Evidence of Transmission of Plasmodium vivax 210 and Plasmodium vivax 247 by Anopheles gambiae and An. coluzzii, Major Malaria Vectors in Benin/West Africa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
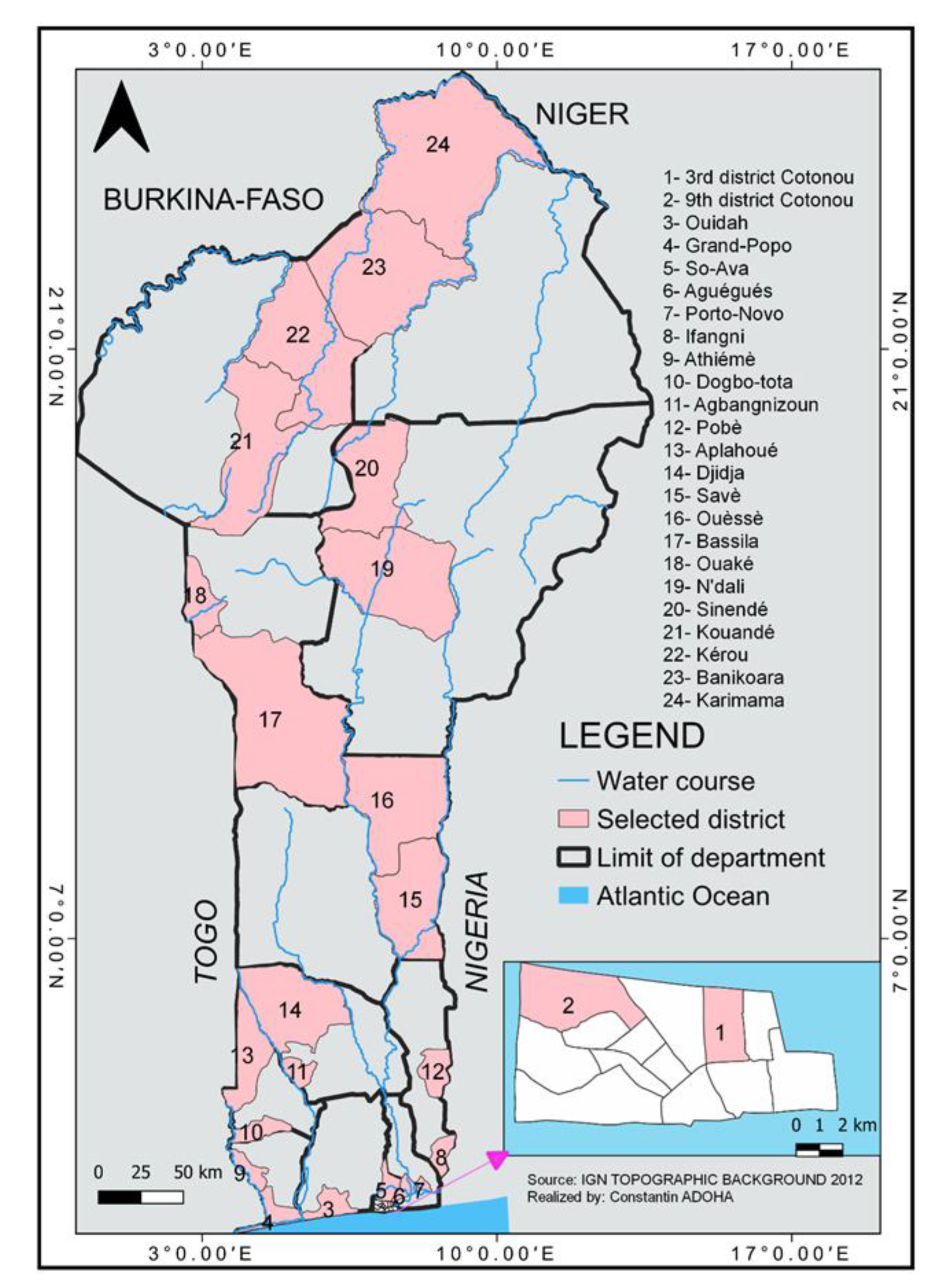
2.1. Study Sites
2.2. Mosquito Collection
2.3. Blood Meal Origin in An. gambiae s.l. Collected by PSC
2.4. Circumsporozoite Protein ELISA for the Detection of P. falciparum, P. vivax 210 and P. vivax 247
2.5. DNA Extraction of Plasmodium Species and Detection of P. vivax Serotypes
2.6. Molecular Species Identification in the Anopheles gambiae Complex
2.7. Data Analysis
2.8. Ethical Consideration
3. Results
3.1. Mosquito Species Composition
3.2. Molecular Species Identification of the Sigbling Species within the Anopheles gambiae Complex
3.3. Blood Meal Origin in An. gambiae s.l. Collected through PSC
3.4. Prevalence of P. falciparum, P. vivax 210 and 247 CSP Antibodies in An. gambiae s.l.
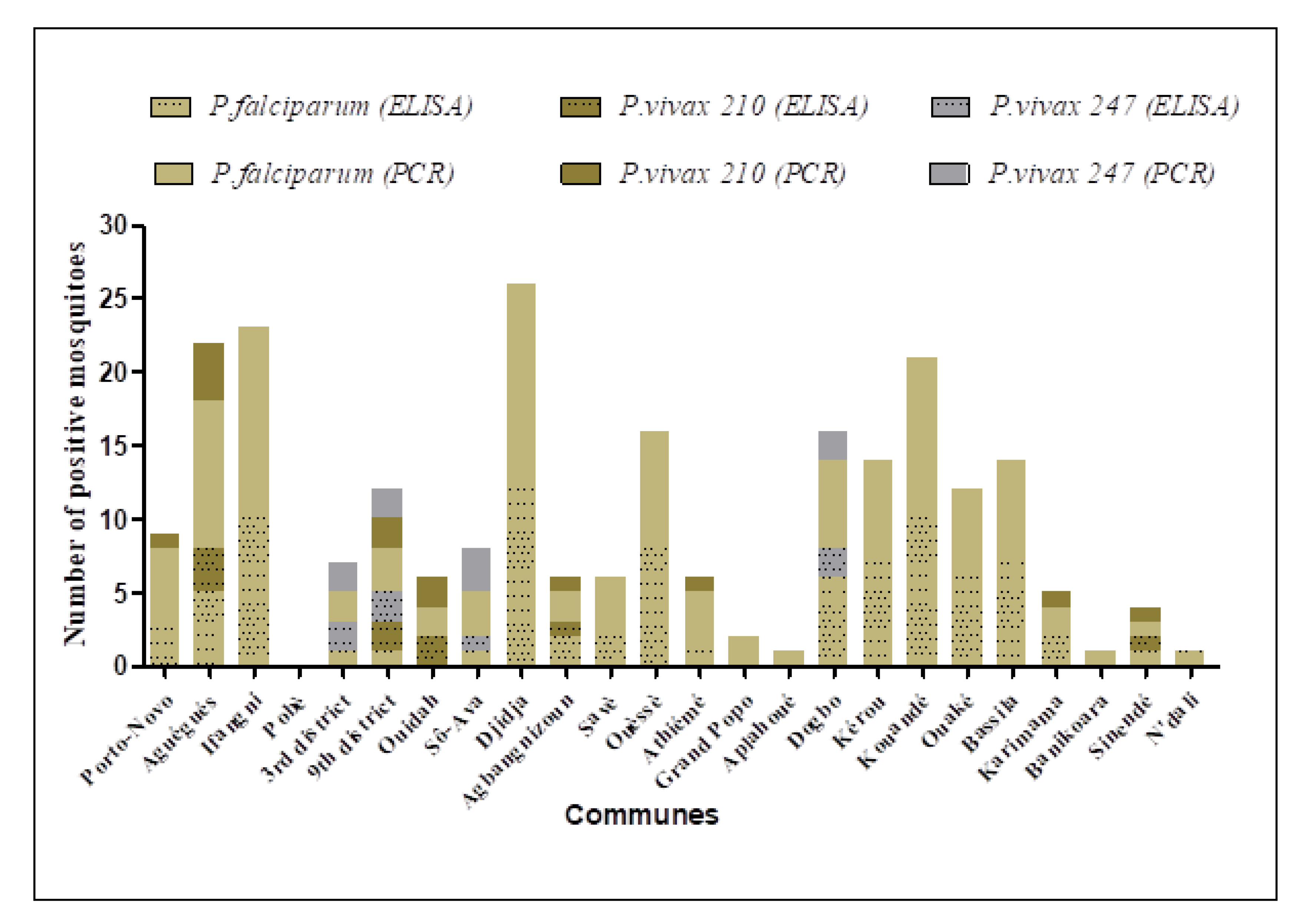
3.4.1. Plasmodium Detection by ELISA
3.4.2. Plasmodium Detection by PCR
3.5. Distribution of the Plasmodium Species in the Sibling Species of the Anopheles gambiae Complex
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Damien, G.B.; Djènontin, A.; Rogier, C.; Vincent Corbel, V.; Bio Bangana, S.; Chandre, F.; Akogbéto, M.; Kindé-Gazard, D.; Massougbodji, A.; Henry, M.-C. Malaria infection and disease in an area with pyrethroid-resistant vectors in southern Benin. Malar. J. 2010, 9, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Ministère de la Santé. Annuaire des Statistiques Sanitaires; Direction de la Programmation et de la Prospective: Cotonou, Benin, 2020; p. 278. [Google Scholar]
- Ossè, R.A.; Tokponnon, F.; Padonou, G.G.; Sidick, A.; Aïkpon, R.; Fassinou, A.; Koukpo, C.Z.; Sèwadé, W.; Akinro, B.; Sovi, A.; et al. Involvement of Anopheles nili in Plasmodium falciparum transmission in North Benin. Malar. J. 2019, 18, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Djènontin, A.; Bio-Bangana, S.; Moiroux, N.; Henry, M.; Bousari, O.; Chabi, J.; Ossè, R.; Koudenoukpo, S.; Corbel, V.; Akogbéto, M.; et al. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vector in Ouidah-Kpomasse-Tori district from Benin (West Africa): A pre-intervention study. Parasites Vectors 2010, 3, 83–84. [Google Scholar] [CrossRef] [Green Version]
- Kantele, A.; Jokiranta, T.S. Review of cases with the emerging fifth human malaria parasite, Plasmodium Knowlesi. Clin. Infect. Dis. 2011, 52, 1356–1362. [Google Scholar] [CrossRef]
- Yap, N.J.; Hossain, H.; Nada-Raja, T.; Ngui, R.; Muslim, A.; Hoh, B.-P.; Khaw, L.T.; Abdul Kadir, K.; Paul Divis, C.S.; Vythilingam, I.; et al. Natural human infections with Plasmodium cynomolgi, P. inui, and 4 other simian malaria parasites, Malaysia. Emerg. Infect. Dis. 2021, 27, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef] [Green Version]
- Loeffel, M.; Ross, A. The relative impact of interventions on sympatric Plasmodium vivax and Plasmodium falciparum malaria: A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010541. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.; Elyazar, I.R.F.; Moyes, C.; Smith, D.; Battle, K.; Guerra, C.A.; Patil, A.P.; Tatem, A.J.; Howes, R.E.; Myers, M.F.; et al. A Long Neglected World Malaria Map: Plasmodium vivax Endemicity in 2010. PLoS Negl. Trop. Dis. 2012, 6, e1814. [Google Scholar] [CrossRef]
- Geleta, G.; Ketema, T. Severe malaria associated with Plasmodium falciparum and P. vivax among children in Pawe Hospital, Northwest Ethiopia. Malar. Res. Treat. 2016, 2016, 1240962. [Google Scholar]
- Asua, V.; Mugenyi, L.; Rosenthal, P.J.; Nsobya, S.L.; Nankabirwa, J.I.; Walakira, A.; Kamya, M.R.; Tukwasibwe, S.; Conrad, M. Plasmodium Species Infecting Children Presenting with Malaria in Uganda. Am. J. Trop. Med. Hyg. 2017, 97, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Roucher, C.; Rogier, C.; Sokhna, C.; Tall, A.; Trape, J.-F. A 20-Year Longitudinal Study of Plasmodium ovale and Plasmodium malariae Prevalence and Morbidity in a West African Population. PLoS ONE 2014, 9, e87169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox-Singh, J.; Singh, B. Knowlesi malaria: Newly emergent and of public health importance? Trends Parasitol. 2008, 24, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, T.N.; Burrows, J.N.; Baird, J.K. Targeting the hypnozoite reservoir of Plasmodium vivax: The hidden obstacle to malaria elimination. Trends Parasitol. 2010, 26, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Doderer-Lang, C.; Atchade, P.S.; Lemoine, J.P.; Coquelin, M.L.; Abou-bacar, A.; Pfaff, A.W.; Brunet, J.; Arnoux, L.; Haar, E.; et al. The hide and seek of Plasmodium vivax in West Africa: Report from a large-scale study in Beninese asymptomatic subjects. Malar. J. 2016, 15, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercereau-Puijalon, O.; Ménard, D. Plasmodium vivax and the Duffy antigen: A paradigm revisited. Transfus. Clin. Et Biol. 2010, 17, 176–183. [Google Scholar] [CrossRef]
- Miller, L.H.; Mason, S.J.; Clyde, D.F.; McGinniss, M.H. The Resistance Factor to Plasmodium vivax in Blacks. N. Engl. J. Med. 1976, 295, 302–304. [Google Scholar] [CrossRef]
- Moreira, C.M.; Abo-Shehada, M.; Price, R.N.; Drakeley, C.J. A systematic review of sub-microscopic Plasmodium vivax infection. Malar. J. 2015, 14, 360. [Google Scholar] [CrossRef] [Green Version]
- Badou, D.F.; Afouda, A.A.; Diekkrüger, B.; Kapangaziwiri, E. Investigation on the 1970s and 1980s drought in four tributaries of the Niger River Basin (West Africa). In Proceedings of the 36th IAHR World Congress, The Hague, The Netherlands, 28 June–3 July 2015; pp. 1–5. [Google Scholar]
- Bossa, A.Y.; Diekkrüger, B.; Agbossou, E.K. Scenario-Based Impacts of Land Use and Climate Change on Land and Water Degradation from the Meso to Regional Scale. Water 2014, 6, 3152–3181. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.T.; Coetzee, M.A. Supplement to the Anophelinae of Africa South of the Sahara, 2nd ed. S. Afr. Inst. Med. Res. 1987, 55, 143. [Google Scholar]
- Beier, J.C.; Vaughan, J.A.; Madani, A.; Noden, B.H. Plasmodium falciparum: Release of circum sporozoïte protein by sporozoïtes in the mosquito vector. Exp. Parasitol. 1992, 75, 248–256. [Google Scholar] [CrossRef]
- Burkot, T.R.; Graves, P.M.; Wirtz, R.A.; Gibson, F.D. The efficiency of sporozoïtes transmission in the human malarias Plasmodium falciparum and P. vivax. Bull. World Health Organ. 1987, 65, 375–380. [Google Scholar]
- MR4. Methods in Anopheles Research, 2nd ed.; CDC: Atlanta, GA, USA, 2010; p. 343. [Google Scholar]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttel, J. Note sur la répartition des moustiques dans le Bas-Dahomey. Bull. Soc. Path. Exot. 1950, 43, 563–566. [Google Scholar]
- Salako, A.S.; Ossè, R.; Padonou, G.G.; Dagnon, F.; Aïkpon, R.; Kpanou, C.; Sagbohan, H.; Sovi, A.; Sèzonlin, M.; Akogbeto, M.C. Population Dynamics of Anopheles gambiae s.l. and Culex quinquefasciatus in Rural and Urban Settings Before an Indoor Residual Spraying Campaign in Northern Benin. Vector Borne Zoonotic Dis. 2019, 19, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Aïkpon, R.; Sèzonlin, M.; Tokponon, F.; Okè, M.; Oussou, O.; Oké-Agbo, F.; Beach, R.; Akogbéto, M. Good performances but short lasting efficacy of Actellic 50 EC Indoor Residual Spraying (IRS) on malaria transmission in Benin, West Africa. Parasites Vectors 2014, 7, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanguenon, V.; Govoétchan, R.; Agossa, F.R.; Ossè, R.; Oke-Agbo, F.; Azondekon, R.; Sovi, A.; Attolou, R.; Badirou, K.; Tokponnon, F.T.; et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar. J. 2014, 13, 444. [Google Scholar] [CrossRef] [Green Version]
- Salako, A.S.; Ahogni, I.; Kpanou, C.; Sovi, A.; Azondekon, R.; Sominahouin, A.A.; Tokponnon, F.; Gnanguenon, V.; Dagnon, F.; Iyikirenga, L.; et al. Baseline entomologic data on malaria transmission in prelude to an indoor residual spraying intervention in the regions of Alibori and Donga, Northern Benin, West Africa. Malar. J. 2018, 17, 392. [Google Scholar] [CrossRef]
- Yovogan, B.; Sovi, A.; Padonou, G.G.; Adoha, C.J.; Akinro, B.; Chitou, S.; Accrombessi, M.; Dangbénon, E.; Akpovi, H.; Messenger, L.A.; et al. Pre-intervention characteristics of the mosquito species in Benin in preparation for a randomized controlled trial assessing the efficacy of dual active-ingredient long-lasting insecticidal nets for controlling insecticide-resistant malaria vectors. PLoS ONE 2021, 16, e0251742. [Google Scholar] [CrossRef]
- Aikpon, R.; Missihoun, A.; Lokossou, A.; Aikpon, G.; Salifou, S.; Dansi, A.; Agbangla, C. Hétérogénéité génétique et résistance des vecteurs du paludisme (Anopheles gambiae s.l) aux insecticides en zone Cotonnière au Benin. Int. J. Biol. Chem. Sci. 2020, 14, 2724–2736. [Google Scholar] [CrossRef]
- Akogbeto, M. Entomological study on the malaria transmission in coastal and lagoon areas: The case of a village built on a brackish lake. Ann. Soc. Belge Med. Trop. 1995, 75, 219–227. [Google Scholar]
- Joy, D.A.; Gonzalez-Ceron, L.; Carlton, J.M.; Gueye, A.; Fay, M.; McCutchan, T.F.; Su, X. Local Adaptation and Vector-Mediated Population Structure in Plasmodium vivax Malaria. Mol. Biol. Evol. 2008, 25, 1245–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandeu, M.M.; Moussiliou, A.; Moiroux, N.; Padonou, G.G.; Massougbodji, A.; Corbel, V.; Ndam, N.T. Optimized Pan-species and Speciation Duplex Real-time PCR Assays for Plasmodium Parasites Detection in Malaria Vectors. PLoS ONE 2012, 7, e52719. [Google Scholar] [CrossRef] [Green Version]
- Howes, R.E.; Reiner Jr, R.C.; Battle, K.E.; Longbottom, J.; Mappin, B.; Ordanovich, D.; Tatem, A.J.; Drakeley, C.; Gething, P.W.; Zimmerman, P.A.; et al. Plasmodium vivax transmission in Africa. PLoS Negl. Trop. Dis. 2015, 9, e0004222. [Google Scholar] [CrossRef] [Green Version]
- Goupeyou-Youmsi, J.; Rakotondranaivo, T.; Puchot, N.; Peterson, I.; Girod, R.; Vigan-Womas, I.; Paul, R.; Ousmane Ndiath, M.; Bourgouin, C. Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar. Parasites Vectors 2020, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Pignatelli, P.; Elaagip, A.; Abdel Hamid, M.M.; Fateh Alrahman, O.; Weetman, D. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016–2018. Emerg. Infect. Dis. 2021, 27, 2952–2954. [Google Scholar] [CrossRef]
- Carter, T.E.; Yared, S.; Gebresilassie, A.; Bonnell, V.; Damodaran, L.; Lopez, K.; Ibrahim, M.; Mohammed, S.; Janies, D. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018, 188, 180–186. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Initiative to Stop the Spread of Anopheles stephensi in Africa; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Collins, K.; Hochberg, L.; Ryan, P.; Collins, W.; Wirtz, R.; Ryan, J. Quantification of Plasmodium malariae infection in mosquito vectors. Ann. Trop. Med. Parasitol. 2004, 98, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bassene, H.; Kengne, P.; Ndiath, M.O.; Sokhna, C.; Dupressoir, T.; Fontenille, D.; Trape, J.-F. Comparaison des méthodes de la PCR, d’ELISA-CSP et d’observation microscopique directe pour la détection des sporozoïtes de Plasmodium falciparum chez Anopheles gambiae au Sénégal. Bull. Soc. Pathol. Exot. 2009, 102, 233–237. [Google Scholar] [PubMed]


| Species | Primers | Sequences (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| Universal reverse Plasmodium spp. | UNR PLF | GACGGTATCTGATCGTCTTC AGTGTGTATCAATCGAGTTTC | |
| P. falciparum | FAR | AGTTCCCCTAGAATA GTTACA | 395 |
| P. vivax | VIR | AGGACTTCCAAGCCGAAGC | 499 |
| P. malariae | MAR | GCCCTCCAA TTGCCT TCT G | 269 |
| P. ovale | OVR | GCATAAGGAATGCAAAGAACAG | 436 |
| P. vivax 210 | PVCSP1 | CCAGTGCTATGGAAGTTCGTC | 789 |
| P. vivax 247 | PVCSP2 | CCAATTTTCCTGTTTCCCATAA | 834 |
| An. coluzzii | 200X6.1F | TCG CCT TAG ACC TTG CGT TA | 479 |
| An. gambiae | 200X6.1R | CGC TTC AAG AAT TCG AGA TAC | 249 |
| An. arabiensis | 223 |
| Ouémé | Plateau | Littoral | Atlantique | Colline | Zou | Mono | Couffo | Atacora | Donga | Alibori | Borgou | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Porto-Novo | Aguégués | Ifangni | Pobè | 3rd District | 9th District | Ouidah | Sô-Ava | Savè | Ouèssè | Djidja | Agbangni Zoun | Athiémé | Grand Popo | Aplahoué | Dogbo | Kérou | Kouandé | Bassila | Ouaké | Karimama | Banikoara | N’dali | Sinendé | |
| Anopheles gambiae s.l. | 524 | 909 | 490 | 20 | 116 | 577 | 169 | 391 | 99 | 280 | 340 | 93 | 1469 | 39 | 82 | 169 | 170 | 321 | 174 | 166 | 89 | 19 | 78 | 73 | 6857 |
| Anopheles funestus gr | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 682 | 299 | 17 | 9 | 6 | 0 | 28 | 60 | 34 | 29 | 55 | 40 | 16 | 9 | 0 | 0 | 1292 |
| Anopheles nili gr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 187 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 189 |
| Anopheles pharoensis | 7 | 16 | 0 | 2 | 0 | 2 | 2 | 83 | 1 | 1 | 0 | 1 | 16 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 5 | 0 | 0 | 1 | 143 |
| Anopheles ziemanni | 2 | 2 | 0 | 28 | 0 | 0 | 0 | 12 | 3 | 0 | 0 | 0 | 1 | 10 | 0 | 2 | 30 | 2 | 2 | 15 | 18 | 1 | 0 | 1 | 129 |
| Aedes aegypti | 30 | 2 | 3 | 1 | 68 | 70 | 334 | 113 | 116 | 57 | 1 | 14 | 0 | 12 | 11 | 16 | 2 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 855 |
| Other Aedes | 0 | 0 | 43 | 34 | 0 | 0 | 1 | 6 | 1 | 3 | 1 | 4 | 3 | 0 | 1 | 12 | 6 | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 121 |
| Culex quinquefasciatus | 817 | 2291 | 364 | 806 | 1736 | 1419 | 194 | 3597 | 17 | 5 | 60 | 82 | 622 | 425 | 182 | 65 | 11 | 118 | 35 | 41 | 118 | 58 | 98 | 105 | 13,266 |
| Other Culex | 24 | 126 | 8 | 22 | 2 | 22 | 2738 | 550 | 8 | 0 | 15 | 13 | 22 | 309 | 3 | 5 | 1 | 8 | 3 | 22 | 4 | 0 | 12 | 0 | 3917 |
| Mansonia africana | 149 | 1116 | 17 | 2607 | 1 | 0 | 2 | 457 | 9 | 31 | 1 | 27 | 319 | 599 | 8 | 74 | 22 | 5 | 16 | 20 | 443 | 13 | 3 | 49 | 5988 |
| Total | 1553 | 4462 | 925 | 3520 | 1923 | 2090 | 3448 | 5209 | 936 | 676 | 435 | 243 | 2458 | 1398 | 315 | 403 | 466 | 501 | 289 | 309 | 694 | 100 | 191 | 229 | 32,757 |
| Blood Meal Source in An. gambiae s.l. females | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Departments | Communes | Total Tested | N. Human (%) | N. Cow (%) | N. Sheep (%) | N. Pig (%) | N. Human + Cow (%) | N. Human + Sheep (%) | N. Human + Pig |
| Ouémé | Porto-Novo | 5 | 5 (100) | ||||||
| Aguégués | 59 | 58 (98.31) | 1 (1.69) | ||||||
| Plateau | Ifangni | 37 | 37 (100) | ||||||
| Pobè | 0 | ||||||||
| Littoral | 3rd district Cotonou | 1 | 1 (100) | ||||||
| 9th district Cotonou | 8 | 8 (100) | |||||||
| Atlantique | Ouidah | 13 | 13 (100) | ||||||
| Sô-Ava | 24 | 21 (87.5) | 2 (8.33) | 1 (4.17) | |||||
| Zou | Djidja | 26 | 26 (100) | ||||||
| Agbangnizoun | 6 | 6 (100) | |||||||
| Collines | Savè | 2 | 2 (100) | ||||||
| Ouèssè | 1 | 1 (100) | |||||||
| Mono | Athiémé | 62 | 58 (93.55) | 3 (4.84) | 1 (1.61) | ||||
| Grand Popo | 2 | 2 (100) | |||||||
| Couffo | Aplahoué | 5 | 5 (100) | ||||||
| Dogbo | 13 | 13 (100) | |||||||
| Atacora | Kérou | 2 | 2 (100) | ||||||
| Kouandé | 6 | 5 (83.33) | 1 (16.67) | ||||||
| Donga | Ouaké | 7 | 7 (100) | ||||||
| Bassila | 1 | 1 (100) | |||||||
| Alibori | Karimama | 28 | 23 (82.14) | 2 (7.14) | 1 (3.57) | 2 (7.14) | |||
| Banikoara | 0 | ||||||||
| Borgou | Sinendé | 2 | 2 (100) | ||||||
| N’dali | 1 | 1 (100) | |||||||
| Total | 311 | 297 (95.5) | 3 (0.96) | 4 (1.29) | 4 (1.29) | 2 (0.64) | 0 | 1 (0.32) | |
| Anopheles gambiae s.l. Species | N. Tested | P. falciparum | P. vivax 210 | P. vivax 247 |
|---|---|---|---|---|
| An. gambiae | 463 | 74 | 3 | 0 |
| An. coluzzii | 474 | 40 | 10 | 9 |
| An. arabiensis | 3 | 1 | 0 | 0 |
| Total | 940 | 115 | 13 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossè, R.A.; Tokponnon, F.; Padonou, G.G.; Glitho, M.E.; Sidick, A.; Fassinou, A.; Koukpo, C.Z.; Akinro, B.; Sovi, A.; Akogbéto, M. Evidence of Transmission of Plasmodium vivax 210 and Plasmodium vivax 247 by Anopheles gambiae and An. coluzzii, Major Malaria Vectors in Benin/West Africa. Insects 2023, 14, 231. https://doi.org/10.3390/insects14030231
Ossè RA, Tokponnon F, Padonou GG, Glitho ME, Sidick A, Fassinou A, Koukpo CZ, Akinro B, Sovi A, Akogbéto M. Evidence of Transmission of Plasmodium vivax 210 and Plasmodium vivax 247 by Anopheles gambiae and An. coluzzii, Major Malaria Vectors in Benin/West Africa. Insects. 2023; 14(3):231. https://doi.org/10.3390/insects14030231
Chicago/Turabian StyleOssè, Razaki A., Filémon Tokponnon, Germain Gil Padonou, Mariette E. Glitho, Aboubakar Sidick, Arsène Fassinou, Come Z. Koukpo, Bruno Akinro, Arthur Sovi, and Martin Akogbéto. 2023. "Evidence of Transmission of Plasmodium vivax 210 and Plasmodium vivax 247 by Anopheles gambiae and An. coluzzii, Major Malaria Vectors in Benin/West Africa" Insects 14, no. 3: 231. https://doi.org/10.3390/insects14030231





