Energy Allocation of the Wolf Spider Pardosa pseudoannulata under Dietary Restriction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Dietary Restriction Setting
2.3. Experiment 1: Effects of Dietary Restriction on the Growth of P. pseudoannulata
2.3.1. Determination of Developmental Period
2.3.2. Determination of Carapace Length, Carapace Width, Body Length, and Weight
2.4. Experiment 2: Effects of Dietary Restriction on Longevity of P. pseudoannulata
2.5. Experiment 3: Effects of Dietary Restriction on the Predation Function of P. pseudoannulata
2.6. Experiment 4: Effects of Dietary Restriction on Water, Fat and Protein Content in the Body of P. pseudoannulata
2.7. Data Analyses
in chloroform)/(dry weight before soaking in chloroform) × 100%
3. Results
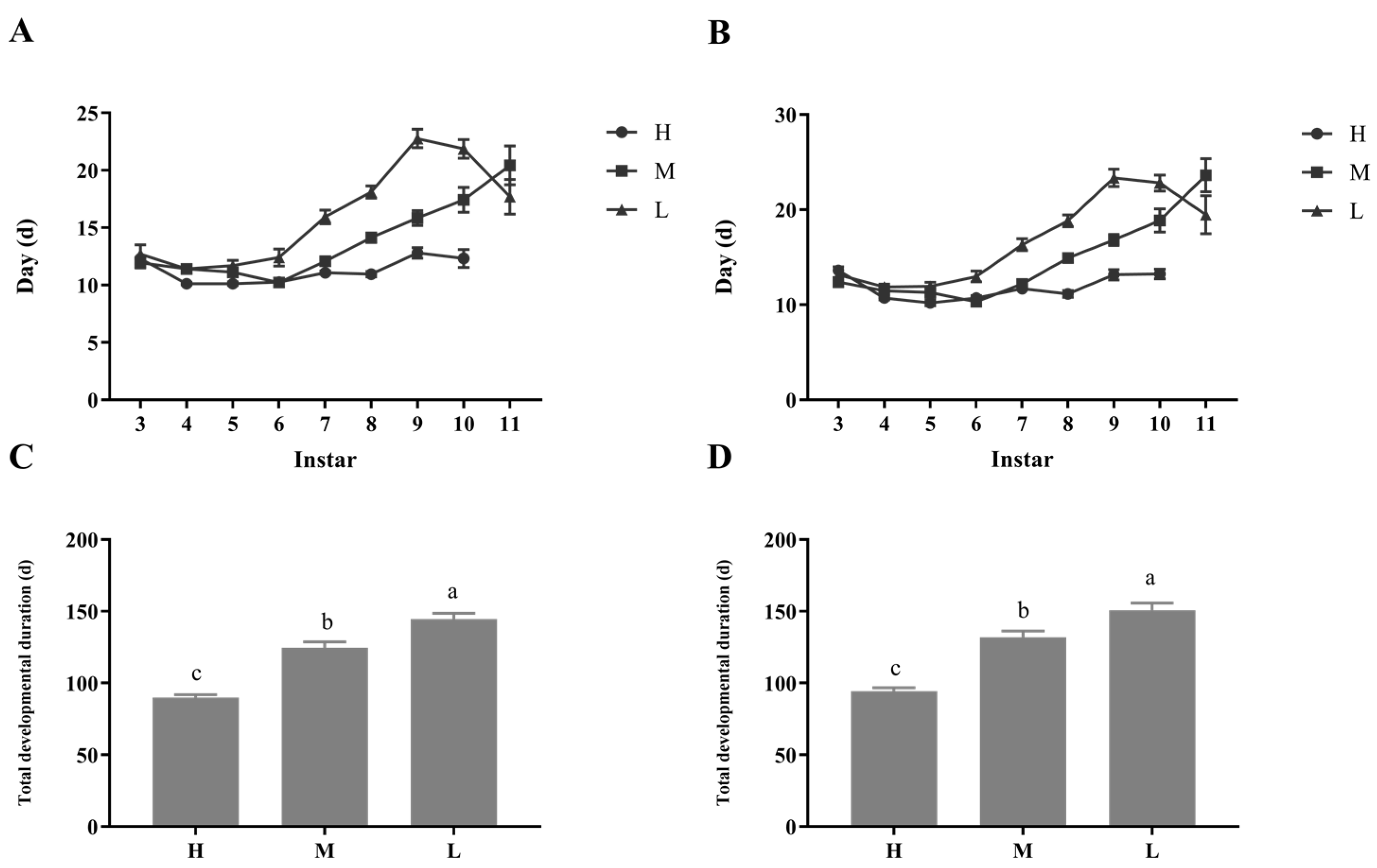
3.1. Experiment 1: Effects of Dietary Restriction on the Growth of P. pseudoannulata
Determination of Developmental Period
3.2. Determination of Carapace Length, Carapace Width, Body Length, and Weight
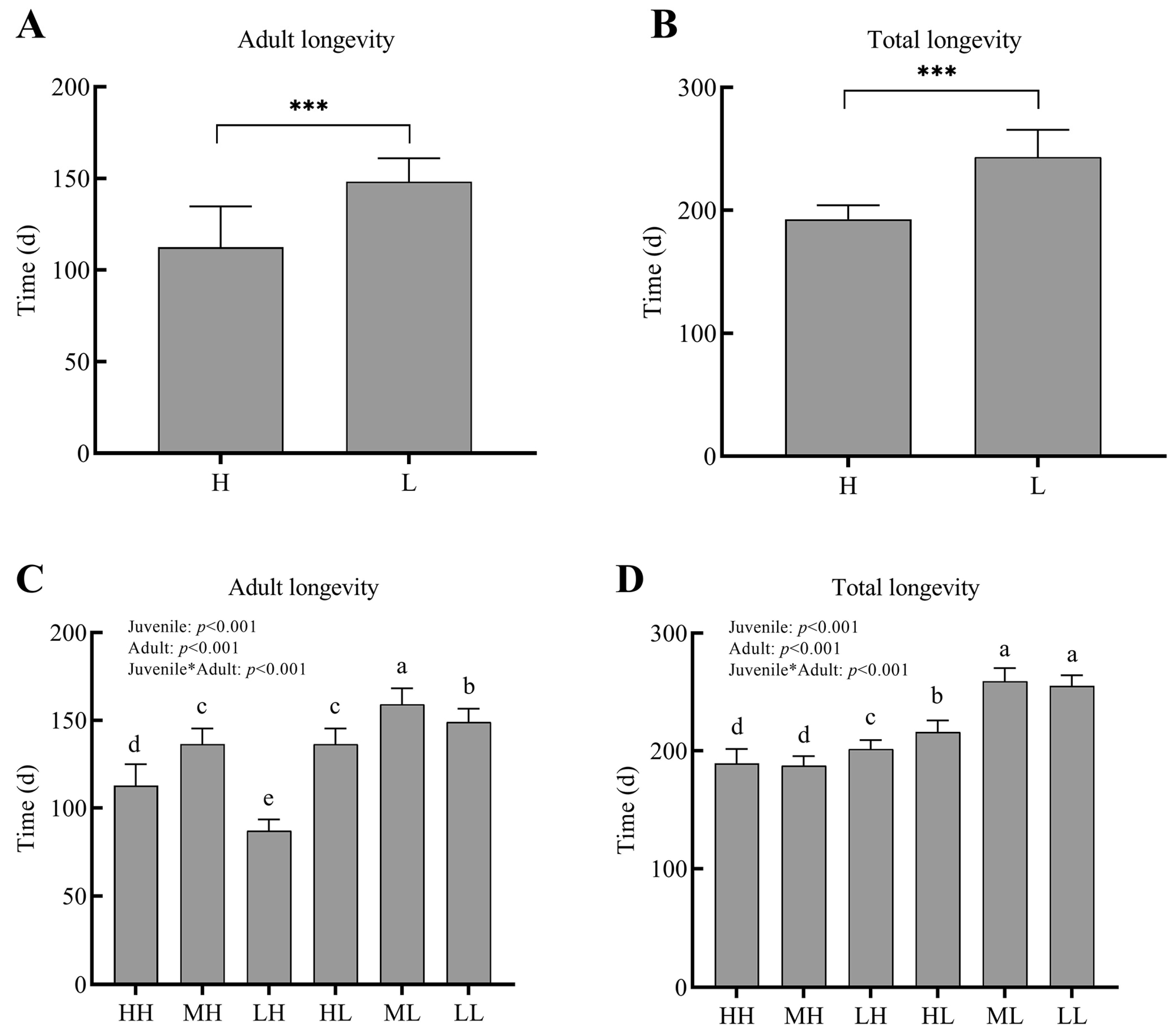
3.3. Experiment 2: Effects of Dietary Restrictions on the Longevity of P. pseudoannulata
3.4. Experiment 3: Effects of Dietary Restrictions on the Predation Function of P. pseudoannulata
3.5. Experiment 4: Effects of Dietary Restriction on Water, Fat and Protein Content in P. pseudoannulata
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chung, K.W.; Kim, D.H.; Park, M.H.; Choi, Y.J.; Kim, N.D.; Lee, J.; Yu, B.P.; Chung, H.Y. Recent advances in calorie restriction research on aging. Exp. Gerontol. 2013, 48, 1049–1053. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapahi, P.; Kaeberlein, M.; Hansen, M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev. 2017, 39, 3–14. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.J.; Jung, H.J.; Noh, S.G.; Yu, B.P.; Choi, Y.J.; Chung, H.Y. Anti-aging effects of calorie restriction (CR) and CR mimetics based on the senoinflammation concept. Nutrients 2020, 12, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.R. Why does caloric restriction increase life and healthspan? The ‘clean cupboards’ hypothesis. Natl. Sci. Rev. 2020, 7, 1153–1156. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Diet studies challenge thinking on proteins versus carbs. Science 2014, 343, 1068. [Google Scholar] [CrossRef]
- Lee, B.C.; Kaya, A.; Ma, S.; Kim, G.; Gerashchenko, M.V.; Yim, S.H.; Hu, Z.; Harshman, L.G.; Gladyshev, V.N. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014, 5, 3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 1989, 5, 155–171. [Google Scholar]
- Klass, M.R. Aging in the nematode Caenorhabditis elegans: Major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977, 6, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.C.; Jaruga, E.; Repnevskaya, M.V.; Jazwinski, S.M. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000, 14, 2135–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, J.R.; Liedo, P.; Harshman, L.; Zhang, Y.; Müller, H.-G.; Partridge, L.; Wang, J.-L. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell 2002, 1, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletcher, S.D.; Macdonald, S.J.; Marguerie, R.; Certa, U.; Stearns, S.C.; Goldstein, D.B.; Partridge, L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 2002, 12, 712–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, W.; Goymer, P.; Scott, P.D.; Partridge, L. Demography of dietary restriction and death in Drosophila. Science 2003, 301, 1731–1733. [Google Scholar] [CrossRef] [Green Version]
- Cooper, T.M.; Mockett, R.J.; Sohal, B.H.; Sohal, R.S.; Orr, W.C. Effect of caloric restriction on the life span of housefly, Musca domestica. FASEB J. 2004, 18, 1591. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.D.S.; Gil, L.H.S. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar. J. 2012, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Segoli, M.; Lubin, Y.; Harari, A.R. The effect of dietary restriction on the lifespan of males in a web-building spider. Evol. Ecol. Res. 2007, 9, 697–704. [Google Scholar]
- Austad, S.N. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp. Gerontol. 1989, 24, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kasumovic, M.M.; Brooks, R.C.; Andrade, M.C.B. Body condition but not dietary restriction prolongs lifespan in a semelparous capital breeder. Biol Lett. 2009, 5, 636–638. [Google Scholar] [CrossRef]
- Auer, S.K.; Arendt, J.D.; Chandramouli, R.; Reznick, D.N. Juvenile compensatory growth has negative consequences for reproduction in Trinidadian guppies (Poecilia reticulata). Ecol. Lett. 2010, 13, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F. Responses to starvation in the spiders Lycosa lenta hentz and Filistata hibernalis (Hentz). Ecology 1974, 55, 576–585. [Google Scholar] [CrossRef]
- Totzke, U.; Fenske, M.; Hüppop, O.; Raabe, H.; Schach, N. The influence of fasting on blood and plasma composition of herring gulls (Larus argentatus). Physiol. Biochem. Zool. 1999, 72, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Platt, B.; Delibegović, M. Effects of dietary restriction on metabolic and cognitive health. Proc. Nutr. Soc. 2020, 80, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Maghami, R.; Rafiee-Dastjerdi, H.; Golizadeh, A.; Yazdanian, M.; Enkegaard, A. Predation activity of Chrysoperla carnea (Neuroptera: Chrysopidae) upon Aphis fabae (Hemiptera: Aphididae): Effect of different hunger levels. J. Asia Pac. Entomol. 2015, 18, 297–302. [Google Scholar] [CrossRef]
- Kang, N.Y.; Powers, D.R.; Vermette, M.L.; Tsai, O.H.-I.; Williams, T.D. Physiological adjustments to high foraging effort negatively affect fecundity but not final reproductive output in captive zebra finches. J. Exp. Biol. 2021, 224, jeb.235820. [Google Scholar]
- Roux, O.; Renault, D.; Mouline, K.; Diabaté, A.; Simard, F. Living with predators at the larval stage has differential long-lasting effects on adult life history and physiological traits in two anopheline mosquito species. J. Insect. Physiol. 2021, 131, 104234. [Google Scholar] [CrossRef]
- Treidel, L.A.; Clark, R.M.; Lopez, M.T.; Williams, C.M. Physiological demands and nutrient intake modulate a trade-off between dispersal and reproduction based on age and sex of field crickets. J. Exp. Biol. 2021, 224, jeb237834. [Google Scholar] [CrossRef] [PubMed]
- Scharf, I. The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 2016, 119, 37–48. [Google Scholar] [CrossRef]
- Eric, C. Yip and Yael Lubin. Effects of diet restriction on life history in a sexually cannibalistic spider. Biol. J. Linn. Soc. 2016, 118, 410–420. [Google Scholar]
- Liu, J.; Sun, L.; Fu, D.; Zhu, J.; Liu, M.; Xiao, F.; Xiao, R. Herbivore-Induced Rice Volatiles Attract and Affect the Predation Ability of the Wolf Spiders, Pirata subpiraticus and Pardosa pseudoannulata. Insects 2022, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Effendi, K.; Munif, A.; Winasa, I.W. Survey of rice pests, diseases and natural enemies on “Upsus” program in Karawang district, West Java Province. J. Perlindungan Tanam. Indones. 2020, 24, 17–27. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [Green Version]
- Lv, B.; Yang, H.L.; Wang, J.; Zeng, Z.; Li, N.; Tang, Y.E.; Peng, Y.D.; Wang, Z.; Song, Q.S. Cadmium exposure alters expression of protective enzymes and protein processing genes in venom glands of the wolf spider Pardosa pseudoannulata. Environ. Pollut. 2021, 268, 115847. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.Y.; Peng, Y.D.; Huang, T.; Yang, H.L.; Peng, X.J.; Xie, C.L.; Xiang, X.; Sun, Z.Y.; Wang, Z.; et al. Transcriptome analysis reveals the molecular response to cadmium toxicity in P. pseudoannulata. Environ. Sci. Pollut. Res. 2018, 25, 34294–34305. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, L.; Ma, J.; Yu, L.; Li, C.; Jiao, X. The effects of prey lipid on female mating and reproduction of a wolf spider. Curr. Zool. 2022, 68, 726–733. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Zhang, G.; Zhao, Y.; Peng, Y.; Yun, Y. Transmission of nanoplastics from Culex quinquefasciatus to Pardosa pseudoannulata and its impact on predators. Sci. Total Environ. 2022, 820, 153331. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C. Some Characteristics of Simple Types of Predation and Parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Saeed, T.; Guirao, J.L.; Sabir, Z.; Alsulami, H.H.; Sánchez, Y.G. A computational approach to solve the nonlinear biological prey-predator system. Fractals 2022, 30, 2240267. [Google Scholar] [CrossRef]
- Ding, Y.X. Insect Mathematical Ecology; Science Press: Beijing, China, 1994. [Google Scholar]
- Luo, J.; Cheng, Y.; Guo, L.; Wang, A.; Lu, M.; Xu, L. Variation of gut microbiota caused by an imbalance diet is detrimental to bugs’ survival. Sci. Total Environ. 2021, 771, 144880. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Holmes, A.J.; Zhang, W.; Hu, D.; Shao, Q.; Wang, Z.; Lu, J.; Raubenheimer, D. Seasonal diet and microbiome shifts in wild rhesus macaques are better correlated at the level of nutrient components than food items. Integr. Zool. 2022, 17, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.S. Caloric restriction in nonmammalian models. J. Anti. Aging Med. 2001, 4, 205–213. [Google Scholar] [CrossRef]
- Mayntz, D.; Toft, S.; Vollrath, F. Effects of prey quality and availability on the life history of a trap-building predator. Oikos 2003, 101, 631–638. [Google Scholar] [CrossRef]
- Fairbairn, D.J.; Wolf, U. Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Grula, C.C.; Rinehart, J.P.; Greenlee, K.J.; Bowsher, J.H. Body size allometry impacts flight-related morphology and metabolic rates in the solitary bee Megachile rotundata. J. Insect Physiol. 2021, 133, 104275. [Google Scholar] [CrossRef]
- Hagstrum, D.W. Carapace width as a tool for evaluating the rate of development of spiders in the laboratory and the field. Ann. Entomol. Soc. Am. 1971, 64, 757–760. [Google Scholar] [CrossRef]
- Gonzaga, M.D.O.; Vasconcellos-Neto, J. Influence of collective feeding on weight gain and size variability of Anelosimus jabaquara Levi 1956 (Araneae: Theridiidae). Behaviour 2002, 139, 1431–1442. [Google Scholar] [CrossRef] [Green Version]
- Kleinteich, A.; Wilder, S.M.; Schneider, J.M. Contributions of juvenile and adult diet to the lifetime reproductive success and lifespan of a spider. Oikos 2015, 124, 130–138. [Google Scholar] [CrossRef]
- Yip, E.C.; Levy, T.; Lubin, Y. Bad neighbors: Hunger and dominance drive spacing and position in an orb-weaving spider colony. Behav. Ecol. Sociobiol. 2017, 71, 128. [Google Scholar] [CrossRef]
- Jespersen, L.B.; Toft, S. Compensatory growth following early nutritional stress in the wolf spider Pardosa prativaga. Funct. Ecol. 2003, 17, 737–746. [Google Scholar] [CrossRef]
- Pijpe, J.; Brakefield, P.M.; Zwaan, B.J. Increased life span in a polyphenic butterfly artificially selected for starvation resistance. Am. Nat. 2008, 171, 81–90. [Google Scholar] [CrossRef]
- Dmitriew, C.; Rowe, L. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef]
- He, Y.P.; Chen, L.; Chen, J.M.; Chen, L.Z.; Zhang, Y.F. Effects of pymetrozine on the feeding behaviour of rice brown planthopper, Nilaparvata lugens (Still). Zhongguo Shuidao Kexue 2010, 24, 635–640. (In Chinese) [Google Scholar]
- Moya-Laraño, J.; Orta-Ocaña, J.M.; Barrientos, J.A.; Bach, C.; Wish, D.H. Intriguing compensation by adult female spiders for food limitation experienced as juveniles. Oikos 2003, 101, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.L.; Findsen, A.; Overgaard, J. Feeding impairs chill coma recovery in the migratory locust (Locusta migratoria). J. Insect Physiol. 2013, 59, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, É. Fasting can protect young and middle-aged Drosophila melanogaster flies against a severe cold stress. Biogerontology 2013, 14, 513–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandi, C.; Pinelo-Nava, M.T. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural. Plast. 2007, 2007, 078970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, C.R.; Anisman, H. Acute and chronic stress effects on performance in a forced-swim task. Behav. Neural Biol. 1984, 42, 99–119. [Google Scholar] [CrossRef]
- Rehman, N.; Varghese, J. Larval nutrition influences adult fat stores and starvation resistance in Drosophila. PLoS ONE 2021, 16, e0247175. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Dutil, J.D.; Godbout, G.; Munro, J. Survival and condition of hard shell male adult snow crabs (Chionoecetes opilio) during fasting at different temperatures. Aquaculture 2000, 189, 259–275. [Google Scholar] [CrossRef]
- Xue, M.; Ke, C.H.; Wei, Y.J. Effects of starvation on biochemical compositions and digestive enzyme activities of spotted babylon, Babylonia areolata juveniles. Re Dai Hai Yang Xue Bao 2010, 29, 120–125. (In Chinese) [Google Scholar]

| Treatment | Fitting Equation | R2 | a′ ± SE | Th | 1/Th | a′/Th |
|---|---|---|---|---|---|---|
| HH | Na = 0.89N/(1 + 0.014 N) | 0.957 | 0.89 ± 0.06 c | 0.016 | 62.5 | 55.63 |
| HL | Na = N/(1 + 0.005 N) | 0.986 | 1 ± 0.03 b | 0.005 | 200 | 200 |
| MH | Na = 1.2N/(1 + 0.014 N) | 0.889 | 1.2 ± 0.64 a | 0.012 | 83 | 100 |
| ML | Na = N/(1 + 0.014 N) | 0.983 | 1 ± 0.56 b | 0.014 | 71.43 | 71.43 |
| LH | Na = 0.95N/(1 + 0.037 N) | 0.900 | 0.95 ± 0.33 c | 0.039 | 25.64 | 24.36 |
| LL | Na = 0.89N/(1 + 0.016 N) | 0.953 | 0.89 ± 0.14 c | 0.018 | 55.56 | 49.44 |
| Treatments | Water (% of Wet Weight) | Fat (% of Dry Weight) | Protein (g/L) |
|---|---|---|---|
| HH | 69.92 ± 0.007 b | 14.66 ± 0.22 a | 4.88 ± 0.88 a |
| HL | 73.85 ± 0.006 a | 10.49 ± 0.02 ab | 4.36 ± 0.23 a |
| MH | 70.36 ± 0.01 b | 10.49 ± 0.02 ab | 1.42 ± 0.22 b |
| ML | 75.31 ± 0.01 a | 8.5 ± 0.02 ab | 1.25 ± 0.13 b |
| LH | 69.94 ± 0.005 b | 14.52 ± 0.008 a | 3.5 ± 0.23 a |
| LL | 75.19 ± 0.009 a | 5.6 ± 0.02 b | 3.1 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Song, L.; Chen, L.; Yun, Y.; Zhang, W.; Zhao, Y.; Peng, Y. Energy Allocation of the Wolf Spider Pardosa pseudoannulata under Dietary Restriction. Insects 2023, 14, 579. https://doi.org/10.3390/insects14070579
Zhu Y, Song L, Chen L, Yun Y, Zhang W, Zhao Y, Peng Y. Energy Allocation of the Wolf Spider Pardosa pseudoannulata under Dietary Restriction. Insects. 2023; 14(7):579. https://doi.org/10.3390/insects14070579
Chicago/Turabian StyleZhu, Yang, Li Song, Limi Chen, Yueli Yun, Wang Zhang, Yao Zhao, and Yu Peng. 2023. "Energy Allocation of the Wolf Spider Pardosa pseudoannulata under Dietary Restriction" Insects 14, no. 7: 579. https://doi.org/10.3390/insects14070579






