Comparative Transcriptomic Analysis Reveals Adaptation Mechanisms of Bean Bug Riptortus pedestris to Different Food Resources
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Plant
2.2. Observation of Salivary Sheath
2.3. Sample Collection and Transcriptome Sequencing
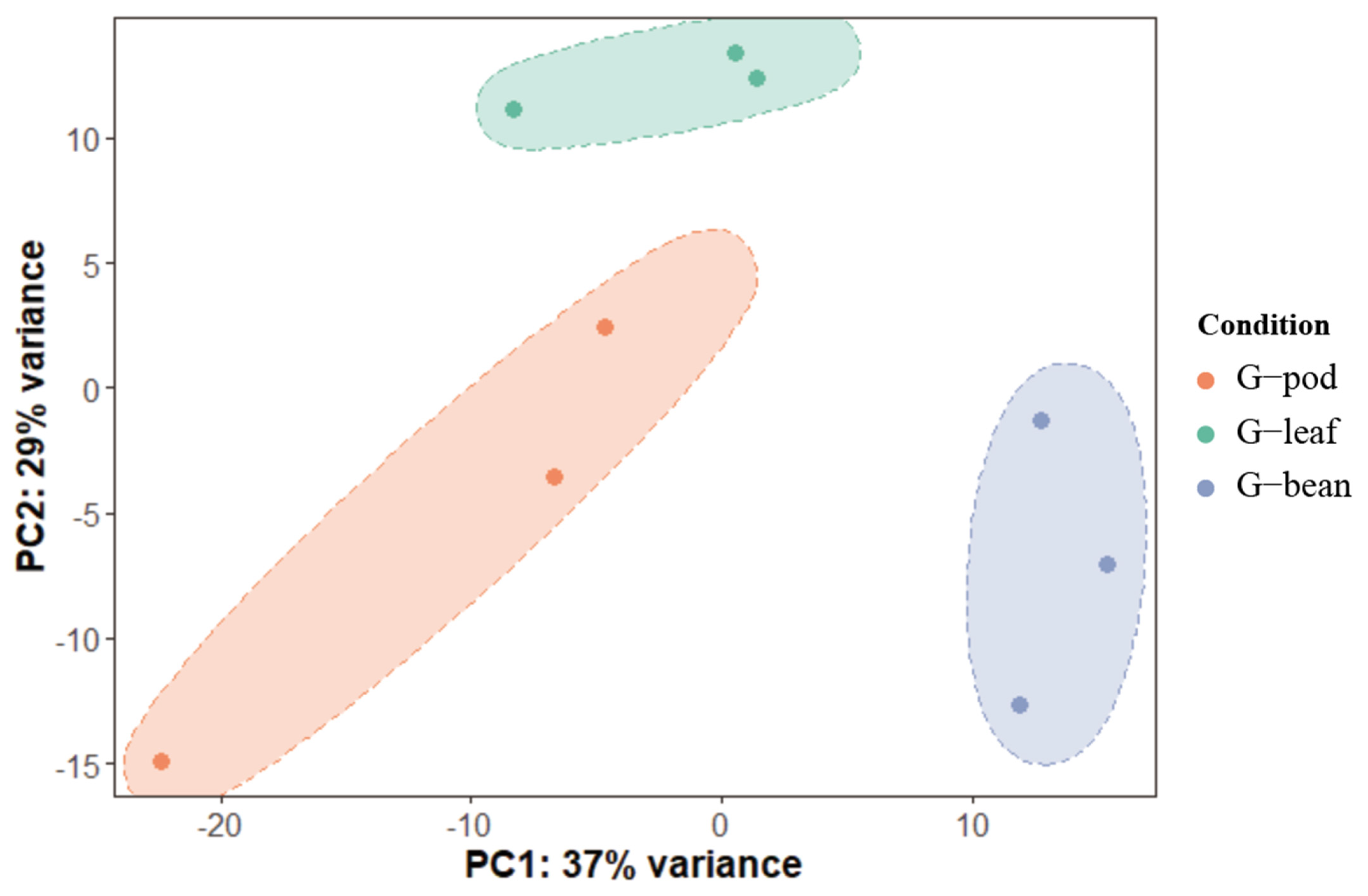
2.4. Identification of Differentially Expressed Genes (DEGs) and Principal Component Analysis (PCA)
2.5. Enrichment Analysis
2.6. qRT-PCR Analysis
3. Results
3.1. Infestation of R. pedestris on Different Food Resources
3.2. Overview of RNA Sequencing Data
3.3. Analysis of Differently Expressed Genes (DEGs)
3.4. Expression Profiles of DEGs Accsociated with Detoxificatiion and Digestion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Huang, J.Y.; Li, L.Y.; Huang, K.Y.; Zhang, Y.T.; Li, Y.L.; Deng, Z.Y.; Ni, X.Z.; Li, X.C. Inductive and synergistic interactions between plant allelochemical flavone and Bt toxin Cry1Ac in Helicoverpa armigera. Insect Sci. 2021, 28, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Vontas, J.; Van Leeuwen, T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Curr. Opin. Insect Sci. 2018, 27, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, Y.Y.; Zeng, R.S. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Chen, M.S.; Liu, S.; Wang, H.; Cheng, X.; El Bouhssini, M.; Whitworth, R.J. Genes expressed differentially in Hessian fly larvae feeding in resistant and susceptible plants. Int. J. Mol. Sci. 2016, 17, 1324. [Google Scholar] [CrossRef]
- Mao, K.K.; Ren, Z.J.; Li, W.H.; Cai, T.W.; Qin, X.Y.; Wan, H.; Jin, B.R.; He, S.; Li, J.H. Carboxylesterase genes in nitenpyram-resistant brown planthoppers, Nilaparvata lugens. Insect Sci. 2021, 28, 1049–1060. [Google Scholar] [CrossRef]
- Wang, M.M.; Long, G.J.; Guo, H.; Liu, X.Z.; Wang, H.; Dewer, Y.; Li, Z.Q.; Liu, K.; Zhang, Q.L.; Ma, Y.F.; et al. Two carboxylesterase genes in Plutella xylostella associated with sex pheromones and plant volatiles degradation. Pest Manag. Sci. 2021, 77, 2737–2746. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, S.; Tan, M.T.; Wang, Q.; Zheng, L.; Yan, S.C. The high adaptability of Hyphantria cunea larvae to cinnamic acid involves in detoxification, antioxidation and gut microbiota response. Pestic. Biochem. Phys. 2021, 174, 104805. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Yan, X.T.; Wei, Z.Y.; Wang, Y.Z.; Chen, J.P.; Li, J.M.; Sun, Z.T.; Zhang, C.X. Identification of Riptortus pedestris salivary proteins and their roles in inducing plant defenses. Biology 2021, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Gao, Y.; Hu, Y.L.; Chen, J.H.; Zhang, J.P.; Shi, S.S. Field cage assessment of feeding damage by Riptortus pedestris on soybeans in China. Insects 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lim, U.T. Effect of food deprivation period on the development and reproduction of Riptortus pedestris (Hemiptera: Alydidae), and its egg parasitism. J. Econ. Entomol. 2014, 107, 1785–1791. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.X.; Guo, J.Q.; Penn, H.; Wu, T.T.; Li, L.; Jiang, H.; Chang, L.D.; Wu, C.X.; Han, T.F. Feeding of Riptortus pedestris on soybean plants, the primary cause of soybean staygreen syndrome in the Huang-Huai-Hai river basin. Crop J. 2019, 7, 360–367. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, Y.; Wang, Z.B.; Ding, W.L.; Xu, K.D.; Li, L.L.; Wang, Y.Y.; Li, J.B.; Yang, M.S.; Liu, X.M.; et al. Modelling the current and future potential distribution of the bean bug Riptortus pedestris with increasingly serious damage to soybean. Pest Manag. Sci. 2022, 78, 4340–4352. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, Y.P.; Wang, X.J.; Li, X.M.; Dong, S.K. Physiology and metabonomics reveal differences in drought resistance among soybean varieties. Bot. Stud. 2022, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, D.S.; Li, M.X.; Shi, L.X. Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef]
- Lu, H.B.; Lu, J.B.; Li, L.L.; Zhang, Z.L.; Chen, J.P.; Li, J.M.; Zhang, C.X.; Huang, H.J. Functional analysis of neutral lipases in bug feeding and reproduction. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef]
- Huang, H.-J.; Cui, J.-R.; Hong, X.-Y. Comparative analysis of diet-associated responses in two rice planthopper species. BMC Genom. 2020, 21, 565. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, Y.Z.; Li, L.L.; Lu, H.B.; Lu, J.B.; Wang, X.; Ye, Z.X.; Zhang, Z.L.; He, Y.J.; Lu, G.; et al. Planthopper salivary sheath protein LsSP1 contributes to manipulation of rice plant defenses. Nat. Commun. 2023, 14, 737. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Babraham, UK, 2014. [Google Scholar]
- Huang, H.J.; Ye, Y.X.; Ye, Z.X.; Yan, X.T.; Wang, X.; Wei, Z.Y.; Chen, J.P.; Li, J.M.; Sun, Z.T.; Zhang, C.X. Chromosome-level genome assembly of the bean bug Riptortus pedestris. Mol. Ecol. Resour. 2021, 21, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhou, S.; Jander, G. Engineering insect resistance using plant specialized metabolites. Curr. Opin. Biotechnol. 2021, 70, 115–121. [Google Scholar] [CrossRef]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M.; Hao, P.; Yang, Z.; Zhu, L.; He, G. Penetration into rice tissues by brown planthopper and fine structure of the salivary sheaths. Entomol. Exp. Appl. 2008, 129, 295–307. [Google Scholar] [CrossRef]
- Tian, X.Y.; Hu, Y.L.; Li, W.B.; Gao, Y.; Shi, S.S. Effects of developmental stages soybean pods on the adult longevity and fecundity of Riptortus pedestris (Hemiptera: Alydidae). Acta Entomol. Sin. 2022, 65, 749–756. [Google Scholar]
- Rahman, M.M.; Lim, U.T. Evaluation of mature soybean pods as a food source for two pod-sucking bugs, Riptortus pedestris (Hemiptera: Alydidae) and Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2017, 12, e0176187. [Google Scholar] [CrossRef] [PubMed]
- Dvoryakova, E.A.; Vinokurov, K.S.; Tereshchenkova, V.F.; Dunaevsky, Y.E.; Belozersky, M.A.; Oppert, B.; Filippova, I.Y.; Elpidina, E.N. Primary digestive cathepsins L of Tribolium castaneum larvae: Proteomic identification, properties, comparison with human lysosomal cathepsin L. Insect Biochem. Mol. Biol. 2022, 140, 103679. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.C.; Dias, R.O.; Bifano, T.D.; Genta, F.A.; Ferreira, C.; Terra, W.R. Cathepsins L and B in Dysdercus peruvianus, Rhodnius prolixus, and Mahanarva fimbriolata. Looking for enzyme adaptations to digestion. Insect Biochem. Mol. Biol. 2020, 127, 103488. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tian, Y.; Fang, Y.; Chen, M.L.; Smagghe, G.; Niu, J.; Wang, J.J. A saliva α-glucosidase MpAgC2-2 enhance the feeding of green peach aphid Myzus persicae via extra-intestinal digestion. Insect Biochem. Mol. Biol. 2022, 150, 103846. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Garg, S.; Gupta, L.; Gupta, K.; Diagne, C.T.; Missé, D.; Pompon, J.; Kumar, S.; Saxena, V. Delineating the role of Aedes aegypti ABC transporter gene family during mosquito development and arboviral infection via transcriptome analyses. Pathogens 2021, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Koenig, C.; Bretschneider, A.; Heckel, D.G.; Grosse-Wilde, E.; Hansson, B.S.; Vogel, H. The plastic response of Manduca sexta to host and non-host plants. Insect Biochem. Mol. Biol. 2015, 63, 72–85. [Google Scholar] [CrossRef]
- Strauss, A.S.; Wang, D.; Stock, M.; Gretscher, R.R.; Groth, M.; Boland, W.; Burse, A. Tissue-specific transcript profiling for ABC transporters in the sequestering larvae of the phytophagous leaf beetle Chrysomela populi. PLoS ONE 2014, 9, e98637. [Google Scholar] [CrossRef]
- Birnbaum, S.S.L.; Rinker, D.C.; Gerardo, N.M.; Abbot, P. Transcriptional profile and differential fitness in a specialist milkweed insect across host plants varying in toxicity. Mol. Ecol. 2017, 26, 6742–6761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Wang, Y.; Xie, D. Jasmonate action in plant defense against insects. J. Exp. Bot. 2019, 70, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Kalinger, R.S.; Pulsifer, I.P.; Hepworth, S.R.; Rowland, O. Fatty Acyl synthetases and thioesterases in plant lipid metabolism: Diverse functions and biotechnological applications. Lipids 2020, 55, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.Y.; Kang, Y.G.; Kim, E.H.; Kim, M.; Park, N.H.; Choi, H.T.; Go, G.H.; Lee, J.H.; Park, J.S.; Hong, Y.S. Metabolomics approach for understanding geographical dependence of soybean leaf metabolome. Food Res. Int. 2018, 106, 842–852. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Liu, Y.; Chen, F.; Han, L. Differences in midgut transcriptomes between resistant and susceptible strains of Chilo suppressalis to Cry1C toxin. BMC Genom. 2020, 21, 634. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.D.; Arraes, F.B.M.; Antonino, J.D.; Macedo, L.L.P.; Fonseca, F.C.A.; Togawa, R.C.; Grynberg, P.; Silva, M.C.M.; Negrisoli, A.S., Jr.; Morgante, C.V.; et al. Comparative gut transcriptome analysis of Diatraea saccharalis in response to the dietary source. PLoS ONE 2020, 15, e0235575. [Google Scholar] [CrossRef] [PubMed]
- Herde, M.; Howe, G.A. Host plant-specific remodeling of midgut physiology in the generalist insect herbivore Trichoplusia ni. Insect Biochem. Mol. Biol. 2014, 50, 58–67. [Google Scholar] [CrossRef]
- Fei, S.G.; Xia, J.M.; Ma, G.Y.; Zhang, M.M.; Sun, J.C.; Feng, M.; Wang, Y.Y. Apolipoprotein D facilitate the proliferation of BmNPV. Int. J. Biol. Macromol. 2022, 223, 830–836. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Li, Y.H.; Li, X.T.; Li, R.Q.; Xu, Y.S.; Shi, L.G.; Wang, H.B. Apolipoprotein D in Lepidoptera: Evolution and functional divergence. Biochem. Biophys. Res. Commun. 2020, 526, 472–478. [Google Scholar] [CrossRef]
- Noveen, A.; Jiang, T.X.; Chuong, C.M. cAMP, an activator of protein kinase A, suppresses the expression of sonic hedgehog. Biochem. Biophys. Res. Commun. 1996, 219, 180–185. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-L.; Wang, X.-J.; Lu, H.-B.; Huang, H.-J. Comparative Transcriptomic Analysis Reveals Adaptation Mechanisms of Bean Bug Riptortus pedestris to Different Food Resources. Insects 2023, 14, 739. https://doi.org/10.3390/insects14090739
Zhang Z-L, Wang X-J, Lu H-B, Huang H-J. Comparative Transcriptomic Analysis Reveals Adaptation Mechanisms of Bean Bug Riptortus pedestris to Different Food Resources. Insects. 2023; 14(9):739. https://doi.org/10.3390/insects14090739
Chicago/Turabian StyleZhang, Ze-Long, Xiao-Jing Wang, Hai-Bin Lu, and Hai-Jian Huang. 2023. "Comparative Transcriptomic Analysis Reveals Adaptation Mechanisms of Bean Bug Riptortus pedestris to Different Food Resources" Insects 14, no. 9: 739. https://doi.org/10.3390/insects14090739




