Temperature-Dependent Biology and Population Performances of the Coffee Berry Borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) on Artificial Diet
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of H. hampei
2.2. Survival and Fecundity at Different Observation Intervals
2.3. Temperature-Dependent Life History
2.4. Statistical Analysis
3. Results
3.1. Survival and Fecundity at Different Observation Intervals
3.2. Development Time
3.3. Development Rate
3.4. Longevity and Fecundity
3.5. Population Parameters
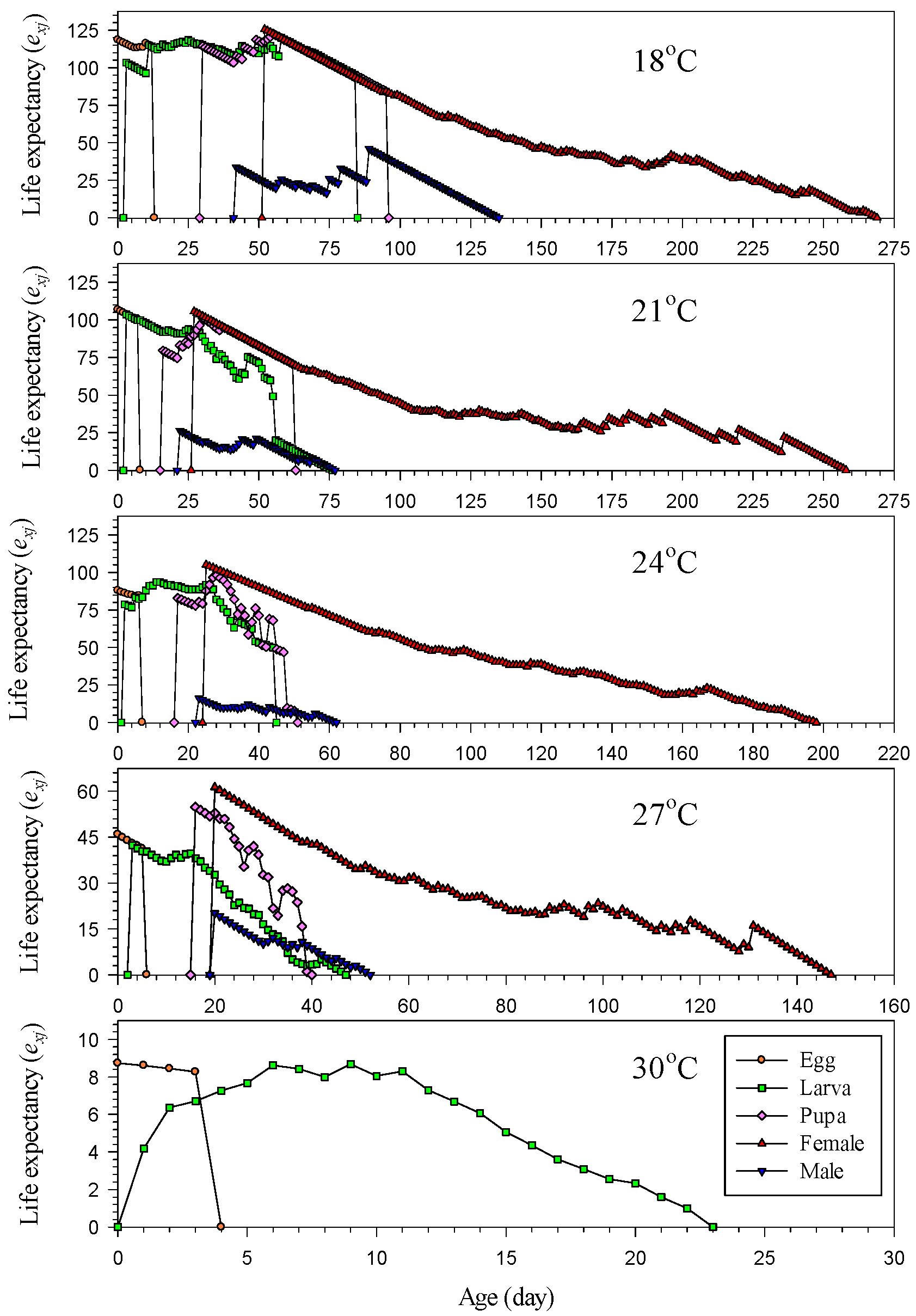
3.6. Life Expectancy and Reproductive Value
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Infante, F. Pest management strategies against the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food Chem. 2018, 66, 5275–5280. [Google Scholar] [CrossRef] [PubMed]
- Annual report of the agricultural statistics of the Republic of China. Council of Agriculture, Executive Yuan, Taipei, Taiwan. Available online: https://agrstat.coa.gov.tw/sdweb/public/book/Book.aspx (accessed on 31 October 2021).
- Waller, J.M.; Bigger, M.; Hillocks, R.J. Berry-feeding Insects. In Coffee Pests, Diseases and Their Management; Waller, J.M., Bigger, M., Hillocks, R.J., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 68–76. [Google Scholar]
- Lin, M.Y.; Wu, Y.F.; Chen, S.K. Monitoring survey of coffee berry borer, Hypothenemus hampei, and its control in the field. Research Bull. Tainan Dist. Agric. Improv. Stn. 2010, 56, 35–44. (In Chinese) [Google Scholar] [CrossRef]
- Lin, M.Y.; Chen, S.K. Study on the control efficiency of insecticides against coffee berry borer, Hypothenemus hampei. Res. Bull. Tainan Dist. Agric. Improv. Stn. 2015, 65, 38–44. (In Chinese) [Google Scholar]
- Damon, A. A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 2000, 90, 453–465. [Google Scholar] [CrossRef]
- Jaramillo, J.; Borgemeister, C.; Baker, P. Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): Searching for sustainable control strategies. Bull. Entomol. Res. 2006, 96, 223–233. [Google Scholar] [CrossRef]
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147. [Google Scholar] [CrossRef]
- Vega, F.E.; Infante, F.; Johnson, A.J. The Genus Hypothenemus, with Emphasis on H. hampei, the Coffee Berry Borer. In Bark Beetles; Vega, F.E., Hofstetter, R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 427–494. [Google Scholar]
- Invasive Species Compendium. CABI, Wallingford, UK. Available online: http://www.cabi.org/isc (accessed on 31 October 2021).
- Portilla, M.; Mumford, J.; Baker, P. Reproductive potential response to continuous rearing of Hypothenemus hampei (Coleoptera: Scolytidae) developed using Cenibroca-artificial diet. Rev. Colomb. Entomol. 2000, 26, 99–105. [Google Scholar] [CrossRef]
- Ruiz-Cárdenas, R.; Baker, P. Life table of Hypothenemus hampei (Ferrari) in relation to coffee berry phenology under Colombian field conditions. Sci. Agric. 2010, 67, 658–668. [Google Scholar] [CrossRef]
- Vega, F.E.; Kramer, M.; Jaramillo, J. Increasing coffee berry borer (Coleoptera: Curculionidae: Scolytinae) female density in artificial diet decreases fecundity. J. Econ. Entomol. 2011, 104, 87–93. [Google Scholar] [CrossRef]
- Hamilton, L.J.; Hollingsworth, R.G.; Sabado-Halpern, M.; Manoukis, N.C.; Follett, P.A.; Johnson, M.A. Coffee berry borer (Hypothenemus hampei) (Coleoptera: Curculionidae) development across an elevational gradient on Hawai‘i Island: Applying laboratory degree-day predictions to natural field populations. PLoS ONE 2019, 14, e0218321. [Google Scholar] [CrossRef]
- Portilla, M.; Streett, D. Biological responses of Hypothenemus hampei (Coleoptera: Curculionidae) on Cenibroca artificial diet at different moisture content levels and relative humidities. Fla. Entomol. 2022, 105, 137–144. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Brun, L.O.; Gaudichon, V.; Wigley, P.J. An artificial diet for continuous rearing of the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae). Int. J. Trop. Insect Sci. 1993, 14, 585–587. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide, Version 9.4; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. National Chung Hsing University, Taichung, Taiwan. Available online: http://140.120.197.173/ecology (accessed on 30 June 2021).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table (erratum). Pest Manag. Sci. 2014, 70, 1936. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993; 456p. [Google Scholar]
- Jha, R.K.; Chi, H.; Tang, L.C. Life table of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) with a discussion on jackknife vs. bootstrap techniques and variations on the Euler-Lotka equation. Formos. Entomol. 2012, 32, 355–375. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Azrag, A.G.A.; Yusuf, A.A.; Pirk, C.W.W.; Niassy, S.; Mbugua, K.K.; Babin, R. Temperature-dependent development and survival of immature stages of the coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae). Bull. Entomol. Res. 2020, 110, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.S.; Barrera, J.F.; Rivas, A. Life-history studies of the coffee berry borer (Hypothenemus hampei, Scolytidae) on coffee trees in southern Mexico. J. Appl. Ecol. 1992, 29, 656–662. [Google Scholar] [CrossRef]
- Jaramillo, J.; Chabi-Olaye, A.; Kamonjo, C.; Jaramillo, A.; Vega, F.E.; Poehling, H.M.; Borgemeister, C. Thermal tolerance of the coffee berry borer Hypothenemus hampei: Predictions of climate change impact on a tropical insect pest. PLoS ONE 2009, 4, e6487. [Google Scholar] [CrossRef]
- Fernández, S.; Cordero, J. Biología de la broca del café Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) en condiciones de laboratorio. Bioagro 2007, 19, 35–40. (In Spanish) [Google Scholar]
- Ruiz, L.; Bustillo, A.E.; Flórez, F.J.P.; González, M.T. Ciclode de vida de Hypothenemus hampei en dos dietas merídlcas. Cenicafé 1996, 47, 77–84. (In Spanish) [Google Scholar]
- Chami, A. Biología de la Broca del Café Hypothenemus hampei Ferrari (Coleoptera: Scolytidae). Ph.D. Thesis, Decanato de Agronomía, Universidad Centroccidental Lisandro Alvarado, Barquisimeto, Venezuela, 2003. (In Spanish). [Google Scholar]
- Azrag, A.G.A.; Murungi, L.K.; Tonnang, H.E.Z.; Mwenda, D.; Babin, R. Temperature-dependent models of development and survival of an insect pest of African tropical highlands, the coffee antestia bug Antestiopsis thunbergii (Hemiptera: Pentatomidae). J. Therm. Biol. 2017, 70, 27–36. [Google Scholar] [CrossRef]
- Azrag, A.G.A.; Pirk, C.W.W.; Yusuf, A.A.; Pinard, F.; Niassy, S.; Mosomtai, G.; Babin, R. Prediction of insect pest distribution as influenced by elevation: Combining field observations and temperature-dependent development models for the coffee stink bug, Antestiopsis thunbergii (Gmelin). PLoS ONE 2018, 13, e0199569. [Google Scholar] [CrossRef]
- Tonnang, E.Z.H.; Juarez, H.; Carhuapoma, P.; Gonzales, J.C.; Mendoza, D.; Sporleder, M.; Simon, R.; Kroschel, J. ILCYM-Insect Life Cycle Modeling: A Software Package for Developing Temperature-Based Insect Phenology Models with Applications for Local, Regional and Global Analysis of Insect Population and Mapping; International Potato Center: Lima, Peru, 2013; 193p. [Google Scholar]
- Lin, M.Y. Temperature-dependent life history of Oligonychus mangiferus (Acari: Tetranychidae) on Mangifera indica. Exp. Appl. Acarol. 2013, 61, 403–413. [Google Scholar] [CrossRef]
- Lin, M.Y.; Lin, C.H.; Lin, Y.P.; Tseng, C.T. Temperature-dependent life history of Eutetranychus africanus (Acari: Tetranychidae) on papaya. Syst. Appl. Acarol. 2020, 25, 479–490. [Google Scholar] [CrossRef]
- Rismayani; Ullah, M.S.; Chi, H.; Gotoh, T. Impact of constant and fluctuating temperatures on population characteristics of Tetranychus pacificus (Acari: Tetranychidae). J. Econ. Entomol. 2021, 114, 638–651. [Google Scholar] [CrossRef]
- Baker, P.S. The Coffee Berry Borer in Colombia: Final Report of the DFID-Cenicafé-CABI Bioscience IPM for Coffee Project (CNTR 93/1536A); CABI: Wallingford, UK, 1999; 144p. [Google Scholar]
- Giraldo-Jaramillo, M.; Garcia, A.G.; Parra, J.R. Biology, thermal requirements, and estimation of the number of generations of Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Curculionidae) in the State of São Paulo, Brazil. J. Econ. Entomol. 2018, 111, 2192–2200. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Morgan, D.J.; Hoddle, M.S. The effects of constant and fluctuating temperatures on development of Diaphorina citri (Hemiptera: Liviidae), the Asian citrus psyllid. J. Econ. Entomol. 2020, 113, 633–645. [Google Scholar] [CrossRef]
- Friederichs, K. Bionomische gegevens omtrent den koffiebessenboeboek. Meded. Koffiebessenboeboek-Fonds 1924, 11, 261–286. (In Dutch) [Google Scholar]
- Bergamin, J. Contribuição para o conhecimento da biologia da broca do café Hypothenemus hampei (Ferrari, 1867) (Col. Ipidae). Arq. Inst. Biológico 1943, 14, 31–72. (In Portuguese) [Google Scholar]
- Ding, H.Y.; Lin, Y.Y.; Tuan, S.J.; Tang, L.C.; Chi, H.; Atlıhan, R.; Özgökçe, M.S.; Güncan, A. Integrating demography, predation rate, and computer simulation for evaluation of Orius strigicollis as biological control agent against Frankliniella intonsa. Entomol. Gen. 2020, 41, 179–196. [Google Scholar] [CrossRef]




| Interval for Observation | Cumulative Fecundity (Eggs/Female) | ||
|---|---|---|---|
| Mean ± SE | |||
| 0–10 Days | 0–20 Days | 0–30 Days | |
| OB1 1 | 0.40 ± 0.26 c 2 | 0.55 ± 0.29 c | 0.55 ± 0.29 c |
| OB5 | 5.95 ± 1.39 b | 7.95 ± 1.83 b | 8.45 ± 1.96 b |
| OB10 | 12.95 ± 1.45 a | 14.60 ± 1.64 a | 19.35 ± 2.57 a |
| F | 28.82 | 24.18 | 25.37 |
| df | 2, 57 | 2, 57 | 2, 57 |
| P | <0.0001 | <0.0001 | <0.0001 |
| Temperature (°C) | Developmental Duration (Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | ||||||||
| n | Egg | n | Larva | n | Pupa | n | Immature | |
| 18 | 91 | 10.46 ± 0.22 d | 62 | 37.97 ± 1.17 c | 62 | 11.40 ± 0.14 d | 62 | 59.85 ± 1.17 d |
| 21 | 95 | 6.04 ± 0.10 c | 81 | 23.48 ± 0.88 b | 80 | 6.20 ± 0.06 c | 80 | 35.86 ± 0.89 c |
| 24 | 98 | 5.65 ± 0.11b c | 75 | 20.45 ± 0.62 ab | 73 | 5.71 ± 0.05 b | 73 | 31.93 ± 0.63 b |
| 27 | 106 | 5.19 ± 0.10 b | 54 | 19.15 ± 0.75 a | 45 | 4.58 ± 0.11 a | 45 | 27.64 ± 0.71 a |
| 30 | 71 | 2.51 ± 0.13 a | - | - | - | |||
| F | 397.32 | 89.57 | 1045.92 | 239.19 | ||||
| df | 4, 456 | 3, 268 | 3, 256 | 3, 256 | ||||
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Temperature (°C) | Sex | Developmental Duration (Day) | ||||
|---|---|---|---|---|---|---|
| Mean ± SE | ||||||
| n | Egg | Larva | Pupa | Immature | ||
| 18 | ♀♀ | 52 | 10.29 ± 0.25 a | 40.19 ± 1.15 b | 11.40 ± 0.16 a | 61.88 ± 1.18 b |
| ♂♂ | 10 | 11.50 ± 1.07 a | 26.40 ± 0.86 a | 11.40 ± 0.22 a | 49.30 ± 1.36 a | |
| 21 | ♀♀ | 69 | 6.00 ± 0.12 a | 24.91 ± 0.92 b | 6.23 ± 0.07 a | 37.14 ± 0.92 b |
| ♂♂ | 11 | 6.73 ± 0.27 b | 15.09 ± 1.10 a | 6.00 ± 0.13 a | 27.82 ± 1.16 a | |
| 24 | ♀♀ | 59 | 5.95 ± 0.13 a | 20.73 ± 0.57 a | 5.78 ± 0.05 b | 32.46 ± 0.58 a |
| ♂♂ | 14 | 5.86 ± 0.14 a | 18.43 ± 2.21 a | 5.43 ± 0.14 a | 29.71 ± 2.20 a | |
| 27 | ♀♀ | 38 | 4.97 ± 0.18 a | 18.32 ± 0.82 a | 4.63 ± 0.12 a | 27.92 ± 0.80 a |
| ♂♂ | 7 | 5.86 ± 0.14 b | 16.00 ± 1.40 a | 4.29 ± 0.29 a | 26.14 ± 1.47 a | |
| Sex | Stage | Regression Equation | p | R2 | T0 (°C) | K (DD) |
|---|---|---|---|---|---|---|
| ♀♀ + ♂♂ | Egg | 0.0882 | 0.8314 | 6.88 | 99.11 | |
| Larva | 0.0573 | 0.8887 | 7.31 | 357.14 | ||
| Pupa | 0.0361 | 0.9292 | 10.64 | 73.91 | ||
| Immature | 0.0352 | 0.9309 | 8.91 | 485.44 | ||
| ♀♀ | Egg | 0.0752 | 0.8553 | 5.65 | 73.91 | |
| Larva | 0.0218 | 0.9569 | 9.56 | 308.64 | ||
| Pupa | 0.0379 | 0.9257 | 10.47 | 75.53 | ||
| Immature | 0.0278 | 0.9451 | 9.39 | 476.19 | ||
| ♂♂ | Egg | 0.1087 | 0.7945 | 6.67 | 109.77 | |
| Larva | 0.3664 | 0.4015 | −4.29 | 485.44 | ||
| Pupa | 0.0303 | 0.9404 | 11.41 | 66.01 | ||
| Immature | 0.1729 | 0.6841 | 3.88 | 581.40 |
| Temperature (°C) | Longevity (Day) | Parameters of Fecundity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | ||||||||
| n | ♀♀ | n | ♂♂ | n | APOP 1 (Day) | TPOP 2 (Day) | Ovi-Days 3 (Day) | Fecundity (Eggs/Female) | |
| 18 | 52 | 115.77 ± 6.26 aA | 10 | 26.50 ± 7.89 aB | 17 | 23.82 ± 4.80 b | 83.06 ± 4.51 c | 18.82 ± 4.67 b | 8.18 ± 2.70 c |
| 21 | 69 | 95.51 ± 4.83 bA | 11 | 20.91 ± 4.41 aB | 61 | 14.75 ± 2.49 ab | 51.54 ± 2.63 b | 19.75 ± 2.39 b | 12.33 ± 2.03 bc |
| 24 | 59 | 97.63 ± 4.81 abA | 14 | 12.50 ± 2.81 aB | 45 | 23.00 ± 3.48 b | 56.29 ± 3.41 b | 32.67 ± 3.36 a | 29.00 ± 3.50 a |
| 27 | 38 | 53.42 ± 4.07 cA | 7 | 14.29 ± 3.35 aB | 38 | 8.95 ± 1.91 a | 36.87 ± 2.17 a | 21.71 ± 2.31 ab | 21.84 ± 2.67 ab |
| F | 19.89 | 1.75 | 4.65 | 22.36 | 4.74 | 9.70 | |||
| df | 3, 214 | 3, 38 | 3, 157 | 3, 157 | 3, 157 | 3, 157 | |||
| P | <0.0001 | 0.1739 | 0.0038 | <0.0001 | 0.0034 | <0.0001 | |||
| Temperature (°C) | Population Parameters | |||
|---|---|---|---|---|
| Mean ± SE | ||||
| Net Reproductive Rate R0 (Eggs/Individual) | Intrinsic Rate of Increase r (Day −1) | Finite Rate of Increase λ (Day −1) | Mean Generation Time T (Day) | |
| 18 | 1.53 ± 0.59 c | 0.0036 ± 0.0037 c | 1.0036 ± 0.0037 c | 117.57 ± 13.08 d |
| 21 | 7.92 ± 1.43 b | 0.0284 ± 0.0025 b | 1.0288 ± 0.0026 b | 72.89 ± 4.68 b |
| 24 | 13.32 ± 2.15 a | 0.0297 ± 0.0021 b | 1.0301 ± 0.0022 b | 87.28 ± 4.36 c |
| 27 | 7.83 ± 1.39 b | 0.0401 ± 0.0040 a | 1.0409 ± 0.0041 a | 51.34 ± 2.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.-H.; Wang, L.-J.; Lin, M.-Y. Temperature-Dependent Biology and Population Performances of the Coffee Berry Borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) on Artificial Diet. Insects 2023, 14, 499. https://doi.org/10.3390/insects14060499
Wei S-H, Wang L-J, Lin M-Y. Temperature-Dependent Biology and Population Performances of the Coffee Berry Borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) on Artificial Diet. Insects. 2023; 14(6):499. https://doi.org/10.3390/insects14060499
Chicago/Turabian StyleWei, Shao-Hua, Liang-Jong Wang, and Ming-Ying Lin. 2023. "Temperature-Dependent Biology and Population Performances of the Coffee Berry Borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) on Artificial Diet" Insects 14, no. 6: 499. https://doi.org/10.3390/insects14060499





