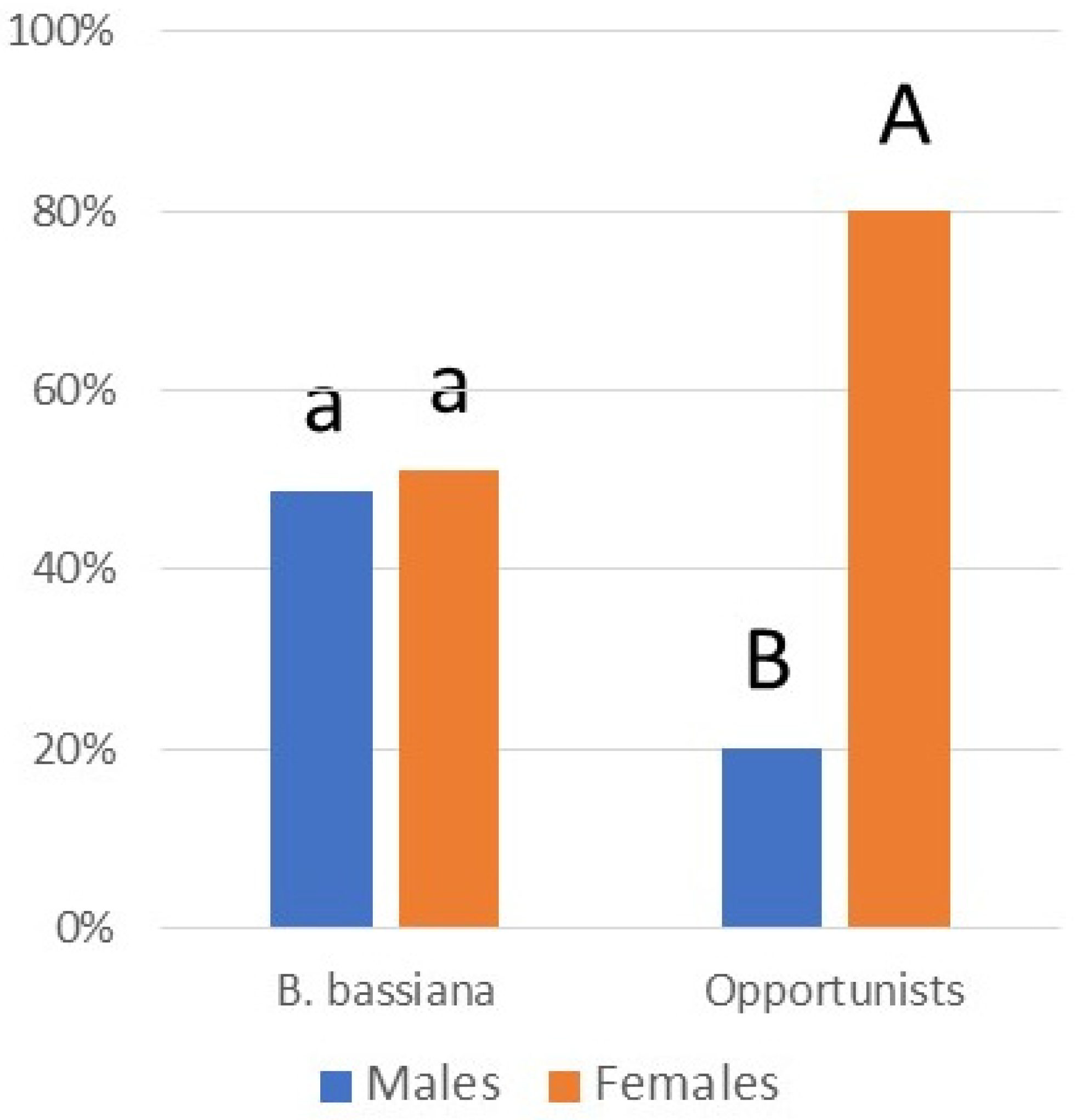
3.2. Pathogen Prevalence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Blumenthal, D.; Jarosik, V.; Puckett, E.E.; Pyšek, P. Controls on pathogen species richness in plants’ introduced and native ranges: Roles of residence time, range size and host traits. Ecol. Lett. 2010, 13, 1525–1535. [Google Scholar] [CrossRef]
- Phillips, B.L.; Kelehear, C.; Pizzatto, L.; Brown, G.P.; Baarton, D.; Shine, R. Parasites and pathogens lag behind their host during periods of host range advance. Ecology 2010, 91, 872–881. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Pathogen accumulation and long-term dynamics of plant invasions. J. Ecol. 2013, 101, 607–613. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Benovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Comm. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Dara, S.K.; Montalva, C.; Barta, M. Microbial control of invasive forest pests with entomopathogenic fungi: A review of the current situation. Insects 2019, 10, 341. [Google Scholar] [CrossRef]
- Araújo, J.P.M.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Gen. 2016, 94, 1–39. [Google Scholar]
- Sacco, N.E.; Hajek, A.E. Diversity and breadth of host specificity among arthropod pathogens in the Entomophthoromycotina. Microorganisms 2023, 11, 1658. [Google Scholar] [CrossRef]
- Domingues, M.M.; dos Santos, P.L.; Costa Gea, B.C.; de Carvalho, V.R.; de Oliveira, F.N.; Solian, E.P.; Serrao, J.E.; Zanuncio, J.C.; Zanetti, R.; Wilcken, C.F. Entomopathogenic fungi, isolated from soils and Bemisia tabaci (Hemiptera: Aleyrodidae) adults, to manage the Eucalyptus red gum lerp psyllid Glycaspis brimblecombei (Hemiptera: Aphalaridae). J. Econ. Entomol. 2022, 115, 1886–1893. [Google Scholar] [CrossRef]
- Biryol, S.; Araz, N.; Eski, A.; Akturk, R.; Aksu, Y.; Gokturk, B.C.; Bilgin, L.; Demir, I. Biodiversity and pathogenicity of entomopathogenic fungi associated with the lesser spruce sawfly, Pristophora abietina. Entomol. Exp. Appl. 2021, 169, 414–423. [Google Scholar] [CrossRef]
- Kovač, M.; Gorczak, M.; Wrzosek, M.; Tkaczuk, C.; Pernek, M. Identification of entomopathogenic fungi as naturally occurring enemies of the invasive oak lace bug, Corythuca arcuata (Say) (Hemiptera: Tingidae). Insects 2020, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Corallo, B.; Simeto, S.; Martinez, G.; Gomez, D.; Abreo, E.; Altier, N.; Lupo, S. Entomopathogenic fungi naturally infecting the eucalypt bronze bug, Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae), in Uruguay. J. Appl. Entomol. 2019, 143, 542–555. [Google Scholar] [CrossRef]
- Sharma, L.; Gonçalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontr. Sci. Technol. 2018, 28, 122–141. [Google Scholar] [CrossRef]
- Dao, H.T.; Beattie, G.A.C.; Rossman, A.Y.; Burgess, L.W.; Holford, P. Four putative entomopathogenic fungi of armoured scale insects on Citrus in Australia. Mycol. Progr. 2016, 15, 47. [Google Scholar] [CrossRef]
- Gouli, V.; Gouli, S.; Marcelino, J.A.P.; Skinner, M.; Parker, B.L. Entomopathogenic fungi associated with exotic invasive insect pests in northeastern forests of the USA. Insects 2013, 4, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.R.; Parker, B.L.; Gouli, S.Y.; Skinner, M.; Gouli, V.V.; Teillon, H.B. Fungi associated with the hemlock woolly adelgid, Adelges tsugae, and assessment of entomopathogenic isolates for management. J. Insect Sci. 2010, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Tanyeli, E.; Sevin, A.; Demirbag, Z.; Eroglu, M.; Demir, I. Isolation and virulence of entomopathogenic fungi against the great spruce bark beetle, Dendroctonus micans (Kugelann) (Coleoptera: Scolytidae). Biocontr. Sci. Technol. 2010, 20, 695–701. [Google Scholar] [CrossRef]
- Venugopal Rao, N.; Reddy, A.S.; Tirumala Rao, K. Natural enemies of cotton whitefly, Bemisia tabaci Gennadius in relation to host population and weather factors. J. Biol. Control 1989, 3, 10–12. [Google Scholar]
- Kaya, H.K.; Vega, F.E. Scope and basic principles of insect pathology. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 1–12. [Google Scholar]
- NYS IPM (New York State Integrated Pest Management Program). Interactive Spotted Lanternfly Map. Available online: http://Go.nysipm.org/slf-map-i (accessed on 26 October 2023).
- Urban, J.M.; Leach, H. Biology and management of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar] [CrossRef]
- Soper, R.S. Pathogens of leafhoppers and planthoppers. In The Leafhoppers and Planthoppers; Nault, L.R., Rodriguez, J.G., Eds.; John Wiley & Sons: New York, NY, USA, 1985; pp. 469–488. [Google Scholar]
- Li, M.-Y.; Lin, H.-F.; Li, S.-G.; Xu, A.; Feng, M.-F. Efficiency of entomopathogenic fungi in the control of eggs of the brown planthopper Nilaparvata lugens Stål (Homoptera: Delphacidae). Afr. J. Microbiol. Res. 2012, 6, 7162–7167. [Google Scholar]
- Clifton, E.H.; Castrillo, L.A.; Gryganskyi, A.; Hajek, A.E. A pair of native fungal pathogens drives decline of a new invasive herbivore. Proc. Natl. Acad. Sci. USA 2019, 116, 9178–9180. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Clifton, E.H.; Stefanik, S.E.; Harris, D.C. Batkoa major infecting the invasive planthopper Lycorma delicatula. J. Invertebr. Pathol. 2022, 194, 107821. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A. The split personality of Beauveria bassiana: Understanding the molecular basis of fungal parasitism and mutualism. mSystems 2021, 6, e00766-21. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Castrillo, L.A.; Hajek, A.E. Discovery of two hypocrealean fungi infecting spotted lanternflies, Lycorma delicatula: Metarhizium pemphigi and a novel species, Ophiocordyceps delicatula. J. Invertebr. Pathol. 2021, 186, 107689. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Hajek, A.E. Efficacy of Beauveria bassiana and Cordyceps javanica mycoinsecticides against spotted lanternflies, Lycorma delicatula, in laboratory bioassays. Biocontr. Sci. Technol. 2021, 32, 824–836. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.S.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef]
- Sung, G.-H.; Sung, J.-M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymer[a]se II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.K.; Steinberg, C.; Persson, P.; Friberg, H. Genus-specific primers for study of Fusarium communities in field samples. Appl. Environ. Microbiol. 2016, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Korabečná, M.; Liška, V.; Fajfrlík, K. Primers ITS1, ITS2 and ITS4 detect the intraspecies variability in the internal transcribed spacers and 5.8S rRNA gene region in clinical isolates of fungi. Folia Microbiol. 2003, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lutzoni, F. Lutzoni Lab Evolution, Ecology, and Genomics of Fungal Symbioses: Primer Sequences. 2014. Available online: https://lutzonilab.org/primer-sequences/ (accessed on 3 September 2023).
- Matheny, P.B.; Liu, Y.J.; Ammirati, J.F.; Hall, B.D. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Amer. J. Bot. 2002, 89, 688–698. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Saidi, A.; Mebdoua, S.; Mecelem, D.; Al-Hoshani, N.; Sadrati, N.; Boufahja, F.; Bendif, H. Dual biocontrol potential of the entomopathogenic fungus Akanthomyces muscarius against Thaumetopoea pityocampa and plant pathogenic fungi. Saudi J. Biol. Sci. 2023, 30, 103719. [Google Scholar] [CrossRef]
- Broumandnia, F.; Rajabpour, A. Morphological and molecular identification of four isolates of the entomopathogenic fungal genus Akanthomyces and their effects against Bemisia tabaci on cucumber. Bull. Entomol. Res. 2021, 111, 628–636. [Google Scholar] [CrossRef]
- Clifton, E.H.; Castrillo, L.A.; Jaronski, S.T.; Hajek, A.E. Cryptic diversity and virulence of Beauveria bassiana recovered from Lycorma delicatula (spotted lanternfly) in eastern Pennsylvania. Front. Insect Sci. 2023, 3, 1127682. [Google Scholar] [CrossRef]
- Hajek, A.E.; Meyling, N.V. Ecology of invertebrate pathogens: Fungi. In Ecology of Invertebrate Diseases; Hajek, A.E., Shapiro-Ilan, D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 327–377. [Google Scholar]
- Montes-Bazurto, L.G.; Bustillo-Pardey, A.E.; Medina-Cárdenas, H.C. Cordyceps cateniannulata, a novel entomopathogenic fugus to control Stenoma impresella Busck (Lepidoptera: Elachistidae) in Colombia. J. Appl. Entomol. 2020, 144, 788–796. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Wang, Y.; Fan, Q.; Duan, D.-E.; Zhang, G.-D.; Dai, R.-Q.; Dai, Y.-D.; Zeng, W.-B.; Chen, Z.-H.; Li, D.-D.; et al. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Div. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Rodrigues, J.; Rocha, L.F.N.; Martinez, J.M.; Montalva, C.; Humber, R.A. Clonostachys spp., natural mosquito antagonists, and their prospects for biological control of Aedes aegypti. Parasitol. Res. 2022, 121, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Q.; Fan, J.; Huang, J.; Wu, Z.; Lin, J.; Bin, S.; Shu, B. Effects of the entomopathogenic fungus Clonostachys rosea on mortality rates and gene expression profiles in Diaphorina citri adults. J. Invertebr. Pathol. 2021, 179, 107539. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Li, S.-D.; Ren, Q.; Xu, J.-L.; Lu, X.; Sun, L.-H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef]
- Batta, Y.A. The first report on entomopathogenic effect of Fusarium avenaceum (Fries) Saccardo (Hypocreales, Ascomycota) against rice weevil (Sitophilus oryzae L.: Curculionidae, Coleoptera). J. Entomol. Acarol. Res. 2012, 44, e11. [Google Scholar] [CrossRef]
- Rajagopal, K.; Suryanarayanan, T.S. Isolation of endophytic fungi from leaves of neem (Azadirachta indica A. Juss.). Curr. Sci. 2000, 78, 1375–1378. [Google Scholar]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Internatl. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Qiu, H.-L.; Fox, E.G.P.; Qin, C.-S.; Yang, H.; Tian, L.-Y.; Wang, D.-S.; Xu, J.-Z. First record of Fusarium concentricum (Hypocreales: Hypocreaceae) isolated from the moth Polychrosis cunninhamiacola (Lepidoptera: Tortricidae) as an entomopathogenic fungus. J. Insect Sci. 2023, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Marques, G. Fusarium, an entomopathogen—A myth or reality? Pathogens 2018, 7, 93. [Google Scholar] [CrossRef]
- Dewing, C.; Van der Nest, M.A.; Santana, Q.C.; Proctor, R.H.; Wingfield, B.D.; Steenkamp, E.T.; De Vos, L. Characterization of host-specific genes from pine- and grass-associated species of the Fusarium fujikuroi species complex. Pathogens 2022, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.K.M. Screening of Fusarium isolates pathogenicity in vitro by using the larvae of Galleria mellonella L. J. Basrah Res. Sci. 2012, 38, 19–28. [Google Scholar]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses. New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Moustafa, A.H.; Hussein, H.A.; El-Sheikh, A.A.; El-Shafrey, S.N.; Fathy, N.A.M.; Enan, G.A. Potential insecticidal activity of Sarocladium strictum, an endophyte of Cynanchum acutum, against Spodoptera littoralis, a polyphagous insect pest. Biocat. Agric. Biotechnol. 2020, 24, 101524. [Google Scholar] [CrossRef]
- Blaszczyk, L.; Waskiewicz, A.; Gromadzka, K.; Mikolajczak, K.; Chelkowski, J. Sarocladium and Lecanicillium associated with maize seeds and their potential to form selected secondary metabolites. Biomolecules 2021, 11, 98. [Google Scholar] [CrossRef]
- Tagne, A.; Neergaard, E.; Hansen, H. Studies of host-pathogen interaction between maize and Acremonium strictum from Cameroon. Eur. J. Plant Pathol. 2002, 108, 93–102. [Google Scholar] [CrossRef]
- Racedo, J.; Salazar, S.M.; Castagnaro, A.P.; Díaz Ricci, J.C. A strawberry disease caused by Acremonium strictum. Eur. J. Plant Pathol. 2013, 137, 649–654. [Google Scholar] [CrossRef]
- Kim, J.-C.; Choi, G.J.; Kim, H.-J.; Kim, H.T.; Ahn, J.W.; Cho, K.Y. Verlamelin, an antifungal compound produced by a mycoparasite, Acremonium strictum. Plant Pathol. J. 2002, 18, 102–105. [Google Scholar] [CrossRef]
- Gonzalez, J.B.; Lambert, C.A.; Foley, A.M.; Hajek, A.E. First report of Colletotrichum fioriniae infections in brown marmorated stink bugs, Halyomorpha halys. J. Invertebr. Pathol. 2023, 199, 107939. [Google Scholar] [CrossRef]
- Marcelino, J.A.P.; Gouli, S.; Giordano, R.; Gouli, V.V.; Parker, B.L.; Skinner, M. Fungi associated with a natural epizootic in Fiorinia externa Ferris (Hemiptera: Diaspididae) populations. J. Appl. Entomol. 2009, 133, 82–89. [Google Scholar] [CrossRef]
- Marcelino, J.A.P.; Gouli, S.; Parker, B.L.; Skinner, M.; Schwarzberg, L.; Giordano, R. Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. J. Insect Sci. 2009, 9, 25. [Google Scholar] [CrossRef]
- Batta, Y. Entomopathogenic effect of Trichothecium roseum (Pers.) Link (Hypocreales: Ascomycota) against Pauropsylla buxtoni (Psylloidea: Hemiptera) infesting Ficus carica leaves and its potential use as biocontrol agent of the insect. J. Appl. Microbiol. 2020, 129, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Karbowy-Thongbai, B. First detection of Trichothecium roseum causing leaf spots on tomato in Germany. Plant Dis. 2023, 107, 1233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Duan, X.; Cai, P.; Zhang, W.; Liu, Y.; Cui, J.; Li, Z.; Qiu, Z. Biocontrol action of Trichothecium roseum against the wheat powdery mildew fungus Blumeria graminis f. sp. tritici. Front. Sustain. Food Syst. 2022, 6, 998830. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Cooperband, M.F.; Murman, K. Responses of adult spotted lanternflies to artificial aggregations composed of all males or females. Front. Insect Sci. 2022, 2, 981832. [Google Scholar] [CrossRef]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 151–187. [Google Scholar]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Mgmt. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef]
- Bartlett, C.R.; O’Brien, L.B.; Wilson, W.W. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Mem. Amer. Entomol. Soc. 2014, 50, 39–69. [Google Scholar]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef]
- NRCC (Northeast Regional Climate Center). NOWData (NOAA Online Weather Data). Available online: https://www.nrcc.cornell.edu/wxstation/nowdata.html (accessed on 15 September 2023).
- Chandler, D. Basic and applied research on entomopathogenic fungi. In Microbial Control of Insect and Mite Pests; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 69–89. [Google Scholar]
- Santos, A.C.S.; Diniz, A.G.; Tiago, P.V.; Oliveira, N.T. Entompathogenic Fusarium species: A review of their potential for biological control of insects, implications and prospects. Fungal Biol. Rev. 2020, 34, 41–57. [Google Scholar] [CrossRef]
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Teetor-Barsch, G.H.; Roberts, D.W. Entomogenous Fusarium species. Mycopathologia 1983, 84, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-ard, J. Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Ariyawansa, H.A.; Becker, J.O.; Yang, J.-I. The evaluation of egg-parasitic fungi Paraboeremia taiwanensis and Samsoniella sp. for the biological control of Meloidogyne enterolobii on Chinese cabbage. Microorganisms 2020, 8, 828. [Google Scholar] [CrossRef]
- Islam, T.; Gupta, D.R.; Surovy, M.Z.; Mahmuc, N.U.; Mazlan, N.; Islam, T. Identification and application of a fungal biocontrol agent Cladosporium cladosporioides against Bemisia tabaci. Biotech. Biotechnol. Equip. 2019, 33, 1698–1705. [Google Scholar] [CrossRef]
- Liu, W.; Yu, S.-H.; Zhang, H.-P.; Fu, Z.-Y.; An, J.-Q.; Zhang, J.-Y.; Yang, P. Two Cladosporium fungi with opposite functions to the Chinese white wax scale insect have different genome characters. J. Fungi 2022, 8, 286. [Google Scholar] [CrossRef]
- Zhu, G.; Ding, W.; Xue, M.; Zhao, Y.; Li, M.; Li, Z. Identification and pathogenicity of a new entomopathogenic fungus, Mucor hiemalis (Mucorales: Mucorales), on the root maggot, Bradysia odoriphaga (Diptera: Sciaridae). J. Insect Sci. 2022, 22, 2. [Google Scholar] [CrossRef]

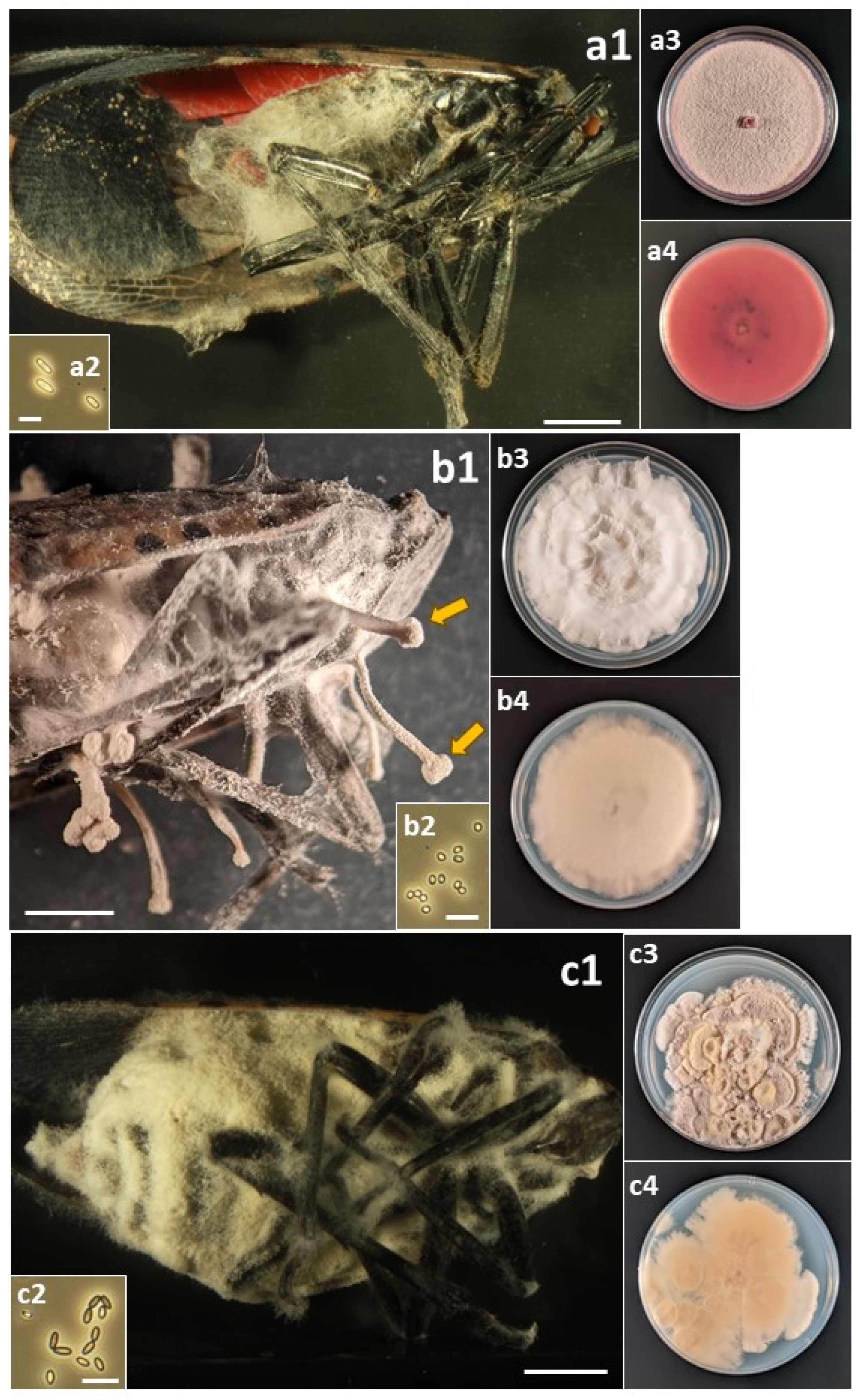
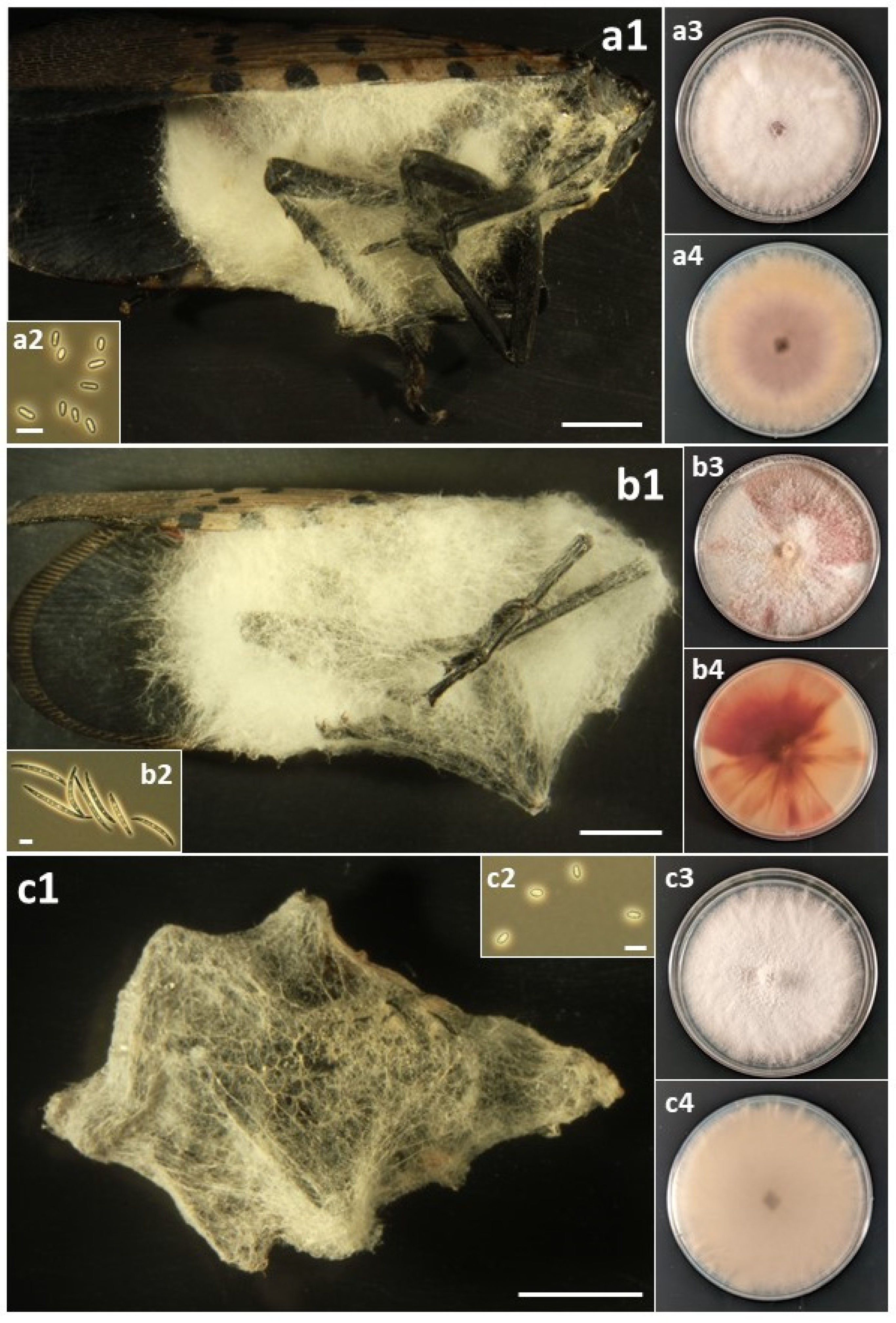
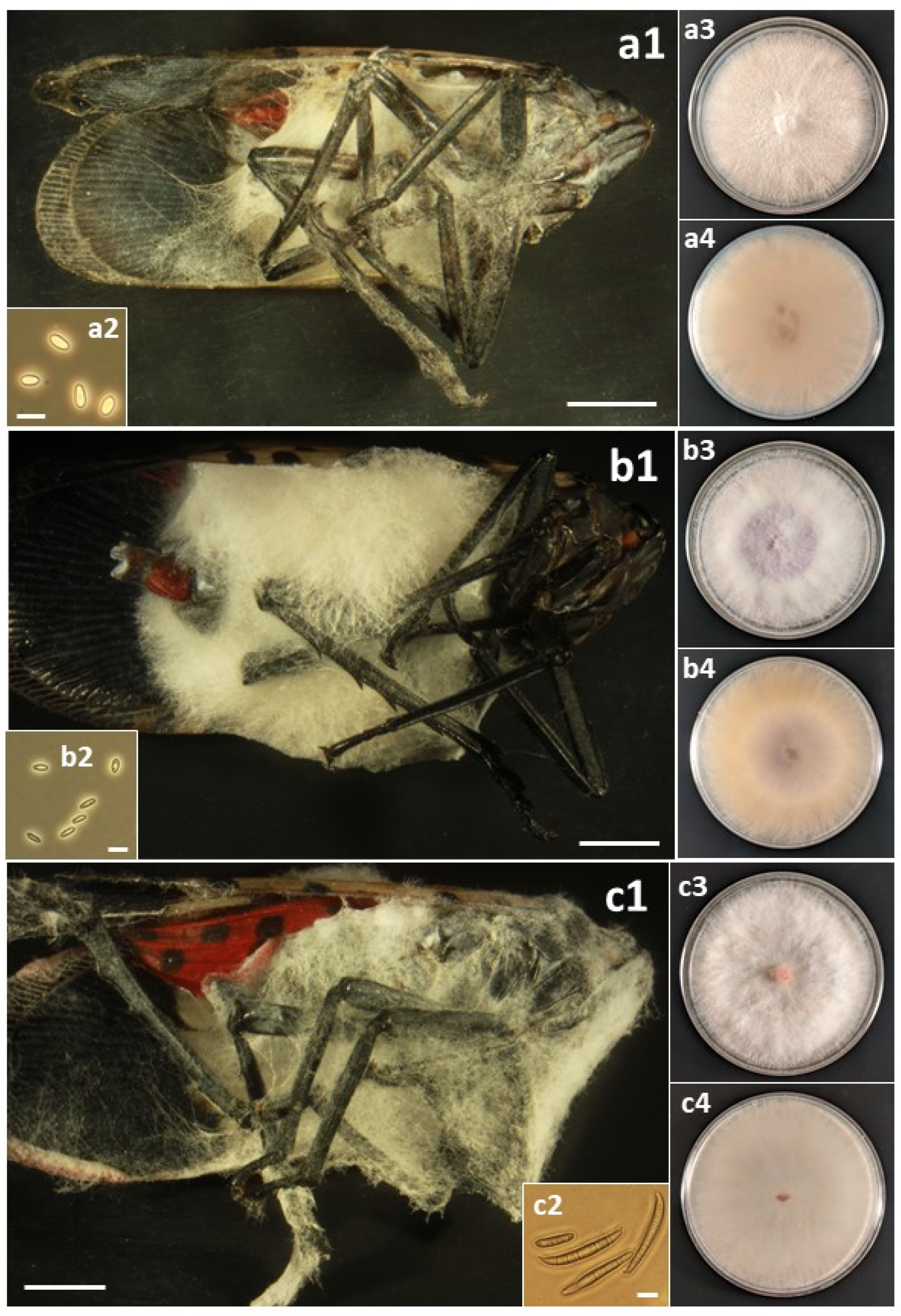


| Site | County | State | GPS | Year(s) Sampled |
|---|---|---|---|---|
| Angora Fruit Farm, Lower Alsace Twp. | Berks | Pennsylvania | 40.35846 N, 75.88323 W | 2021, 2022, 2023 |
| Penn Ave., Sinking Spring, Lower Heidelberg Twp. | Berks | Pennsylvania | 40.32700 N, 76.04683 W | 2022, 2023 |
| Glen Run Nature Preserve, Stroud Twp. | Monroe | Pennsylvania | 40.96950 N, 75.19004 W | 2021, 2022, 2023 |
| Minisink Park, Delaware Water Gap | Monroe | Pennsylvania | 40.98622 N, 75.13695 W | 2022 |
| Cherry Valley Rd., Stroud Twp. | Monroe | Pennsylvania | 40.97055 N, 75.17941 W | 2022 |
| Boys & Girls Club, Main St., Owego | Tioga | New York | 42.11008 N, 76.25717 W | 2023 |
| Locus | Primer | Primer Sequence | Reference |
|---|---|---|---|
| ITS | ITS1 | TCCGTAGGTGAACCTGCGG | White et al., 1990 [29] |
| ITS1 mod | TCCGTAGGTGAACCTTGCGG | Korabečná et al., 2003 [38] | |
| ITS4 | TCCTCCGCTTATTGATATGC | White et al., 1990 [29] | |
| LSU | LR5 | ATCCTGAGGGAAACTTC | Vilgalys and Hester, 1990 [30] |
| LR0R 1 | ACCCGCTGAACTTAAGC | Lutzoni, 2014 [39] | |
| RPB1 | CRPB1 | CCWGGYTTYATCAAGAARGT | Castlebury et al., 2004 [31] |
| RPB1-Cr | CNGCDATNTCRTTRTCCATRTA | Matheny et al., 2002 [40] | |
| RPB2 | RPB2-5F | GAYGAYMGWGATCAYTTYGG | Liu et al., 1999 [34] |
| RPB2-5F2 | GGGGWGAYCAGAAGAAGGC | Sung et al., 2007 [32] | |
| RPB2-7cR | CCCATRGCTTGYTTRCCCAT | Liu et al., 1999 [34] | |
| TEF1-α | EF-1 | ATGGGTAAGGARGACAAGAC | O’Donnell et al., 1998 [41] |
| EF-2 | GGARGTACCAGTSATCATGTT | O’Donnell et al., 1998 [41] | |
| 983F | GCYCCYGGHCAYCGTGAYTTYAT | Rehner and Buckley, 2005 [35] | |
| 2218R | ATGACACCRACRGCRACRGTYTG | Rehner and Buckley, 2005 [35] | |
| TUB2 | T1 | AACATGCGTGAGATTGTAAGT | O’Donnell and Cigelnik, 1997 [42] |
| T2 | TAGTGACCCTTGGCCCAGTTG | O’Donnell and Cigelnik, 1997 [42] |
| Kingdom Fungi | Functional Roles 1 | References for Roles |
|---|---|---|
| Division Ascomycota | ||
| Subdivision Pezizomycotina | ||
| Class Sordariomycetes | ||
| Order Hypocreales | ||
| Family Cordycipitaceae | ||
| Akanthomyces muscarius (Petch) Spatafora, Kepler and B. Shrestha, 2017 | EPF, MP | Saidi et al., 2023 [43]; Broumandnia and Rajabpour, 2021 [44] |
| Beauveria bassiana (Bals.-Criv.) Vuill., 1912 | EPF, PEP | Clifton et al., 2019, 2023 [24,45]; Hajek and Meyling, 2018 [46] |
| Cordyceps cateniannulata (Z.Q. Liang) Kepler, B. Shrestha, and Spatafora, 2017 | EPF | Montes-Bazurto et al., 2020 [47] |
| Cordyceps javanica (Bally) Kepler, B. Shrestha, and Spatafora, 2017 | EPF | Clifton and Hajek, 2021 [28] |
| Flavocillium bifurcatum H. Yu, Y.B. Wang, Y. Wang, Q. Fan, and Zhu L. Yang, 2020 | EPF | Wang et al., 2020 [48] |
| Samsoniella sp. | EPF | This study |
| Family Bionectriaceae | ||
| Clonostachys eriocamporesii R.H. Perera and K.D. Hyde, 2020 | EPF | Rodrigues et al., 2022 [49] |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert, and W. Gams, 1999 | EPF, MP | Yang et al., 2021 [50]; Sun et al., 2020 [51] |
| Family Nectriaceae | ||
| Fusarium avenaceum (Fr.) Sacc., 1886 | EPF, PP, PEP | Batta, 2012 [52]; Rajagopal and Suryanarayanan, 2000 [53]; Uhlig et al., 2007 [54] |
| Fusarium concentricum Nirenberg and O’Donnell, 1998 | EPF, PP | Qiu et al., 2023 [55] |
| Fusarium falsibabinda M.M. Wang and L. Cai, 2022 | EPF | This study |
| Fusarium fujikuroi Nirenberg, 1976 | EPF, PP | Sharma et al., 2018 [56]; Dewing et al., 2022 [57] |
| Fusarium graminearum Schwabe, 1839 | EPF, PP, PEP | Ameen, 2012 [58]; Trail, 2009 [59]; Lofgren et al., 2018 [60] |
| Family Sarocladiaceae | ||
| Sarocladium strictum (W. Gams) Summerb., 2011 | EPF, PP, PEP, MP | El-Sayed et al., 2020 [61]; Blaszczyk et al., 2021 [62]; Tagne et al., 2002 [63]; Racedo et al., 2013 [64]; Kim 2002 [65]; This study |
| Family Glomerellaceae | ||
| Colletotrichum fioriniae (Marcelino and Gouli) Pennycook, 2017 | EPF, PP, PEP | Gonzalez et al., 2023 [66]; Marcelino et al., 2009 [67,68] |
| Family Myrotheciomycetaceae | ||
| Trichothecium roseum (Pers.) Link, 1809 | EPF, PP, MP | Batta, 2020 [69]; Götz and Karbowy-Thongbai, 2023 [70]; Zhu et al., 2022a [71] |
| Fungal Species | ARSEF Number | ITS | LSU | RPB1 | RPB2 | TEF1-α | TUB2 |
|---|---|---|---|---|---|---|---|
| Akanthomyces muscarius | 14661 | OR577160 2 | OR575222 | OR593718 | OR593721 | OR593725 | |
| Clonostachys eriocamporesii | 14696 | OR582991 2 | OR602799 | ||||
| Clonostachys rosea | 14682 | OR577161 | OR593726 | ||||
| Colletotrichum fioriniae | 14695 | OR583017 | OR672136 | ||||
| Cordyceps cateniannulata | 14662 | OR577162 2 | OR575223 | OR593719 | OR593727 | ||
| Cordyceps javanica | 14690 | OR577169 | OR672137 | ||||
| Flavocillium bifurcatum | 14694 | OR582994 2 | OR577040 | OR602848 | OR602852 | OR602802 | |
| Fusarium avenaceum | 14691 | OR577163 | OR593728 | ||||
| Fusarium concentricum | 14687 | OR652385 | OR593729 | ||||
| Fusarium falsibabinda | 14667 | OR577164 2 | OR593730 | ||||
| Fusarium fujikuroi | 14677 | OR577165 2 | OR593722 | OR593731 | |||
| Fusarium graminearum | 14692 | OR577166 2 | OR593723 | OR593732 | |||
| Samsoniella sp. | 14689 | OR577167 | OR593720 | OR593724 | OR672131 | OR672138 | |
| Sarocladium strictum | 14693 | OR577168 2 | OR672132 | ||||
| Trichothecium roseum | 14697 | OR583015 2 | OR672134 |
| Fungal Species | # Sites with Fungus | Instars Infected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | A1 | A2 | A3 | Total by Row | ||
| Akanthomyces muscarius | 3 | 2 | 1 | 1 | 5 | 9 | |||
| Beauveria bassiana | 5 | 1 | 1 | 4 | 32 | 50 | 88 | ||
| Clonostachys eriocamporesii | 1 | 1 | 1 | ||||||
| Clonostachys rosea | 2 | 1 | 2 | 3 | |||||
| Colletotrichum fioriniae | 1 | 1 | 1 | ||||||
| Cordyceps cateniannulata | 1 | 1 | 1 | ||||||
| Cordyceps javanica | 1 | 2 | 2 | ||||||
| Flavocillium bifurcatum | 1 | 1 | 1 | ||||||
| Fusarium avenaceum | 4 | 2 | 1 | 1 | 4 | ||||
| Fusarium concentricum | 3 | 1 | 2 | 1 | 4 | ||||
| Fusarium falsibabinda | 2 | 1 | 4 | 5 | |||||
| Fusarium fujikuroi | 6 | 1 | 6 | 14 | 9 | 30 | |||
| Fusarium graminearum | 3 | 3 | 2 | 5 | |||||
| Samsoniella sp. | 1 | 1 | 1 | ||||||
| Sarocladium strictum | 5 | 3 | 6 | 10 | 3 | 22 | |||
| Trichothecium roseum | 1 | 2 | 2 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajek, A.E.; Everest, T.A.; Clifton, E.H. Accumulation of Fungal Pathogens Infecting the Invasive Spotted Lanternfly, Lycorma delicatula. Insects 2023, 14, 912. https://doi.org/10.3390/insects14120912
Hajek AE, Everest TA, Clifton EH. Accumulation of Fungal Pathogens Infecting the Invasive Spotted Lanternfly, Lycorma delicatula. Insects. 2023; 14(12):912. https://doi.org/10.3390/insects14120912
Chicago/Turabian StyleHajek, Ann E., Thomas A. Everest, and Eric H. Clifton. 2023. "Accumulation of Fungal Pathogens Infecting the Invasive Spotted Lanternfly, Lycorma delicatula" Insects 14, no. 12: 912. https://doi.org/10.3390/insects14120912





