Effects of Sucrose Feeding on the Quality of Royal Jelly Produced by Honeybee Apis mellifera L.
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bees
2.2. Feeding Treatment
2.3. RJ Production
2.4. Stored Food Collection
2.5. Analytical Procedures of RJ and Stored Food
2.6. Scanning Electron Microscopy Analysis
2.7. Determination of Midgut Sucrase Enzyme Activity
2.8. RNA Extraction, cDNA Synthesis, and Reverse Transcription Quantitative PCR
2.9. Statistical Analysis
3. Results
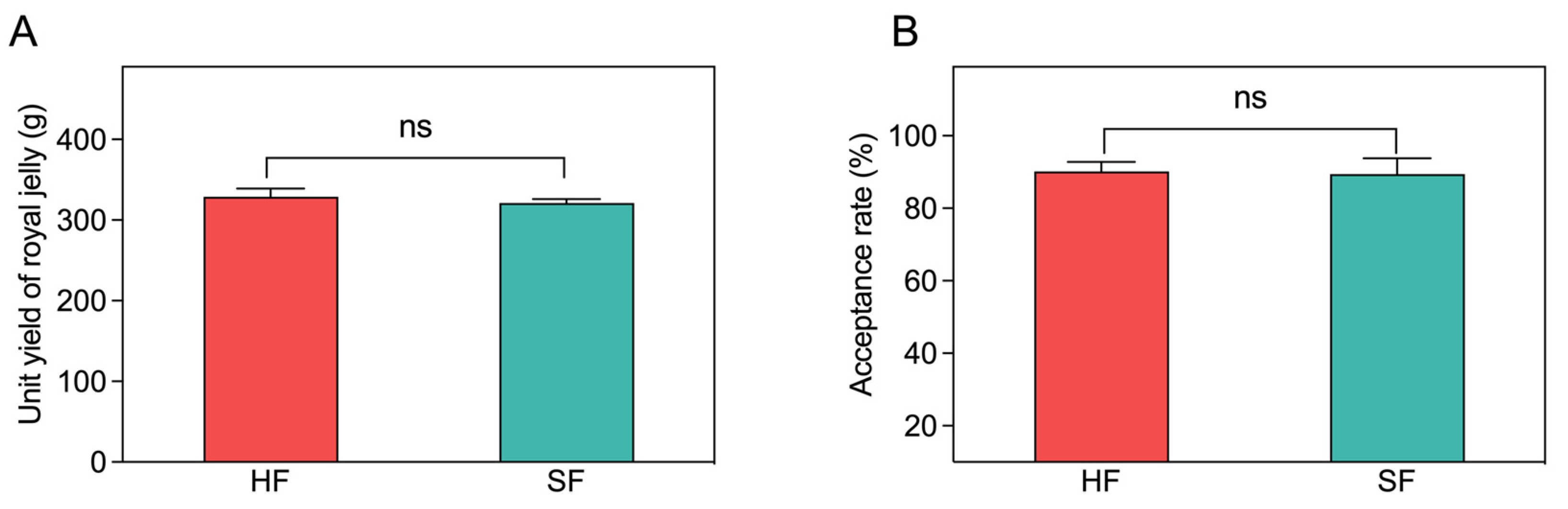
3.1. Unit Yield of Royal Jelly and the Acceptance Rate of the Queen Cells
3.2. Conventional and Mineral Composition of the Royal Jelly
3.3. Conventional and Mineral Composition of the Stored Food
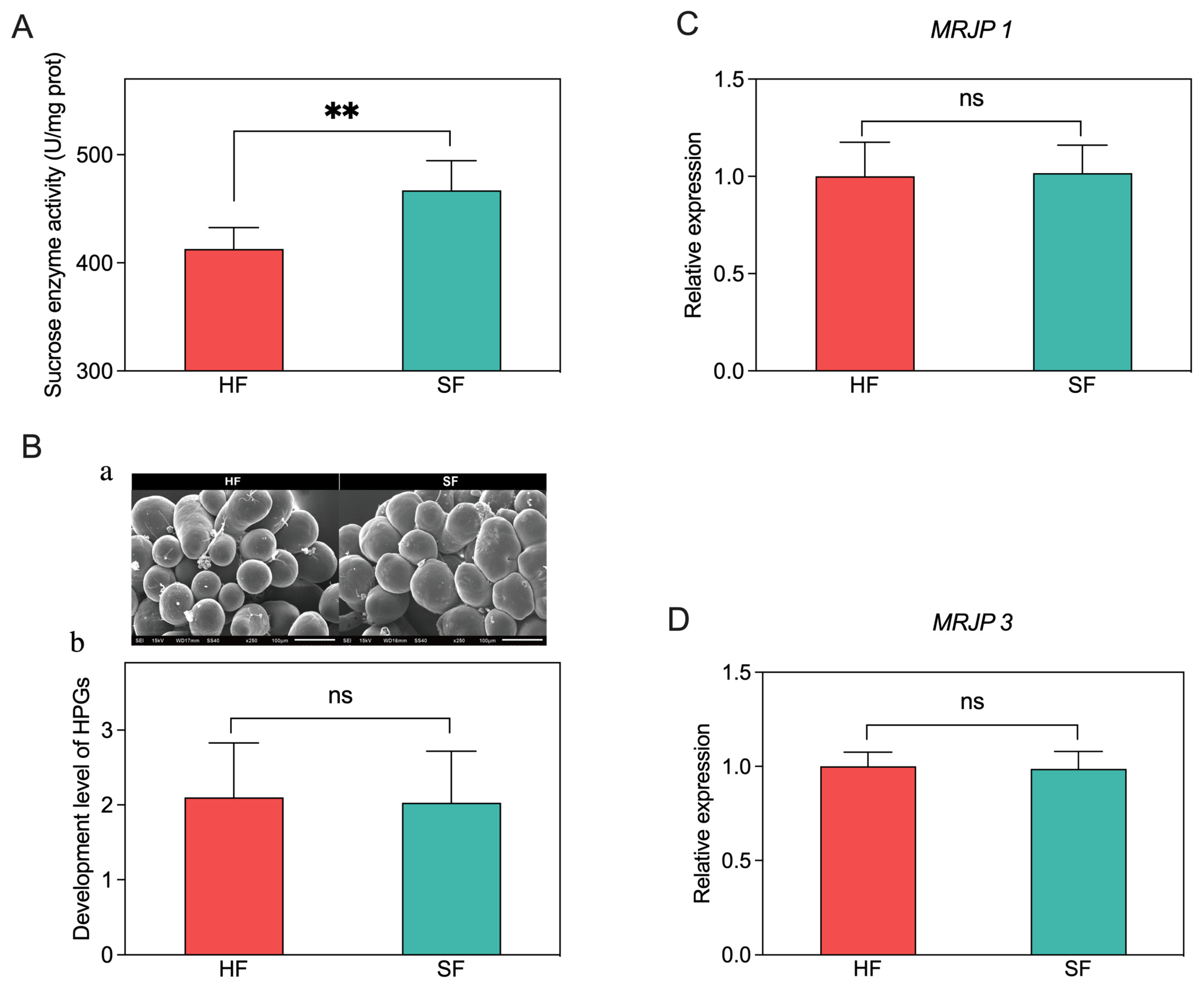
3.4. Sucrose Enzyme Activity, Hypopharyngeal Gland Development and Gene Expression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001, 75, 237–240. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Pavelić, S.K. Bioactives from Bee Products and Accompanying Extracellular Vesicles as Novel Bioactive Components for Wound Healing. Molecules 2021, 26, 3770. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L.B.D. Quality and standardisation of royal jelly. J. ApiProduct ApiMedical Sci. 2009, 1, 16–21. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Uversky, V.N.; Albar, A.H.; Khan, R.H.; Redwan, E.M. Multifunctionality and intrinsic disorder of royal jelly proteome. Proteomics 2021, 21, e2000237. [Google Scholar] [CrossRef]
- Ma, C.; Ma, B.; Li, J.; Fang, Y. Changes in chemical composition and antioxidant activity of royal jelly produced at different floral periods during migratory beekeeping. Food Res. Int. 2022, 155, 111091. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Rodopoulou, M.-A.; Goras, G.; Argena, N.; Thrasyvoulou, A. Investigating the Effect of Supplementary Feeding on Carbohydrate Composition and Quantity of Royal Jelly. Open J. Appl. Sci. 2018, 8, 141–149. [Google Scholar] [CrossRef]
- Daniele, G.; Casabianca, H. Sugar composition of French royal jelly for comparison with commercial and artificial sugar samples. Food Chem. 2012, 134, 1025–1029. [Google Scholar] [CrossRef]
- Wytrychowski, M.; Daniele, G.; Casabianca, H. Combination of sugar analysis and stable isotope ratio mass spectrometry to detect the use of artificial sugars in royal jelly production. Anal. Bioanal. Chem. 2012, 403, 1451–1456. [Google Scholar] [CrossRef]
- Balkanska, R.; Zhelyazkova, I.; Ignatova, M.; Kashamov, B. Effect of Supplementary Honey and Artificial Sugar Feeding of Bees on the Composition of Royal Jelly. Agric. Sci. Technol. 2013, 5, 335–338. [Google Scholar]
- Wang, Y.; Ma, L.-T.; Xu, B.-H. Diversity in life history of queen and worker honey bees, Apis mellifera L. J. Asia-Pac. Entomol. 2015, 18, 145–149. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Jie, H.; Li, P.M.; Zhao, G.J.; Feng, X.L.; Zeng, D.J.; Zhang, C.L.; Lei, M.Y.; Yu, M.; Chen, Q. Amino acid composition of royal jelly harvested at different times after larval transfer. Genet. Mol. Res. 2016, 15, gmr.15038306. [Google Scholar] [CrossRef]
- Stocker, A.; Rossmann, A.; Kettrup, A.; Bengsch, E. Detection of royal jelly adulteration using carbon and nitrogen stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2006, 20, 181–185. [Google Scholar] [CrossRef]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A Member of Complementary Medicinal Food: Anatolian Royal Jellies, Their Chemical Compositions, and Antioxidant Properties. J. Evid.-Based Complement. Altern. Med. 2016, 21, N43–N48. [Google Scholar] [CrossRef]
- Jianke, L.; Mao, F.; Begna, D.; Yu, F.; Aijuan, Z. Proteome comparison of hypopharyngeal gland development between Italian and royal jelly producing worker honeybees (Apis mellifera L.). J. Proteome Res. 2010, 9, 6578–6594. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Ma, L.; Li, G.; Han, K.; Liu, Z.; Wang, H.; Xu, B. The Native Dietary Habits of the Two Sympatric Bee Species and Their Effects on Shaping Midgut Microorganisms. Front. Microbiol. 2021, 12, 738226. [Google Scholar] [CrossRef]
- Shrinivasa, D.J.; Mathur, S.M. Compound feed production for livestock. Curr. Sci. 2020, 118, 553–559. [Google Scholar] [CrossRef]
- Brown, W.H.; Felauer, E.E.; Smith, M.V. Biosynthesis of royal jelly acid from sucrose. Nature 1962, 195, 75–76. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, J.; Li, X.; Wang, W.; Huang, Z. Characterization of sugar composition in Chinese royal jelly by ion chromatography with pulsed amperometric detection. J. Food Compos. Anal. 2019, 78, 101–107. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A.; Brescia, M.; Caprio, E. Assessment of geographical origin and production period of royal jelly by NMR metabolomics. Chem. Biol. Technol. Agric. 2020, 7, 24. [Google Scholar] [CrossRef]
- Pattamayutanon, P.; Peng, C.-C.; Sinpoo, C.; Chantawannakul, P. Effects of Pollen Feeding on Quality of Royal Jelly. J. Econ. Entomol. 2018, 111, 2974–2978. [Google Scholar] [CrossRef]
- SereiaI, M.J.; Toledo, V.d.A.A.d. Quality of royal jelly produced by Africanized honeybees fed a supplemented diet. Food Sci. Technol. 2013, 33, 304–309. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Cui, X.; Xu, B. Zinc nutrition increases the antioxidant defenses of honey bees. Entomol. Exp. Appl. 2015, 156, 201–210. [Google Scholar] [CrossRef]
- Chi, X.; Wei, W.; Zhang, W.; Liu, Z.; Wang, H.; Xu, B. Sodium Selenium Enhances the Antioxidative Activities and Immune Functions of Apis mellifera (Hymenoptera: Apidae) and Increases the Selenium Content in Royal Jelly. Environ. Entomol. 2019, 49, 169–177. [Google Scholar] [CrossRef]
- Al-Kahtani, S.; Taha, E.-K.A. Effect of Harvest Time on Royal Jelly Yield and Chemical Composition. J. Kans. Entomol. Soc. 2020, 93, 132–139. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef]
- Jimenez, D.R.; Gilliam, M. Peroxisomal enzymes in the honey bee midgut. Arch. Insect Biochem. Physiol. 1996, 31, 87–103. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Hang, X.; Wang, H.; Yang, W.; Xu, B. Nutritional effect of alpha-linolenic acid on honey bee colony developement (Apis mellifera L.). J. Apic. Res. 2015, 59, 63–72. [Google Scholar]
- Srisuparbh, D.; Klinbunga, S.; Wongsiri, S.; Sittipraneed, S. Isolation and characterization of major royal jelly cDNAs and proteins of the honey bee (Apis cerana). J. Biochem. Mol. Biol. 2003, 36, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, W.; Li, Z.; Zhang, S.; Chen, S.; Su, S. High-abundance mRNAs in Apis mellifera: Comparison between nurses and foragers. J. Insect Physiol. 2011, 57, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, B.-Y.; Kim, J.-M.; Choi, Y.-S.; Lee, M.-Y.; Lee, K.-S.; Jin, B.-R. Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion. Insects 2022, 13, 334. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Detection of adulterations of honey with high fructose syrups from inulin by GC analysis. J. Food Compos. Anal. 2010, 23, 273–276. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Shan, Y.; Zhang, Z.; Li, G.; Su, D.; Liu, F. Detection of adulterants such as sweeteners materials in honey using near-infrared spectroscopy and chemometrics. J. Food Eng. 2010, 101, 92–97. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chang, Y.-W.; Lu, K.-H.; Yang, E.-C. Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage. Insect Biochem. Mol. Biol. 2017, 88, 12–20. [Google Scholar] [CrossRef]


| Amino Acid | Group | F | P | |
|---|---|---|---|---|
| HF | SF | |||
| Phenylalanine | 0.508 ± 0.029 | 0.534 ± 0.057 | 1.497 | 0.256 |
| Alanine | 0.328 ± 0.022 | 0.410 ± 0.062 * | 10.923 | 0.011 |
| Methionine | 0.052 ± 0.019 | 0.117 ± 0.056 | 3.751 | 0.089 |
| Glycine | 0.350 ± 0.019 | 0.380 ± 0.053 * | 8.533 | 0.019 |
| Glutamic acid | 1.042 ± 0.055 | 1.130 ± 0.113 | 1.473 | 0.26 |
| Cysteine | 0.016 ± 0.003 | 0.026 ± 0.005 | 3.2 | 0.111 |
| Arginine | 0.534 ± 0.042 | 0.602 ± 0.065 | 0.3 | 0.599 |
| Lysine | 0.704 ± 0.068 | 0.772 ± 0.085 | 0.594 | 0.463 |
| Tyrosine | 0.378 ± 0.013 | 0.450 ± 0.041 * | 5.763 | 0.043 |
| Leucine | 0.852 ± 0.054 | 0.938 ± 0.118 | 4.191 | 0.075 |
| Proline | 0.526 ± 0.035 | 0.514 ± 0.027 | 0.123 | 0.735 |
| Serine | 0.606 ± 0.030 | 0.700 ± 0.060 | 0.74 | 0.415 |
| Threonine | 0.492 ± 0.024 | 0.552 ± 0.054 | 1.635 | 0.237 |
| Aspartic acid | 1.922 ± 0.113 | 2.150 ± 0.237 | 1.833 | 0.213 |
| Valine | 0.626 ± 0.050 | 0.708 ± 0.121 * | 5.906 | 0.041 |
| Isoleucine | 0.534 ± 0.048 | 0.576 ± 0.105 * | 7.648 | 0.024 |
| Histidine | 0.264 ± 0.019 | 0.280 ± 0.031 | 1.428 | 0.266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, L.; Wang, H.; Liu, Z.; Chi, X.; Xu, B. Effects of Sucrose Feeding on the Quality of Royal Jelly Produced by Honeybee Apis mellifera L. Insects 2023, 14, 742. https://doi.org/10.3390/insects14090742
Wang Y, Ma L, Wang H, Liu Z, Chi X, Xu B. Effects of Sucrose Feeding on the Quality of Royal Jelly Produced by Honeybee Apis mellifera L. Insects. 2023; 14(9):742. https://doi.org/10.3390/insects14090742
Chicago/Turabian StyleWang, Ying, Lanting Ma, Hongfang Wang, Zhenguo Liu, Xuepeng Chi, and Baohua Xu. 2023. "Effects of Sucrose Feeding on the Quality of Royal Jelly Produced by Honeybee Apis mellifera L." Insects 14, no. 9: 742. https://doi.org/10.3390/insects14090742





