Efficacy of Conventional and Organic Insecticides against Scaphoideus titanus: Field and Semi-Field Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Insecticides Used in Conventional Viticulture
2.2. Experimental Sites and Insecticides Used in Organic Viticulture
2.3. Insecticide Residual Activity in Semi-Field Conditions
2.4. Insecticide Residual Activity in Field Conditions
2.5. Data Analysis
3. Results
3.1. Efficacy of Conventional Insecticides against S. titanus Nymphs in Open Field (Basal Leaves)
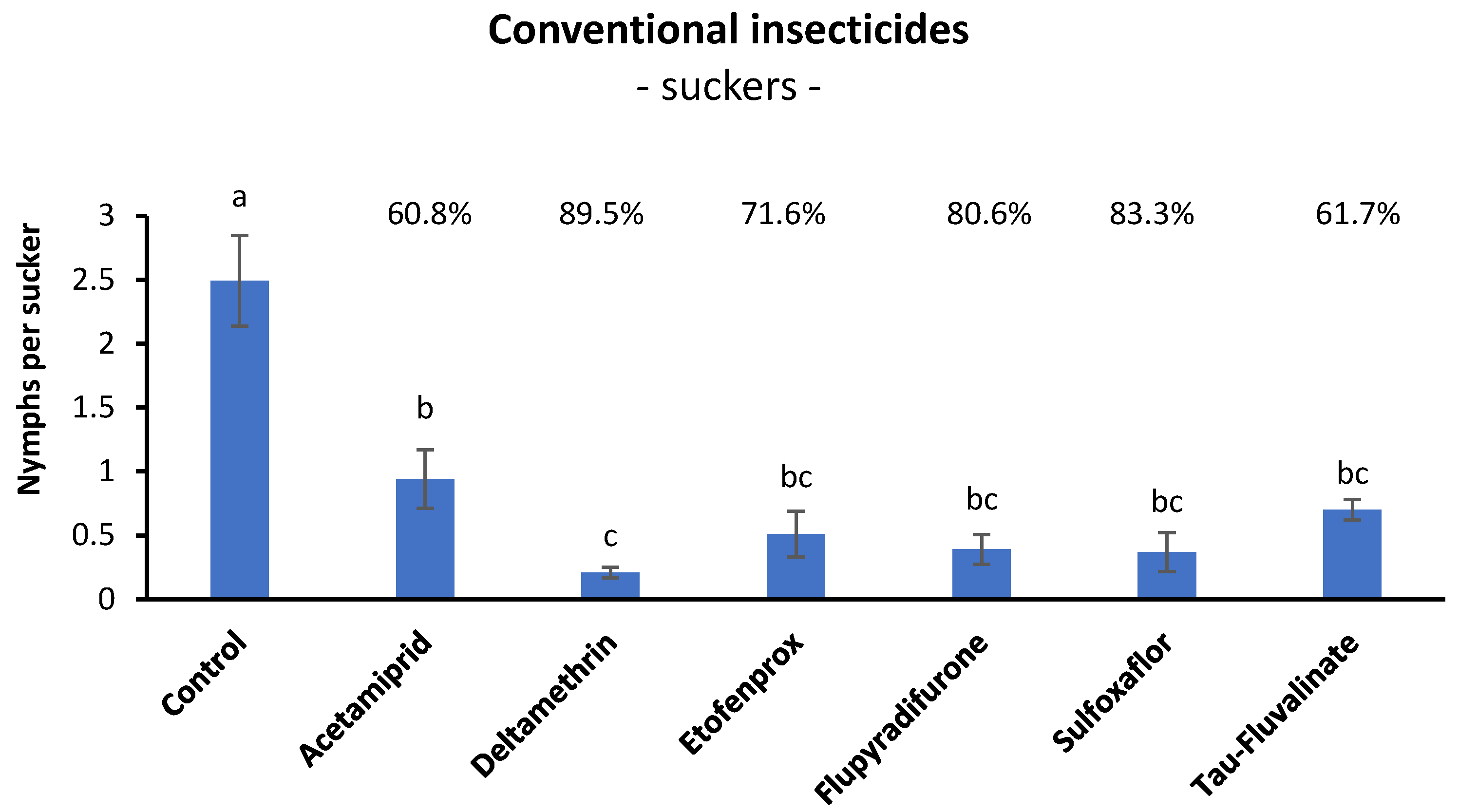
3.2. Efficacy of Conventional Insecticides against S. titanus Nymphs in Open Field (Suckers)
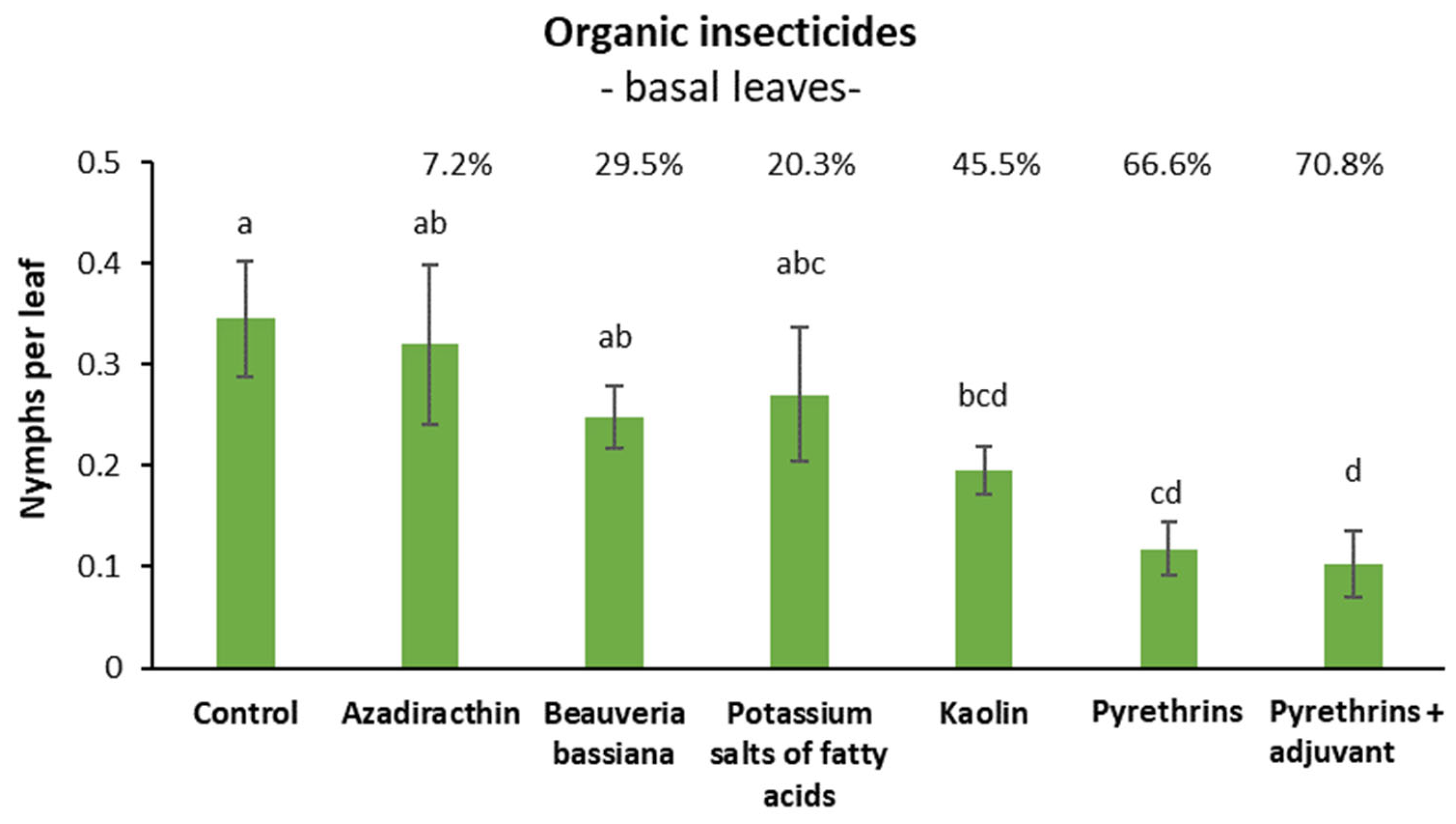
3.3. Efficacy of Organic Insecticides against S. titanus Nymphs in Open Field (Basal Leaves)
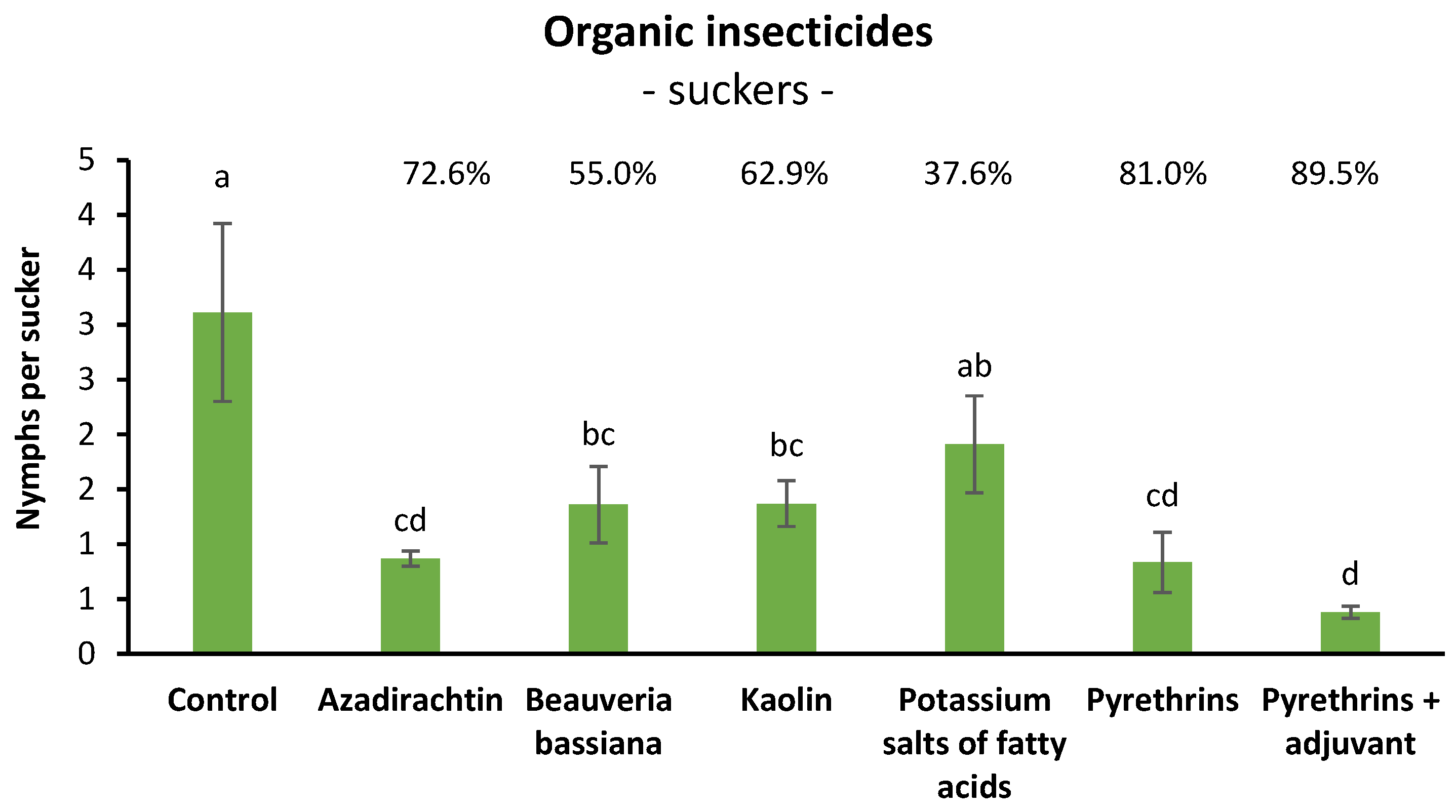
3.4. Efficacy of Organic Insecticides against S. titanus Nymphs in Open Field (Suckers)
3.5. Insecticide Residual Activity in Semi-Field Trials
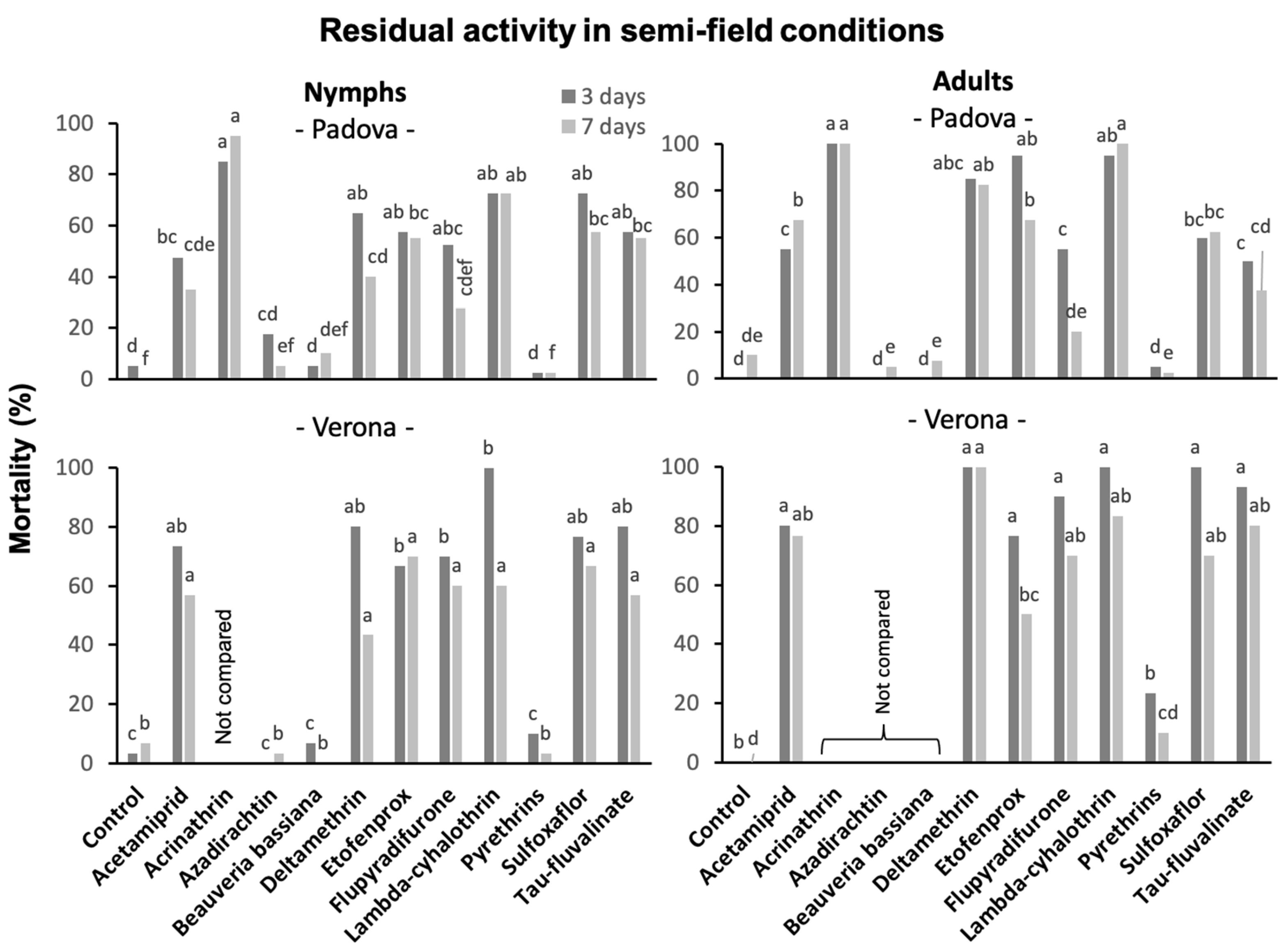
3.5.1. Against S. titanus Nymphs
3.5.2. Against S. titanus Adults
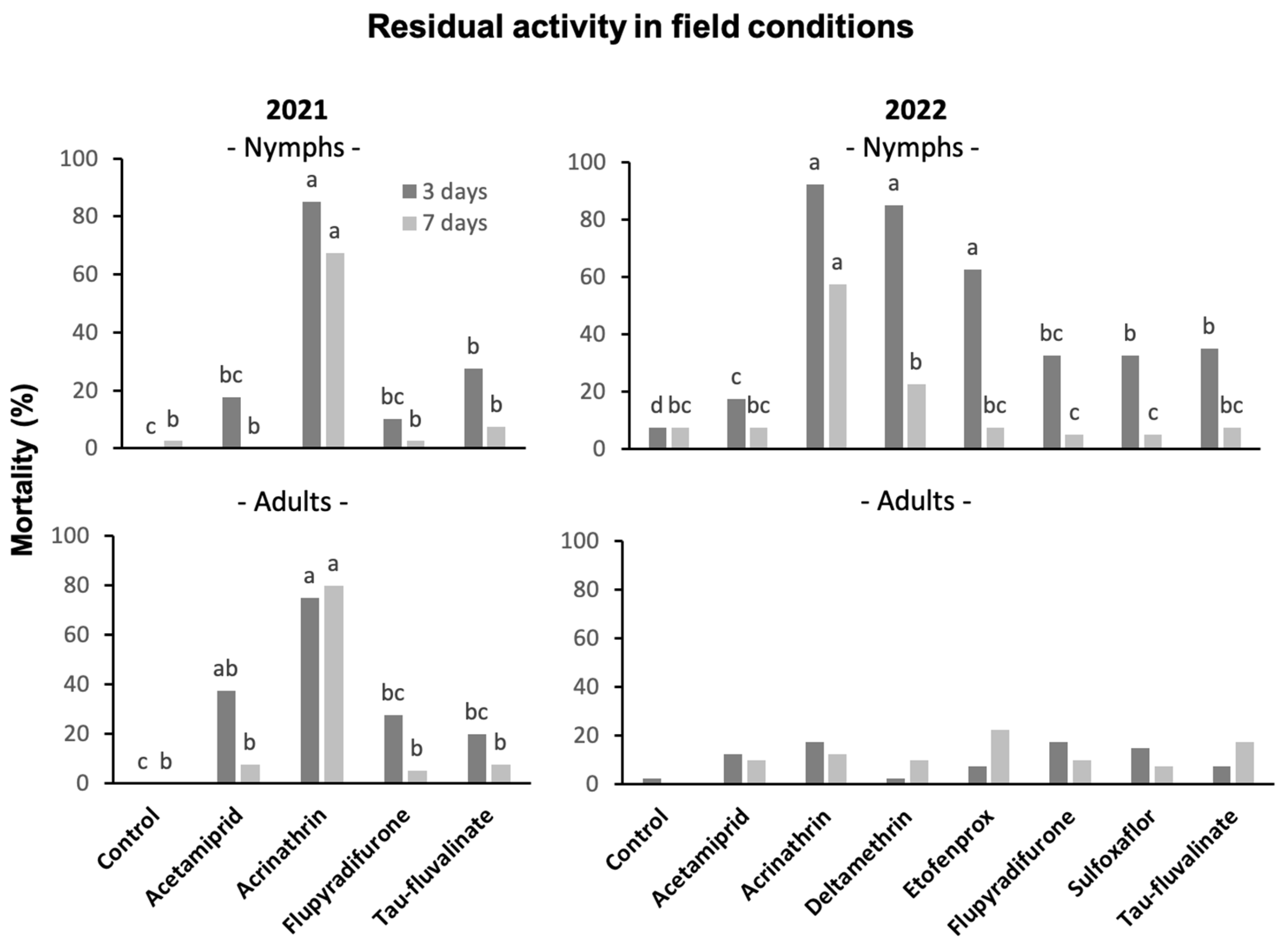
3.6. Insecticide Residual Activity in Field Trials
3.6.1. Against S. titanus Nymphs
3.6.2. Against S. titanus Adults
4. Discussion
4.1. The Efficacy of Conventional Insecticides against Nymphs in Field Conditions
4.2. The Efficacy of Organic Insecticides against S. titanus Nymphs in Field Conditions
4.3. Residual Activity of Insecticides in Semi-Field Conditions
4.4. Residual Activity of Insecticides in Field Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schvester, D.; Moutous, G.; Carle, P. Scaphoideus littoralis Ball (Homopt. Jassidae) cicadelle vectrice de la Flavescence dorée de la vigne. Rev. Zool. Agric. Appl. 1962, 61, 118–131. (In French) [Google Scholar]
- Mori, N.; Bressan, A.; Martini, M.; Guadagnini, M.; Girolami, V.; Bertaccini, A. Experimental transmission by Scaphoideus titanus Ball of two Flavescence dorée-type phytoplasmas. Vitis 2002, 41, 99–102. [Google Scholar]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K.; et al. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Evol. Microbiol. 2022, 72, 005353. [Google Scholar] [CrossRef] [PubMed]
- Belli, G.; Bianco, P.A.; Conti, M. Grapevine yellows: Past, present and future. J. Plant Pathol. 2010, 92, 303–326. [Google Scholar]
- Dermastia, M.; Bertaccini, A.; Constable, F.; Mehle, N. Grapevine Yellows Diseases and Their Phytoplasma Agents: Biology and Detection; Springer: Cham, Switzerland, 2017; ISBN 9783319506487. [Google Scholar]
- Malembic-Maher, S.; Desque, D.; Khalil, D.; Salar, P.; Bergey, B.; Danet, J.L.; Duret, S.; Dubrana-Ourabah, M.P.; Beven, L.; Ember, I.; et al. When a Palearctic bacterium meets a Nearctic insect vector: Genetic and ecological insights into the emergence of the grapevine Flavescence dorée epidemics in Europe. PLoS Pathog. 2020, 16, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfils, J.; Schvester, D. Les cicadelles (Homoptera: Auchenorrhyncha) dans leurs rapports avec la vigne dans le 200 Sud-Ouest de la France. Ann. Épiphyties 1960, 9, 325–336. [Google Scholar]
- Vidano, C. Scoperta in Italia dello Scaphoideus littoralis Ball cicalina americana collegata alla “Flavescence dorée” della vite. L’Italia Agric. 1964, 101, 1031–1049. (In Italian) [Google Scholar]
- Chuche, J.; Thiéry, D. Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381. [Google Scholar] [CrossRef] [Green Version]
- Gnezdilov, V.M.; Orlov, O.V. First Record of Scaphoideus titanus Ball, 1932 (Hemiptera, Auchenorrhyncha: Cicadellidae: Deltocephalinae), a Vector of flavescence dorée of Grapevine, from Ciscaucasia. Entomol. Rev. 2022, 102, 108–109. [Google Scholar] [CrossRef]
- Lessio, F.; Mondino, E.B.; Alma, A. Spatial patterns of Scaphoideus titanus (Hemiptera: Cicadellidae): A geostatistical and neural network approach. Int. J. Pest Manag. 2011, 57, 205–216. [Google Scholar] [CrossRef]
- Alma, A.; Lessio, F.; Gonella, E.; Picciau, L.; Mandrioli, M.; Tota, F. New insights in phytoplasma-vector interaction: Acquisition and inoculation of flavescence dorée phytoplasma by Scaphoideus titanus adults in a short window of time. Ann. Appl. Biol. 2018, 173, 55–62. [Google Scholar] [CrossRef]
- Chuche, J.; Thiéry, D. Cold winter temperatures condition the egg-hatching dynamics of a grape disease vector. Naturwissenschaften 2009, 7, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, F.; Pavanetto, E.; Duso, C. Dinamica di popolazione di Scaphoideus titanus Ball. In Atti del convegno internazionale su “La Flavescenza dorata della vite”; Verona/Vicenza, Italy, 28–29 May 1987; pp. 149–155. (In Italian) [Google Scholar]
- Bocca, F.M.; Picciau, L.; Alma, A. New insights on Scaphoideus titanus biology and their implication for integrated pest management. Entomol. Gen. 2020, 4, 337–349. [Google Scholar] [CrossRef]
- Pavan, F.; Stefanelli, G.; Villani, A.; Mori, N.; Posenato, G.; Bressan, A.; Girolami, V. Controllo di FD attraverso la lotta contro il vettore Scaphoideus titanus Ball. Flavescenza Dorata Altri Giallumi Della Vite Toscana Italia 2005, 3, 91–116. (In Italian) [Google Scholar]
- Trivellone, V.; Jermini, M.; Posenato, G.; Mori, N. Influence of pruning wood management and suckering on Scaphoideus titanus Ball density in two distinct wine-growing area. In Book of Abstracts IOBC-WPRS Meeting of the Working Group on “Integrated Protection and Production in Viticulture”; IOBC-WPRS: Vienna, Austria, 2015; p. 11. [Google Scholar]
- Schvester, D.; Moutous, G.; Bonfilis, J.; Carle, P. Étude biologique des cicadelles de la vigne dans le sud-ouest de la France. Ann. Épiphyt. 1962, 13, 205–237. (In French) [Google Scholar]
- Bernard, P.; Du Fretay, G. Dynamique de population de Scaphoideus titanus, vecteur de la Flavescence dorée dans l’Aude en 1987. Bull. Technol. Inf. 1988, 434, 457–464. (In French) [Google Scholar]
- Caudwell, A. La flavescence dorée de la vigne en France. Phytoma-La Défense Végétaux 1981, 325, 16–19. (In French) [Google Scholar]
- Pavan, F.; Mori, N.; Bigot, G.; Zandigiacomo, P. Border effect in spatial distribution of Flavescence dorée affected grapevines and outside source of Scaphoideus titanus vectors. Bull. Insectol. 2012, 65, 281–290. [Google Scholar]
- Lessio, F.; Portaluri, A.; Paparella, F.; Alma, A. A mathematical model of flavescence dorée epidemiology. Ecol. Model. 2015, 312, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Lessio, F.; Tota, F.; Alma, A. Tracking the dispersion of Scaphoideus titanus Ball (Hemiptera: Cicadellidae) from wild to cultivated grapevine: Use of a novel mark-capture technique. Bull. Entomol. Res. 2014, 104, 432–443. [Google Scholar] [CrossRef]
- Martini, M.; Murari, E.; Mori, N.; Bertaccini, A. Identification and epidemic distribution of two flavescence dorée-related phytoplasmas in Veneto (Italy). Plant Dis. 1999, 83, 925–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girolami, V.; Mori, N.; Borella, E.; Capuzzo, C.; Scopel, C.; Posenato, G. Lotta integrata al vettore della flavescenza dorata. L’Informatore Agrar. 2002, 1, 10–11. (In Italian) [Google Scholar]
- Posenato, G.; Mori, N.; Bressan, A.; Sancassani, G.P.; Girolami, V. Scaphoideus titanus conoscerlo per combatterlo. L’Informatore Agrar. 2001, 15, 91–93. (In Italian) [Google Scholar]
- Žežlina, I.; Škvarč, A.; Bohinc, T.; Trdan, S. Testing the efficacy of single applications of five insecticides against Scaphoideus titanus on common grapevines. Int. J. Pest Manag. 2013, 59, 1–9. [Google Scholar] [CrossRef]
- Matko, B.; Miklavc, J.; Mesl, M. Experiences with controlling American grapevine leafhopper (Scaphoideus titanus Ball) in Northeast Slovenia in the period 2008–2012. Plant Prot. Soc. Slov. 2013, 210–215. [Google Scholar]
- Zidaric, I.; Razinger, J.; Skerlava, V. Field efficacy evaluation of several insecticides against Scaphoideus titanus Ball (1932) in wine-growing region Posavje conducted in years 2011 and 2012. [Conference poster]. Plant Prot. Soc. Slov. 2013, 348–353. [Google Scholar]
- European Parliamen and the Council. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC; European Parliamen and the Council: Brussels, Belgium, 2009; Volume 309.
- Gusberti, M.; Jermini, M.; Wyss, E.; Linder, C. Efficacité d’insecticides contre Scaphoideus titanus en vignobles biologiques et effets secondaires. Rev. Suisse Vitic. Arboric. Hortic. 2008, 40, 173–177. (In French) [Google Scholar]
- Mori, N.; Tonello, D.; Posenato, G.; Pozzebon, A.; Duso, C. Efficacy of biopesticides against Scaphoideus titanus Ball in different experimental conditions. IOBC/WPRS Bull. 2014, 105, 45–48. [Google Scholar]
- Tacoli, F.; Mori, N.; Pozzebon, A.; Cargnus, E.; Da Vià, S.; Zandigiacomo, P.; Duso, C.; Pavan, F. Control of Scaphoideus titanus with natural products in organic vineyards. Insects 2017, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Mazio, P. Control trials on Scaphoideus titanus Ball in organic vineyards. ATTI Giornate Fitopatol. 2004, 6, 109–110. (In Italian) [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brow wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Colleluori, M.; Bernasconi, L.; Cernuschi, M.; Casagrandi, M. Efficacia di Tau-Fluvalinate nel controllo delle cicaline della vite e selettività su acari fitoseidi. ATTI Giornate Fitopatol. 2020, 1, 65–70. (In Italian) [Google Scholar]
- Bosio, G.; Martinez, M.C.; Occhetti, P.; Rovetto, I.; Della Valle, D.; Laiolo, L.; Valota, G. Valutazione dell’efficacia di diversi insetticidi per la lotta alle forme giovanili di Scaphoideus titanus su vite in Piemonte. ATTI Giornate Fitopatol. 2004, 1, 95–102. (In Italian) [Google Scholar]
- Lavezzaro, S.; Deandrea, M.; Pensa, P. Controllo di Scaphoideus titanus in un vigneto dell’Albese. L’informatore Agrar. 2019, 75, 54. (In Italian) [Google Scholar]
- Ladurner, E.; Benuzzi, M.; Fiorentini, F. Efficacy of Beauveria bassiana strain ATCC 74040 (Naturalis®) against the leafhopper Scaphoideus titanus Ball under open-field conditions. IOBC/WPRS Bull. 2020, 150, 26–32. [Google Scholar]
- Forte, V.; Bertazzon, N.; Filippin, L.; Angelini, E.; Chemello, M.; Bacci, L. Prove di efficacia di isoclast nella lotta a Scaphoideus titanus, vettore della flavescenza dorata della vite. ATTI Giornate Fitopatol. 2018, 1, 41–50. (In Italian) [Google Scholar]
- Posenato, G.; Rossi, R.; Gumini, R.; Mori, N. Flavescenza dorata, controllo del vettore con flupyradifurone. L’informatore Agrar. 2019, 75 (Suppl. 10), 9–11. (In Italian) [Google Scholar]
- Georghiou, G.P.; Taylor, C.E. Factors influencing the evolution of resistance. In Pesticide Resistance: Strategies and Tactics for Management; National Research Council—National Academy Press: Washington, DC, USA, 1986; pp. 157–169. [Google Scholar]
- Onstad, D.W. Insect Resistance Management: Biology, Economics, and Prediction; Academic Press: Boston, MA, USA, 2014. [Google Scholar]
- Yu, S. The Toxicology and Biochemistry of Insecticides, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 276. [Google Scholar]
- Tirello, P.; Marchesini, E.; Gherardo, P.; Raniero, D.; Rossetto, F.; Pozzebon, A.; Duso, C. The control of the American leafhopper Erasmoneura vulnerata (Fitch) in European vineyards: Impact of synthetic and natural insecticides. Insects 2021, 12, 85. [Google Scholar]
- Forte, V.; Bertazzon, N.; Filippin, L.; Angelini, E.; Chemello, M. Prove di efficacia di sali potassici di acidi grassi nella lotta a Scaphoideus titanus, vettore della flavescenza dorata della vite. ATTI Giornate Fitopatol. 2018, 1, 75–84. (In Italian) [Google Scholar]
- Bottura, M.; Mori, N.; Posenato, G.; Sancassani, G.P.; Girolami, V. Lotta alle cicaline nei vigneti a conduzione biologica. L’informatore Agrar. 2003, 59, 75–79. (In Italian) [Google Scholar]
- Riskallah, M.R. Influence of posttreatment temperature on the toxicity of pyrethroid insecticides to susceptible and resistant larvae of the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.). Experientia 1984, 40, 188–190. [Google Scholar] [CrossRef]
- Brown, M.A. Temperature-dependent Pyrethroid Resistance in a Pyrethroid-selected Colony of Heliothis virescens (F.) (Lepidoptera: Noctuidae). J. Econ. Entomol. 1987, 80, 330–332. [Google Scholar] [CrossRef]
- Fabellar, L.T.; Mochida, O. Susceptibility of brown planthopper (BPH) and green leafhopper (GLH) to insecticides under different temperatures. Int. Rice Res. Newsl. 1988, 13, 36. [Google Scholar]
- Swain, V.; Seth, R.K.; Raghavendra, K.; Mohanty, S.S. Impact of temperature on susceptible and resistant strains of Culex quinquefasciatus to synthetic pyrethroids. Acta Trop. 2009, 112, 303–307. [Google Scholar] [CrossRef]
- Glunt, K.D.; Oliver, S.V.; Hunt, R.H.; Paaijmans, K.P. The impact of temperature on insecticide toxicity against the malaria vectors Anopheles arabiensis and Anopheles funestus. Malar. J. 2018, 17, 131. [Google Scholar] [CrossRef] [Green Version]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dadzie, S.K. Relationship between temperature and Anopheles gambiae sensu lato mosquitoes’ susceptibility to pyrethroids and expression of metabolic enzymes. Parasites Vectors. 2022, 15, 163. [Google Scholar] [CrossRef]
- Kiaeian Moosavi, F.; Cargnus, E.; Pavan, F.; Zandigiacomo, P. Effects of grapevine bunch exposure to sunlight on berry surface temperature and Lobesia botrana (Lepidoptera: Tortricidae) egg laying, hatching and larval settlement. Agric. For. Entomol. 2018, 20, 420–432. [Google Scholar] [CrossRef]

| Management | Formulation | Active Ingredient | Dose (mL or g/hL) | Experimental Trial |
|---|---|---|---|---|
| Conventional | Decis EVO | Deltamethrin | 50 | FTE, SRAT * |
| Conventional | Closer | Sulfoxaflor | 40 | FTE, SRAT * |
| Conventional | Epik SL | Acetamiprid | 150 | FTE, SRAT * |
| Conventional | Sivanto Prime | Flupyradifurone | 60 | FTE, SRAT * |
| Conventional | Mavrik Smart | Tau-fluvalinate | 30 | FTE, SRAT * |
| Conventional | Trebon UP | Etofenprox | 50 | FTE, SRAT * |
| Conventional | Rufast | Acrinathrin | 60 | SRAT * |
| Conventional | Karate Zeon | Lambda-cyhalothrin | 25 | SRAT * |
| Organic | Biopiren Plus | Pyrethrins | 160 | FTE, SRAT * |
| Organic | Biopiren Plus + Mago | Pyrethrins + ethoxylated sorbitan monooleate | 160 + 150 | FTE * |
| Organic | Naturalis | Beauveria bassiana | 150 | FTE, SRAT * |
| Organic | Flipper | Potassium salts of fatty acids | 1500 | FTE * |
| Organic | Neemik TEN | Azadirachtin | 390 | FTE, SRAT * |
| Organic | Surround WP | Kaolin | 2500 | FTE * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prazaru, S.C.; D’Ambrogio, L.; Dal Cero, M.; Rasera, M.; Cenedese, G.; Guerrieri, E.; Pavasini, M.; Mori, N.; Pavan, F.; Duso, C. Efficacy of Conventional and Organic Insecticides against Scaphoideus titanus: Field and Semi-Field Trials. Insects 2023, 14, 101. https://doi.org/10.3390/insects14020101
Prazaru SC, D’Ambrogio L, Dal Cero M, Rasera M, Cenedese G, Guerrieri E, Pavasini M, Mori N, Pavan F, Duso C. Efficacy of Conventional and Organic Insecticides against Scaphoideus titanus: Field and Semi-Field Trials. Insects. 2023; 14(2):101. https://doi.org/10.3390/insects14020101
Chicago/Turabian StylePrazaru, Stefan Cristian, Lisa D’Ambrogio, Martina Dal Cero, Mirko Rasera, Giovanni Cenedese, Enea Guerrieri, Marika Pavasini, Nicola Mori, Francesco Pavan, and Carlo Duso. 2023. "Efficacy of Conventional and Organic Insecticides against Scaphoideus titanus: Field and Semi-Field Trials" Insects 14, no. 2: 101. https://doi.org/10.3390/insects14020101





