Trends towards Lower Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Teflubenzuron in Brazil: An Evidence for Field-Evolved Resistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Spodoptera frugiperda Populations
2.2. Bioassays
2.3. Concentration-response Curve of S. frugiperda Susceptible Strain to Estimate the Diagnostic Concentration
2.4. Temporal Pattern of S. frugiperda Susceptibility to Teflubenzuron
2.5. Spatial Pattern of S. frugiperda Susceptibility to Teflubenzuron
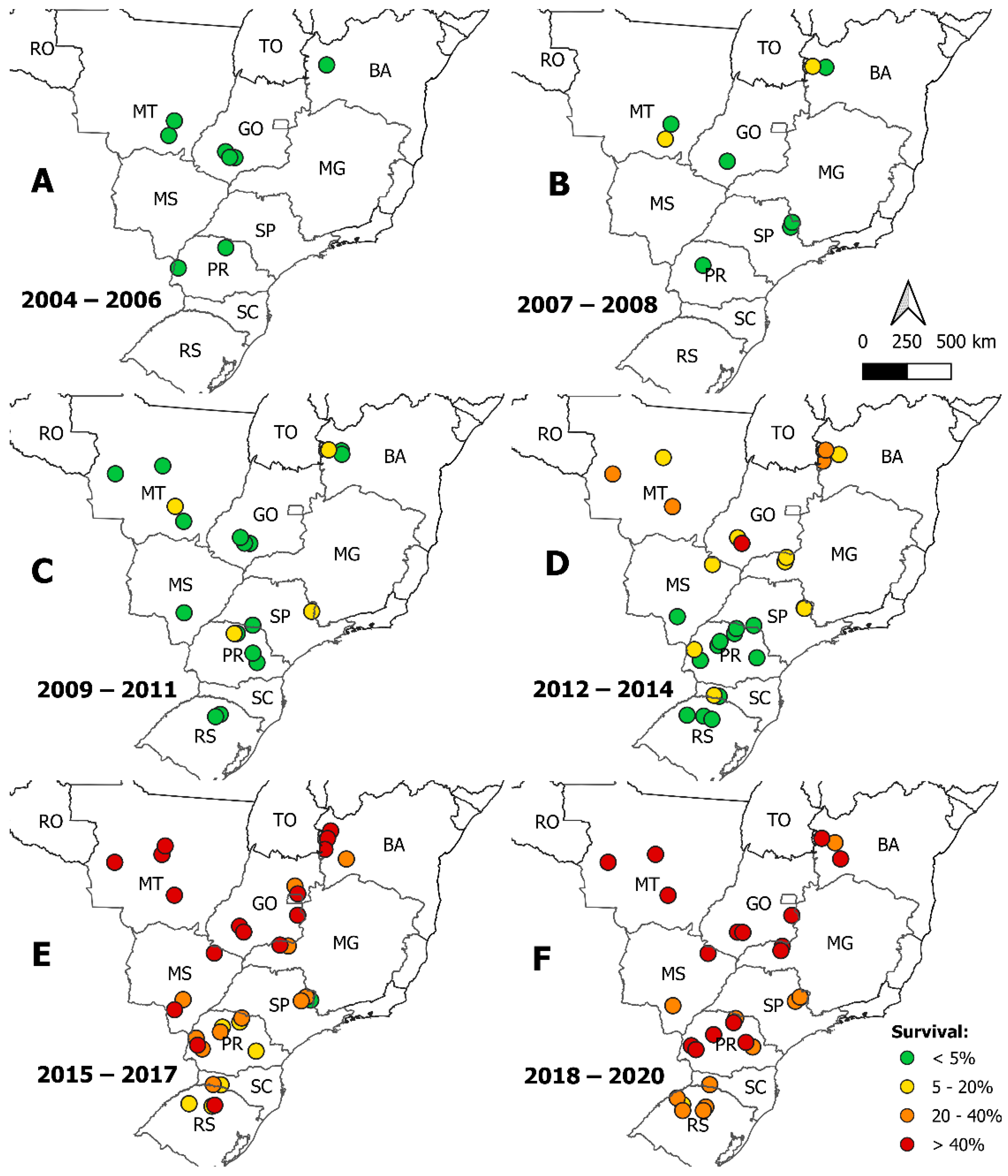
3. Results
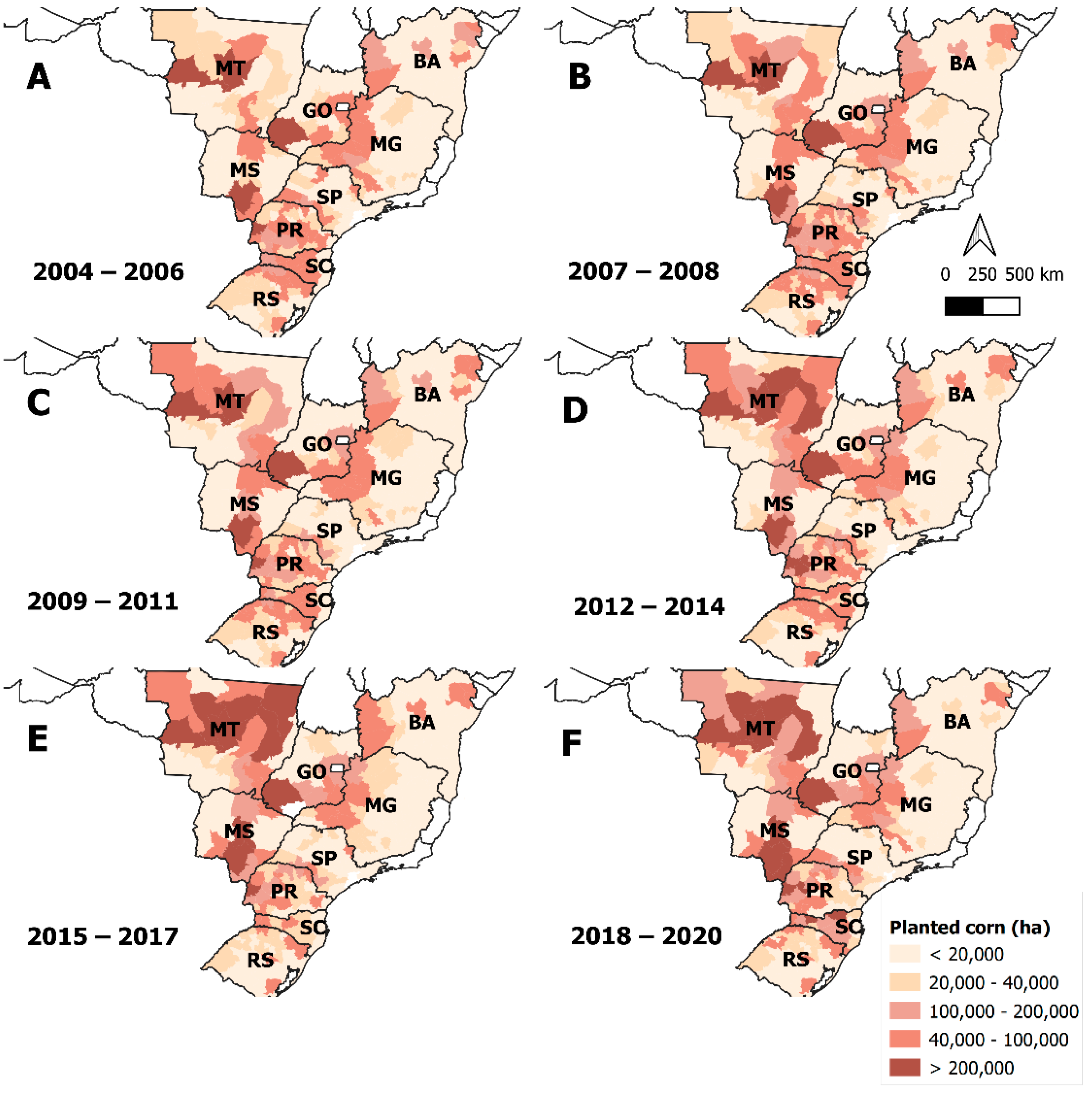
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganiger, P.C.; Yeshwanth, H.M.; Muralimohan, K.; Vinay, N.; Kumar, V.A.R.; Chandrashekara, K. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr. Sci. 2018, 115, 621–623. [Google Scholar] [CrossRef]
- Global action for fall armyworm control | Food and Agriculture Organization of the United Nations (FAO). Available online: http://Www.Fao.Org/Fall-Armyworm/Monitoring-Tools/Faw-Map/En/ (accessed on 12 November 2022).
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Rosas-Garcia, N.M.; Meagher, R.L.; Fleischer, S.J.; Westbrook, J.K.; Sappington, T.W.; Hay-Roe, M.; Thomas, J.M.G.; Murua, G.M. Haplotype profile comparisons between Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Ricco, South America, and the United States and their implications to migratory behavior. J. Econ. Entomol. 2015, 108, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hardke, J.T.; Lorenz, G.M.; Leonard, B.R. Fall armyworm (Lepidoptera: Noctuidae) ecology in southeastern cotton. J. Integr. Pest Manag. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Bueno, R.C.O.F.; Bueno, A.F.; Moscardi, F.; Parra, J.R.P.; Hoffmann-Campo, C.B. Lepidopteran larva consumption of soybean foliage: Basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag. Sci. 2011, 67, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Burtet, L.M.; Bernardi, O.; Melo, A.A.; Pes, M.P.; Strahl, T.T.; Guedes, J.V. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag. Sci. 2017, 73, 2569–2577. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J. Arthropod Pesticide Resistance Database. Available online: https://www.pesticideresistance.org/ (accessed on 12 November 2022).
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; dos Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- do Nascimento, A.R.B.; Fresia, P.; Cônsoli, F.L.; Omoto, C. Comparative transcriptome analysis of lufenuron-resistant and susceptible strains of Spodoptera frugiperda (Lepidoptera: Noctuidae). BMC Genom. 2015, 16, 985. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef]
- Diez-Rodríguez, G.I.; Omoto, C. Inheritance of lambda-cyhalothrin resistance in Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Neotrop. Entomol. 2001, 30, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Okuma, D.M.; Bernardi, D.; Horikoshi, R.J.; Bernardi, O.; Silva, A.P.; Omoto, C. Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manag. Sci. 2018, 74, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.B.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.A.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.C.; Bolzan, A.; Nascimento, A.R.B.; Amaral, F.S.A.; Kanno, R.H.; Kaiser, I.S.; Omoto, C. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: Inheritance and cross-resistance to spinosad. Pest Manag. Sci. 2020, 76, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Muraro, D.S.; Abbade Neto, D.O.; Kanno, R.H.; Kaiser, I.S.; Bernardi, O.; Omoto, C. Inheritance patterns, cross-resistance and synergism in Spodoptera frugiperda (Lepidoptera: Noctuidae) resistant to emamectin benzoate. Pest Manag. Sci. 2021, 77, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Okuma, D.M.; Cuenca, A.; Nauen, R.; Omoto, C. Large-scale monitoring of the frequency of ryanodine receptor target-site mutations conferring diamide resistance in Brazilian field populations of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Kynetec Group, Kynetec. Available online: www.kynetec.com (accessed on 1 June 2022).
- Andersen, S.O. Biochemistry of insect cuticle. Annu. Rev. Entomol. 1979, 24, 29–59. [Google Scholar] [CrossRef]
- van Eck, W.H. Mode of action of two benzoylphenyl ureas as inhibitors of chitin synthesis in insects. Insect Biochem. 1979, 9, 295–300. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Deep, G.; Patil, J.; Saroj, Y.; Brajesh, K.; Ravinder, N. Novel insecticides: A potential tool for the management of insect pest. J. Entomol. Zool. Stud. 2018, 6, 277–281. [Google Scholar]
- Yang, Q.; Fukamizo, T. Targeting chitin-containing organisms. In Advances in Experimental Medicine and Biology; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; Volume 1142, ISBN 978-981-13-7317-6. [Google Scholar]
- Becher, H.M.; Becker, P.; Prokic-Immel, R.; Wirtz, W. CME 134, a new chitin synthesis inhibiting insecticide. In Proceedings of the 10th International Congress of Plant Protection, Brighton, UK, 20–25 November 1983; Volume 1, pp. 408–415. [Google Scholar]
- Stacke, R.F.; Godoy, D.N.; Pretto, V.E.; Führ, F.M.; da S. Gubiani, P.; Hettwer, B.L.; Garlet, C.G.; Somavilla, J.C.; Muraro, D.S.; Bernardi, O. Field-evolved resistance to chitin synthesis inhibitor insecticides by soybean looper, Chrysodeixis includens (Lepidoptera: Noctuidae), in Brazil. Chemosphere 2020, 259, 127499. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.R.B.; Pavinato, V.A.C.; Rodrigues, J.G.; Silva-Brandão, K.L.; Consoli, F.L.; Michel, A.; Omoto, C. There is more than chitin synthase in insect resistance to benzoylureas: Molecular markers associated with teflubenzuron resistance in Spodoptera frugiperda. J. Pest Sci. 2022, 95, 129–144. [Google Scholar] [CrossRef]
- Silva, J.E.; Silva, W.M.; Silva, T.B.M.; Campos, M.R.; Filho, A.B.E.; de Siqueira, H.Á.A. High resistance to insect growth disruptors and control failure likelihood in Brazilian populations of the tomato pinworm Tuta absoluta. Phytoparasitica 2021, 49, 689–701. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing 2022, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 5 November 2022).
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2022. Available online: http://www.qgis.org. (accessed on 5 November 2022).
- INMET—Instituto Nacional de Meteorologia. Available online: https://clima.inmet.gov.br/NormaisClimatologicas/1961-1990/precipitacao_acumulada_mensal_anual (accessed on 10 November 2022).
- IBGE—Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9117-producao-agricola-municipal-culturas-temporarias-e-permanentes.html?=&t=destaques (accessed on 10 November 2022).
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Future climate scenarios project a decrease in the risk of fall armyworm outbreaks. J. Agric. Sci. 2017, 155, 1219–1238. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czepak, C.; Albernaz, K.C.; Vivan, L.M.; Guimarães, H.O.; Carvalhais, T. Primeiro registro de ocorrência de Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) no Brasil. Pesqui. Agropecuária Trop. 2013, 43, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Brasil. Portaria nº 1.109, de 6 de novembro de 2013. Diário Oficial da União, Brasília. Available online: https://www.jusbrasil.com.br/diarios/61394114/dou-secao-1-07-11-2013-pg-5 (accessed on 8 November 2022).
- Muraro, D.S.; Salmeron, E.; Cruz, J.V.S.; Amaral, F.S.A.; Guidolin, A.S.; Nascimento, A.R.B.; Malaquias, J.B.; Bernardi, O.; Omoto, C. Evidence of field-evolved resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) to emamectin benzoate in Brazil. Crop Prot. 2022, 162, 106071. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Belinato, T.A.; Martins, A.J. Insecticide Resistance and Fitness Cost. In Insecticides Resistance; Stanislav, T., Ed.; InTech: Singapore, 2016. [Google Scholar] [CrossRef] [Green Version]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, J.; du Plessis, H. Chemical control and isecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Wengrat, A.P.G.S.; Coelho Junior, A.; Parra, J.R.P.; Takahashi, T.A.; Foerster, L.A.; Corrêa, A.S.; Polaszek, A.; Johnson, N.F.; Costa, V.A.; Zucchi, R.A. Integrative taxonomy and phylogeography of Telenomus remus (Scelionidae), with the first record of natural parasitism of Spodoptera spp. in Brazil. Sci. Rep. 2021, 11, 14110. [Google Scholar] [CrossRef]
- Bentivenha, J.P.F.; Rodrigues, J.G.; Lima, M.F.; Marçon, P.; Popham, H.J.R.; Omoto, C. Baseline susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to SfMNPV and evaluation of cross-resistance to major insecticides and Bt proteins. J. Econ. Entomol. 2019, 112, 91–98. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Reavey, C.E.; Walker, A.S.; Joyce, S.P.; Broom, L.; Willse, A.; Ercit, K.; Poletto, M.; Barnes, Z.H.; Marubbi, T.; Troczka, B.J.; et al. Self-limiting fall armyworm: A new approach in development for sustainable crop protection and resistance management. BMC Biotechnol. 2022, 22, 5. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, F.S.d.A.e.; Kanno, R.H.; Nascimento, A.R.B.d.; Guidolin, A.S.; Omoto, C. Trends towards Lower Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Teflubenzuron in Brazil: An Evidence for Field-Evolved Resistance. Insects 2023, 14, 129. https://doi.org/10.3390/insects14020129
Amaral FSdAe, Kanno RH, Nascimento ARBd, Guidolin AS, Omoto C. Trends towards Lower Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Teflubenzuron in Brazil: An Evidence for Field-Evolved Resistance. Insects. 2023; 14(2):129. https://doi.org/10.3390/insects14020129
Chicago/Turabian StyleAmaral, Fernando Semmelroth de Assunção e, Rubens Hideo Kanno, Antonio Rogério Bezerra do Nascimento, Aline Sartori Guidolin, and Celso Omoto. 2023. "Trends towards Lower Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Teflubenzuron in Brazil: An Evidence for Field-Evolved Resistance" Insects 14, no. 2: 129. https://doi.org/10.3390/insects14020129




