Ecological Factors Associated with the Distribution of Bemisia tabaci Cryptic Species and Their Facultative Endosymbionts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Whitefly Sampling and DNA Extraction
2.2. PCR Amplification
2.3. Sequence Alignment and Phylogenetic Analysis
2.4. Statistical Analyses
3. Results
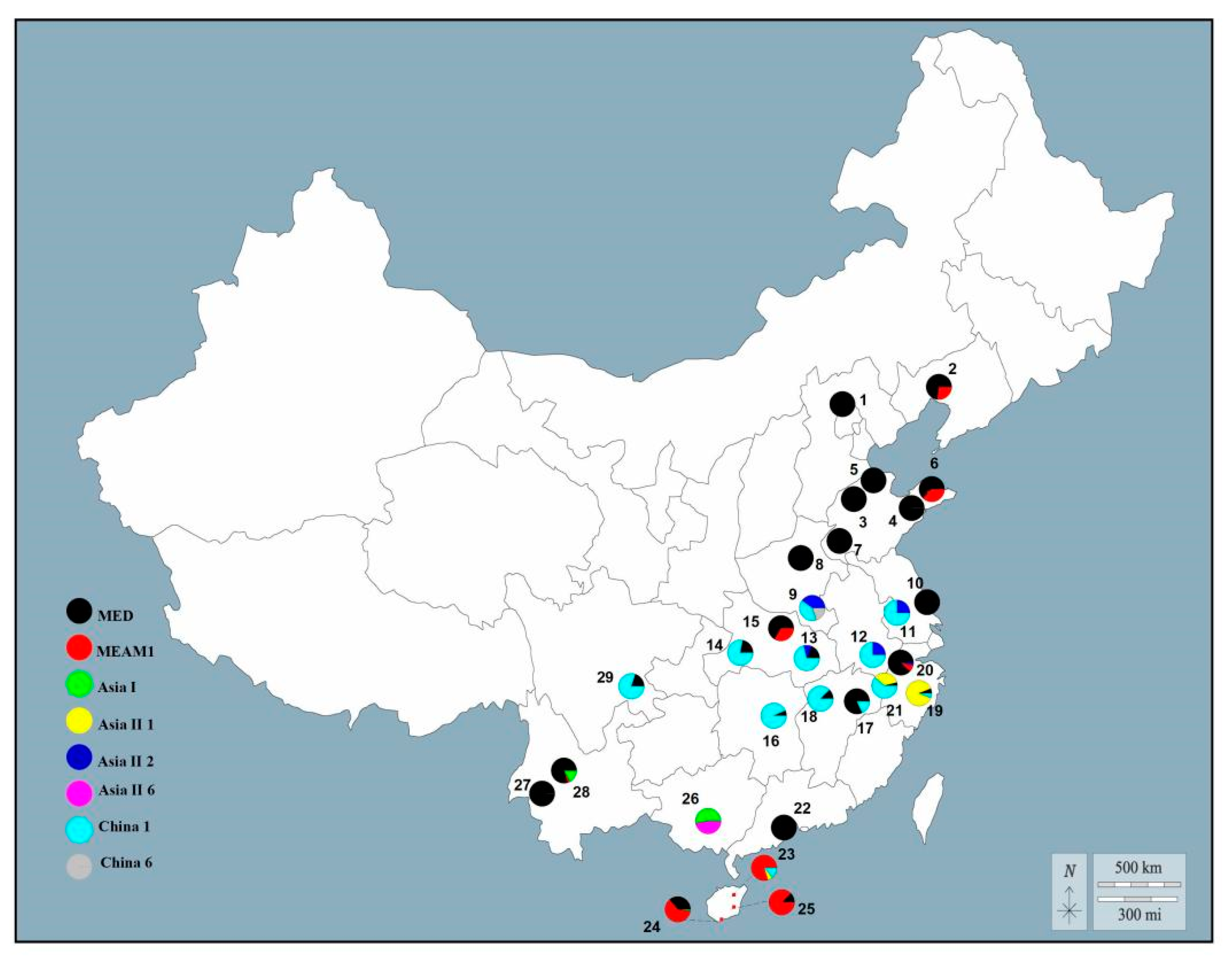
3.1. Identification and Distribution of B. tabaci Cryptic Species
3.2. Habitat Distribution of B. tabaci Cryptic Species in China
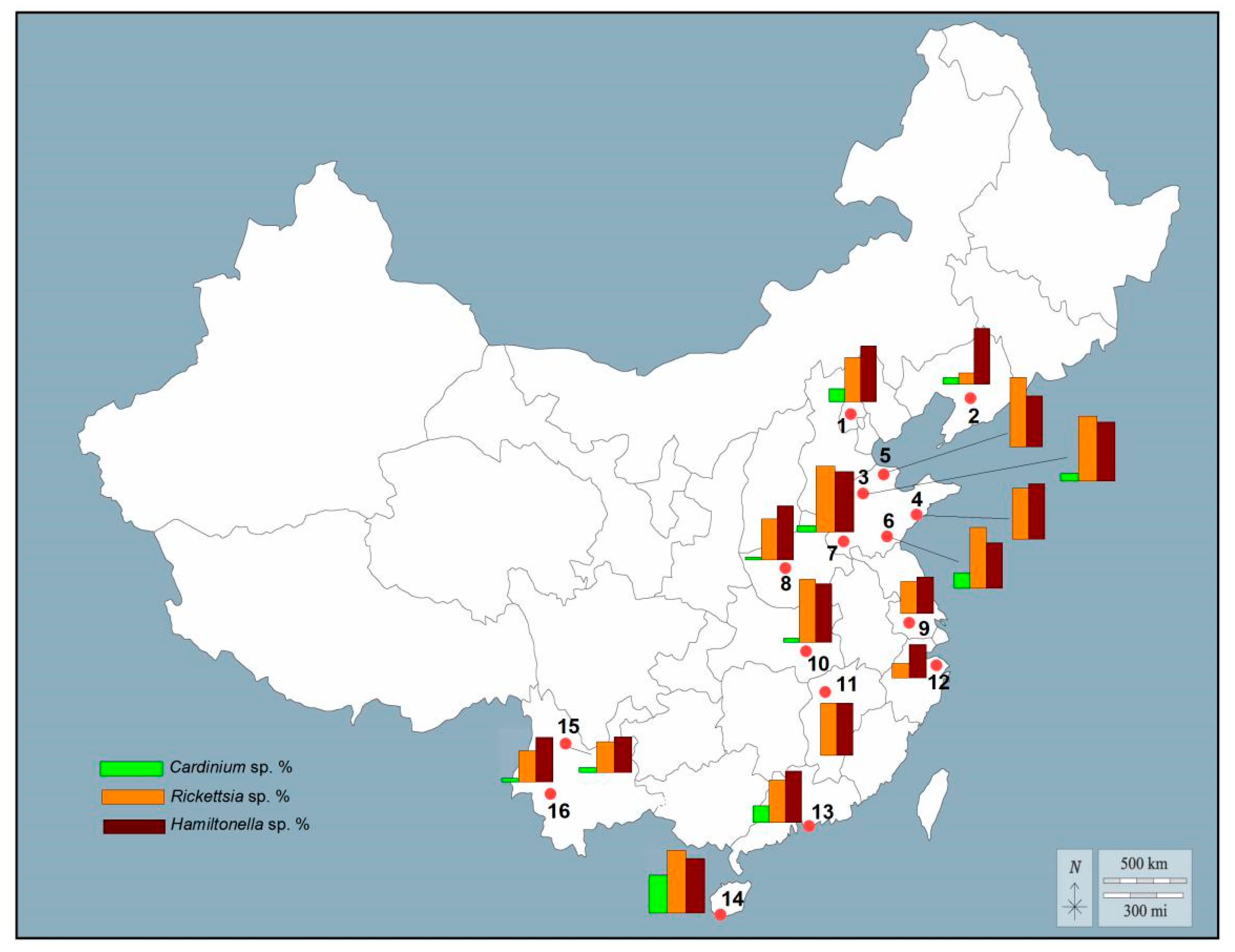
3.3. Geographic Distribution of Endosymbionts in B. tabaci Cryptic Species
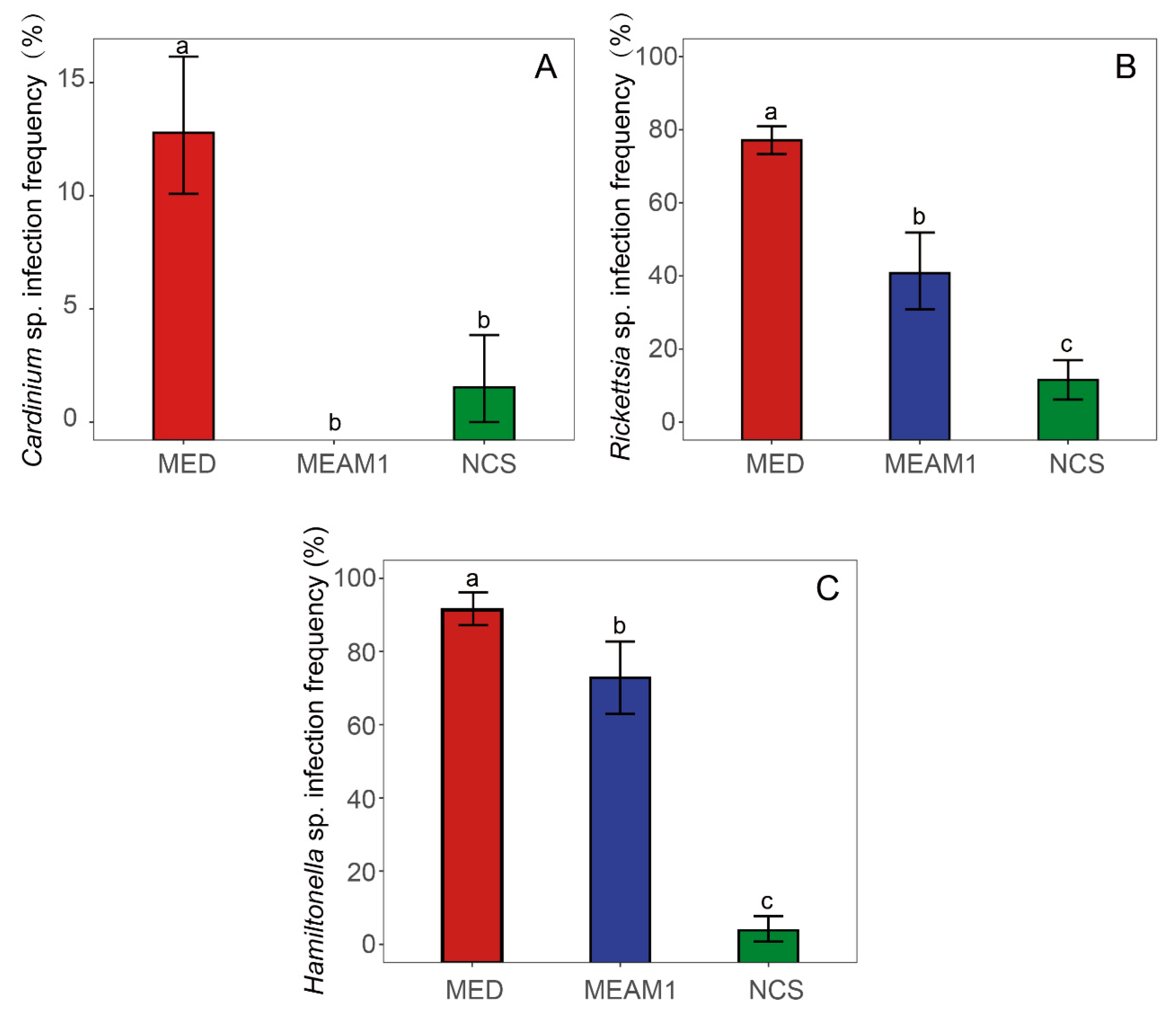
3.4. Infection Frequencies of Facultative Endosymbionts in B. tabaci Cryptic Species
3.5. Multiple Infections of Facultative Endosymbionts in B. tabaci MED
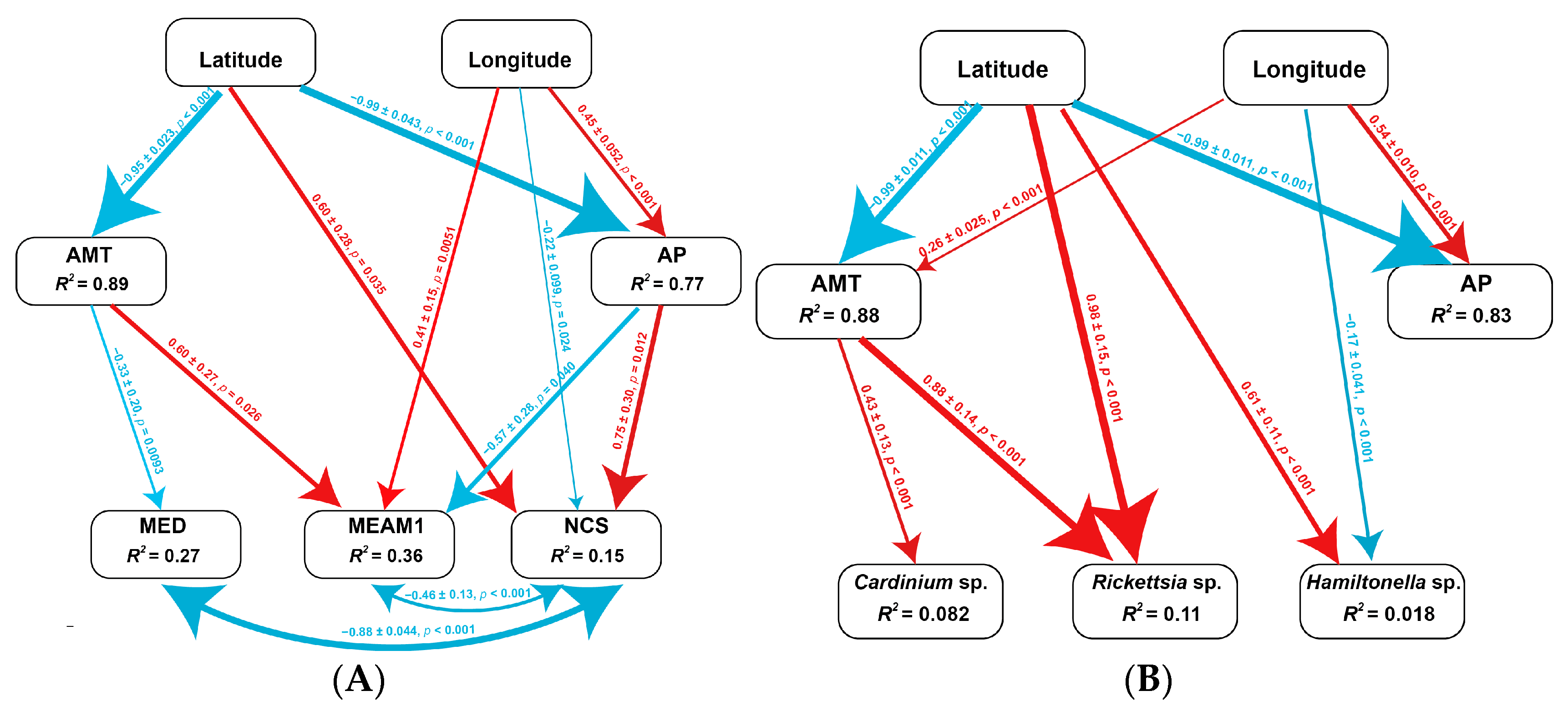
3.6. Association of Environmental Factors on the Frequency of Cryptic Species and Facultative Endosymbionts in MED
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, F.H.; Yang, N.W. Invasion and management of agricultural alien insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Sardain, A.; Sardain, E.; Leung, B. Global forecasts of shipping traffic and biological invasions to 2050. Nat. Sustain. 2019, 2, 274–282. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. Author correction: High and rising economic costs of biological invasions worldwide. Nature 2022, 608, E35. [Google Scholar] [CrossRef]
- Jezkova, T.; Wiens, J.J. Rates of change in climatic niches in plant and animal populations are much slower than projected climate change. Proceedings. Biol. Sci. 2016, 283, 1843. [Google Scholar] [CrossRef] [Green Version]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Early, R.; Sax, D.F. Climatic niche shifts between species native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob. Ecol. Biogeogr. 2014, 23, 1356–1365. [Google Scholar] [CrossRef]
- Lu, M.; Hulcr, J.; Sun, J.H. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [Green Version]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Ayoubi, A.; Talebi, A.A.; Fathipour, Y.; Mehrabadi, M. Coinfection of the secondary symbionts, Hamiltonella defensa and Arsenophonus sp. contribute to the performance of the major aphid pest, Aphis gossypii (Hemiptera: Aphididae). Insect Sci. 2020, 27, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Li, X.; Ge, D.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Chu, D.; Liu, B.; Xu, B.; et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS ONE 2012, 7, e30760. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.X.; Song, Y.L.; Zhang, Y.K.; Hoffmann, A.A.; Zhou, J.C.; Sun, J.T.; Hong, X.Y. Incidence of facultative bacterial endosymbionts in spider mites associated with local environments and host plants. Appl. Environ. Microbiol. 2018, 84, e02546-17. [Google Scholar] [CrossRef]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, C.; Wang, Y.; Zhang, Y.; Ji, L. Ecological niche complexity of invasive and native cryptic species of the Bemisia tabaci species complex in China. J. Pest Sci. 2022, 95, 1245–1259. [Google Scholar] [CrossRef]
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Pan, L.L.; Bouvaine, S.; Fan, Y.Y.; Liu, Y.Q.; Liu, S.S.; Seal, S.; Wang, X.W. Differential transmission of Sri Lankan cassava mosaic virus by three cryptic species of the whitefly Bemisia tabaci complex. Virology 2020, 540, 141–149. [Google Scholar] [CrossRef]
- Perring, T.M. The Bemisia tabaci species complex. Crop Prot. 2001, 20, 725–737. [Google Scholar] [CrossRef]
- Tay, W.T.; Elfekih, S.; Polaszek, A.; Court, L.N.; Evans, G.A.; Gordon, K.H.; De Barro, P.J. Novel molecular approach to define pest species status and tritrophic interactions from historical Bemisia specimens. Sci. Rep. 2017, 7, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.S.; De Barro, P.J.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Pan, H.P.; Tao, Y.L.; Jiang, D.F.; Zhang, Y.J.; Chu, D. Species identification of indigenous Bemisia tabaci in agricultural areas in China. J. Plant Prot. 2016, 43, 84–90. [Google Scholar] [CrossRef]
- Chu, D.; Wan, F.H.; Zhang, Y.J.; Brown, J.K. Change in the biotype composition of Bemisia tabaci in Shandong province of China from 2005 to 2008. Environ. Entomol. 2010, 39, 1028–1036. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Jiang, Z.; Zhang, F.; Liu, Y.; Li, Z. New putative cryptic species detection and genetic network analysis of Bemisia tabaci (Hempitera: Aleyrodidae) in China based on mitochondrial COI sequences. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2018, 29, 474–484. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhu, Y.Z.; Zhang, Y.J.; Keller, M.A.; Liu, T.X.; Chu, D. Invasion biology and management of sweetpotato whitefly (Bemisia tabaci) in China. J. Integr. Pest Manag. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, A.R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 2006, 72, 3646–3652. [Google Scholar] [CrossRef] [Green Version]
- Bing, X.L.; Ruan, Y.M.; Rao, Q.; Wang, X.W.; Liu, S.S. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci. 2013, 20, 194–206. [Google Scholar] [CrossRef]
- Moran, N.A.; Russell, J.A.; Koga, R.; Fukatsu, T. Evolutionary relationships of three new species of enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005, 71, 3302–3310. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.W.; Liu, L.Y.; Zhang, H.L.; Jiang, D.; Chu, D. Competitive ability and fitness differences between two introduced lines of the invasive whitefly Bemisia tabaci Q in China. PLoS ONE 2014, 9, e100423. [Google Scholar] [CrossRef]
- Karut, K.; Castle, S.J.; Karut, S.T.; Karaca, M.M. Secondary endosymbiont diversity of Bemisia tabaci and its parasitoids. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 78, 104104. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Cong, B.; Zhang, Y.J.; Xu, B.Y.; Wu, Q.J.; Zhu, G.R. Detection and phylogenetic analysis of Wolbachia in different Bemisia tabaci biotypes. Acta Entomol. Sin. 2005, 4, 518–525. [Google Scholar] [CrossRef]
- Ji, H.L.; Qi, L.D.; Hong, X.Y.; Xie, H.F.; Li, Y.X. Effects of host sex, plant species, and putative host species on the prevalence of Wolbachia in natural populations of Bemisia tabaci (Hemiptera: Aleyrodidae): A modified nested PCR study. J. Econ. Entomol. 2015, 108, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, D.R.; Torres-Jerez, I.I.; Bedford, I.D.; Markham, P.G.; Brown, J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA 6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Sánchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef] [Green Version]
- Li, H.R.; Pan, H.P.; Tao, Y.L.; Zhang, Y.J.; Chu, D. Population genetics of an alien whitefly in China: Implications for its dispersal and invasion success. Sci. Rep. 2017, 7, 2228. [Google Scholar] [CrossRef] [Green Version]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- De Barro, P.; Ahmed, M.Z. Genetic Networking of the Bemisia tabaci Cryptic species complex reveals pattern of biological invasions. PLoS ONE 2011, 6, e25579. [Google Scholar] [CrossRef] [Green Version]
- Nishimaki, T.; Sato, K. An extension of the Kimura Two-Parameter model to the natural evolutionary process. J. Mol. Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Drew, C.A.; Wiersma, Y.F.; Huettmann, F. Predictive species and habitat modeling in landscape ecology. Model. Species Distrib. Change Using Random For. 2011, 8, 139–159. [Google Scholar] [CrossRef]
- Hayduk, L.A. Structural equation modeling with LISREL: Essentials and advances. Can. Stud. Popul. 1987, 14, 257. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. World Clim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hu, J.; De Barro, P.; Zhao, H.; Wang, J.; Nardi, F.; Liu, S.S. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS ONE 2011, 6, e16061. [Google Scholar] [CrossRef]
- Jiu, M.; Hu, J.; Wang, L.J.; Dong, J.F.; Song, Y.Q.; Sun, H.Z. Cryptic species identification and composition of Bemisia tabaci (Hemiptera: Aleyrodidae) complex in Henan Province, China. J. Insect Sci. 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lestari, S.M.; Khatun, M.F.; Acharya, R.; Sharma, S.R.; Shrestha, Y.K.; Jahan, S.M.H.; Kil, E.J.; Lee, S.; Kim, S.M.; Lee, K.Y. Genetic diversity of cryptic species of Bemisia tabaci in Asia. Arch. Insect Biochem. Physiol. 2023, 112, e21981. [Google Scholar] [CrossRef]
- Acharya, R.; Shrestha, Y.K.; Sharma, S.R.; Lee, K.Y. Genetic diversity and geographic distribution of Bemisia tabaci species complex in Nepal. J. Asia Pac. Entomol. 2020, 23, 509–515. [Google Scholar] [CrossRef]
- Shadmany, M.; Boykin, L.M.; Muhamad, R.; Omar, D. Genetic diversity of Bemisia tabaci (Hemiptera: Aleyrodidae) species complex across Malaysia. J. Econ. Entomol. 2019, 112, 75–84. [Google Scholar] [CrossRef]
- Götz, M.; Winter, S. Diversity of Bemisia tabaci in Thailand and Vietnam and indications of species replacement. J. Asia Pac. Entomol. 2016, 19, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, A.; Maekawa, K.; Tsuchida, T. Genetic groups and endosymbiotic microbiota of the Bemisia tabaci species complex in Japanese agricultural sites. J. Appl. Entomol. 2015, 139, 55–66. [Google Scholar] [CrossRef]
- Islam, W.; Lin, W.; Qasim, M.; Islam, S.U.; Ali, H.; Adnan, M.; Arif, M.; Du, Z.; Wu, Z. A nation-wide genetic survey revealed a complex population structure of Bemisia tabaci in Pakistan. Acta Trop. 2018, 183, 119–125. [Google Scholar] [CrossRef]
- Ellango, R.; Singh, S.T.; Rana, V.S.; Priya, N.G.; Raina, H.; Chaubey, R. Distribution of Bemisia tabaci genetic groups in India. Environ. Entomol. 2015, 44, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, S.; Vosman, B.; Hidayati, N.; Supena, E.D.J.; Visser, R.G.F.; van Heusden, A.W. The Bemisia tabaci species complex: Additions from different parts of the world. Insect Sci. 2013, 20, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, J.M.; Yuki, V.A.; Rocha, K.C.G.; Mituti, T.; Pelegrinotti, F.M.; Ferreira, F.Z. At least two indigenous species of the Bemisia tabaci complex are present in Brazil. J. Appl. Entomol. 2012, 137, 113–121. [Google Scholar] [CrossRef]
- da F Barbosa, L.; Marubayashi, J.M.; De Marchi, B.R.; Yuki, V.A.; Pavan, M.A.; Moriones, E.; Navas-Castillo, J.; Krause-Sakate, R. Indigenous American species of the Bemisia tabaci complex are still widespread in the Americas. Pest Manag. Sci. 2014, 70, 1440–1445. [Google Scholar] [CrossRef]
- Gautam, S.; Mugerwa, H.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Differential transmission of Old and New World Begomoviruses by Middle East-Asia Minor 1 (MEAM1) and Mediterranean (MED) cryptic species of Bemisia tabaci. Viruses 2022, 14, 1104. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Jiang, K.; Zhou, X.P.; Liu, S.S. Differential indirect effects of two plant viruses on an invasive and an indigenous whitefly vector: Implications for competitive displacement. Ann. Appl. Biol. 2009, 155, 439–448. [Google Scholar] [CrossRef]
- Alemandri, V.; De Barro, P.; Bejerman, N.; Argüello Caro, E.B.; Dumón, A.D.; Mattio, M.F.; Rodriguez, S.M.; Truoli, G. Species within the Bemisia tabaci (Hemiptera: Aleyrodidae) complex in soybean and bean crops in Argentina. J. Econ. Entomol. 2012, 105, 48–53. [Google Scholar] [CrossRef]
- Nabugoomu, J.; Seruwagi, G.K.; Corbett, K.; Kanyesigye, E.; Horton, S.; Hanning, R. Needs and barriers of teen mothers in rural eastern Uganda: Stakeholders’ perceptions regarding maternal/child nutrition and health. Int. J. Environ. Res. Public Health 2018, 15, 2776. [Google Scholar] [CrossRef] [Green Version]
- Gueguen, G.; Vavre, F.; Gnankine, O.; Peterschmitt, M.; Charif, D.; Chiel, E.; Gottlieb, Y.; Ghanim, M.; Zchori-Fein, E.; Fleury, F. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 2010, 19, 4365–4376. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.W.; Liu, S.S. The costs and benefits of two secondary symbionts in a whitefly host shape their differential prevalence in the field. Front. Microbiol. 2021, 12, 739521. [Google Scholar] [CrossRef]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Yang, K.; Yuan, M.Y.; Liu, Y.; Guo, C.L.; Liu, T.X.; Zhang, Y.J.; Chu, D. First evidence for thermal tolerance benefits of the bacterial symbiont Cardinium in an invasive whitefly, Bemisia tabaci. Pest Manag. Sci. 2021, 77, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Brown, J.K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2002, 95, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Thierry, M.; Becker, N.; Hajri, A.; Reynaud, B.; Lett, J.M.; Delatte, H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011, 20, 2172–2187. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Giordano, R.; Colbert, A.M.; Karr, T.L.; Robertson, H.M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 1992, 89, 2699–2702. [Google Scholar] [CrossRef] [Green Version]
- Thao, M.L.; Baumann, P. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 2004, 48, 140–144. [Google Scholar] [CrossRef]
- Everett, K.D.E.; Thao, M.; Horn, M.; Dyszynski, G.E.; Baumann, P. Novel chlamydiae in whiteflies and scale insects: Endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 2005, 55, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Hu, X.; Gao, C.; Zhao, H.; Nichols, R.L.; Li, X. Use of mitochondrial cytochrome oxidase I polymerase chain reaction-restriction fragment length polymorphism for identifying subclades of Bemisia tabaci Mediterranean group. J. Econ. Entomol. 2012, 105, 242–251. [Google Scholar] [CrossRef] [PubMed]


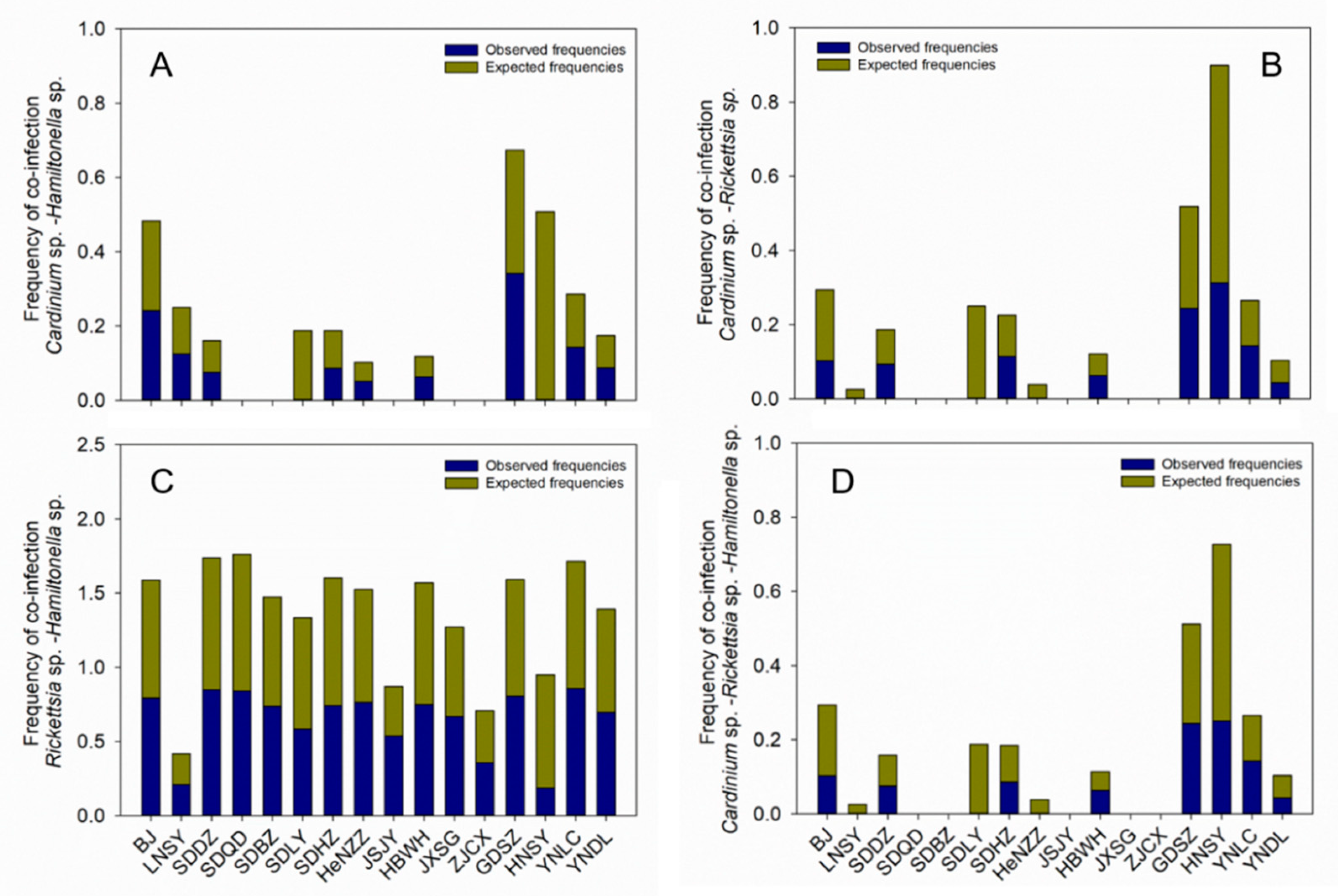
| Comparison Group | Observed Value (%) | Expected Value (%) | Rank of W | p |
|---|---|---|---|---|
| Cardinium sp.–Hamiltonella sp. | 7.58 ± 2.46 | 11.98 ± 3.53 | 17 | 0.31 |
| Cardinium sp.–Rickettsia sp. | 6.98 ± 2.40 | 11.33 ± 3.86 | 11 | 0.056 |
| Rickettsia sp.–Hamiltonella sp. | 64.84 ± 5.46 | 69.54 ± 5.34 | 53 | 0.46 |
| Effects | MED | MEAM1 | NCS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient ± SE | z | p | Coefficient ± SE | z | p | Coefficient ± SE | z | p | |
| Geographical factors | −0.26 ± 0.32 | 0.83 | 0.41 | −0.21 ± 0.30 | 0.70 | 0.48 | 0.37 ± 0.24 | 1.53 | 0.13 |
| Climate factors | 0.61 ± 0.31 | 1.93 | 0.054 | −0.22 ± 0.34 | 0.63 | 0.53 | −0.47 ± 0.19 | 2.44 | 0.015 |
| Total effects | 0.34 ± 0.097 | 3.52 | <0.001 | −0.42 ± 0.18 | 0.18 | 0.019 | −0.094 ± 0.16 | 0.60 | 0.55 |
| Effects | Cardinium sp. | Rickettsia sp. | Hamiltonella sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient ± SE | z | p | Coefficient ± SE | z | p | Coefficient ± SE | z | p | |
| Geographical factors | 0.17 ± 0.12 | 1.45 | 0.15 | 0.61 ± 0.11 | 5.31 | <0.001 | −0.062 ± 0.039 | 1.60 | 0.11 |
| Climate factors | −0.35 ± 0.11 | 3.39 | <0.001 | −0.11 ± 0.059 | 1.94 | 0.052 | −0.026 ± 0.001 | 0.19 | 0.85 |
| Total effects | −0.19 ± 0.058 | 3.24 | <0.001 | 0.49 ± 0.15 | 3.38 | 0.001 | −0.088 ± 0.040 | 1.46 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Jiang, Z.; Zhou, J.; Liu, X.; Zhang, Y.; Chu, D. Ecological Factors Associated with the Distribution of Bemisia tabaci Cryptic Species and Their Facultative Endosymbionts. Insects 2023, 14, 252. https://doi.org/10.3390/insects14030252
Li H, Jiang Z, Zhou J, Liu X, Zhang Y, Chu D. Ecological Factors Associated with the Distribution of Bemisia tabaci Cryptic Species and Their Facultative Endosymbionts. Insects. 2023; 14(3):252. https://doi.org/10.3390/insects14030252
Chicago/Turabian StyleLi, Hongran, Zhihui Jiang, Jincheng Zhou, Xin Liu, Youjun Zhang, and Dong Chu. 2023. "Ecological Factors Associated with the Distribution of Bemisia tabaci Cryptic Species and Their Facultative Endosymbionts" Insects 14, no. 3: 252. https://doi.org/10.3390/insects14030252







