Honey vs. Mite—A Trade-Off Strategy by Applying Summer Brood Interruption for Varroa destructor Control in the Mediterranean Region
Abstract
:Simple Summary
Abstract
1. Introduction
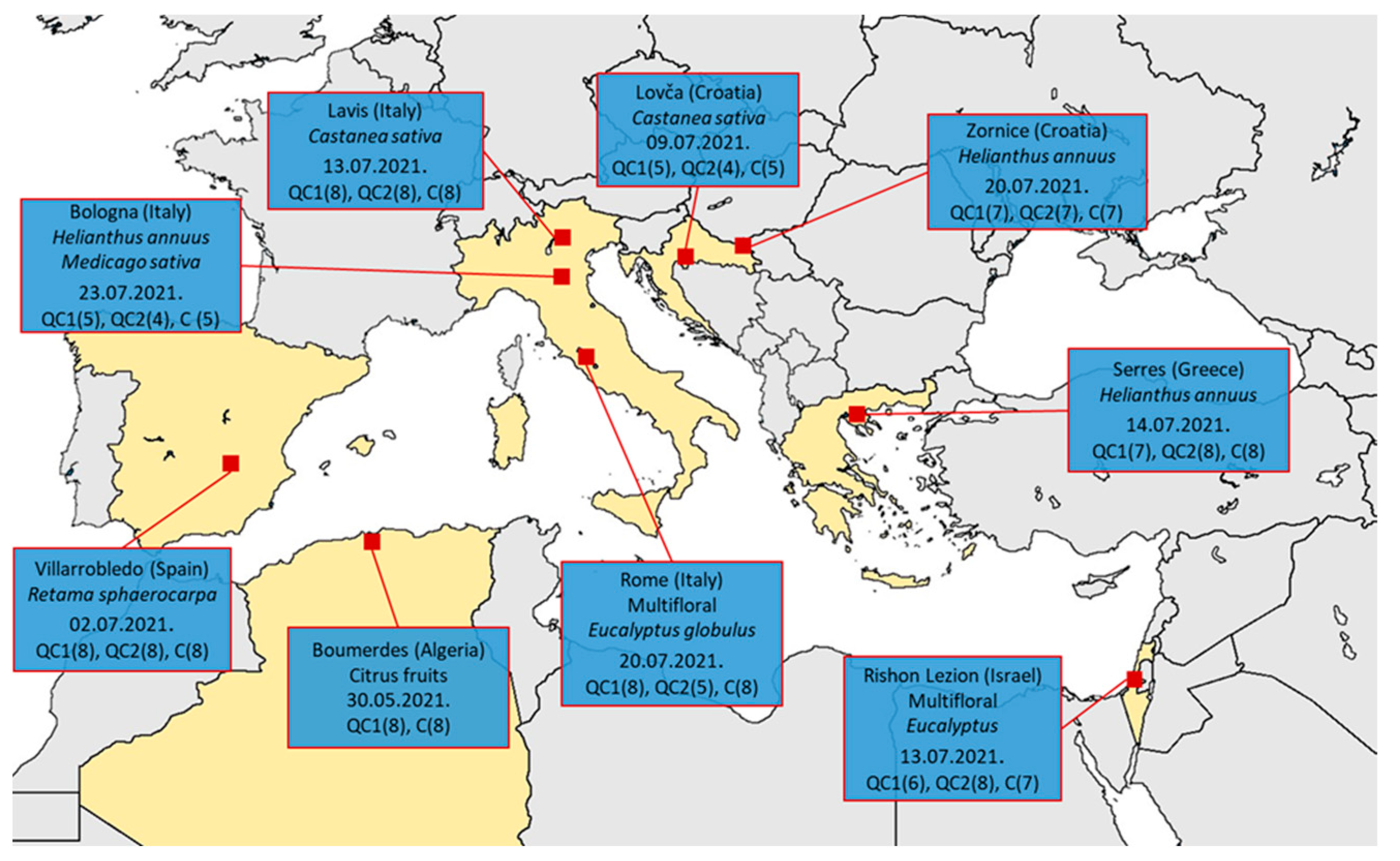
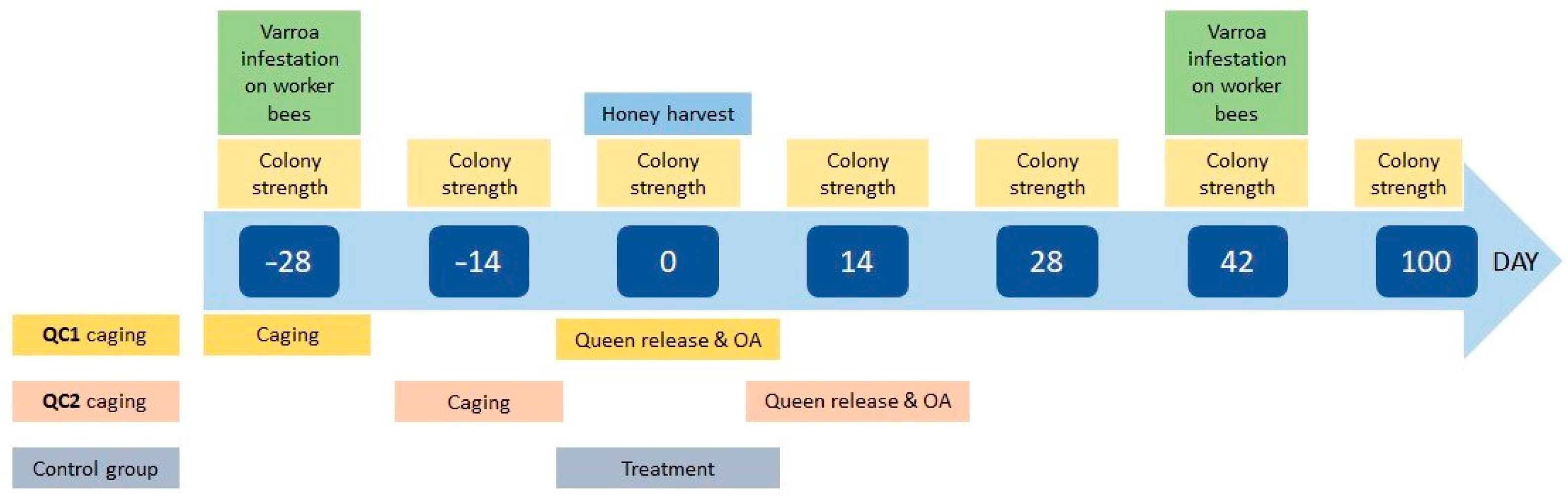
2. Materials and Methods
3. Results
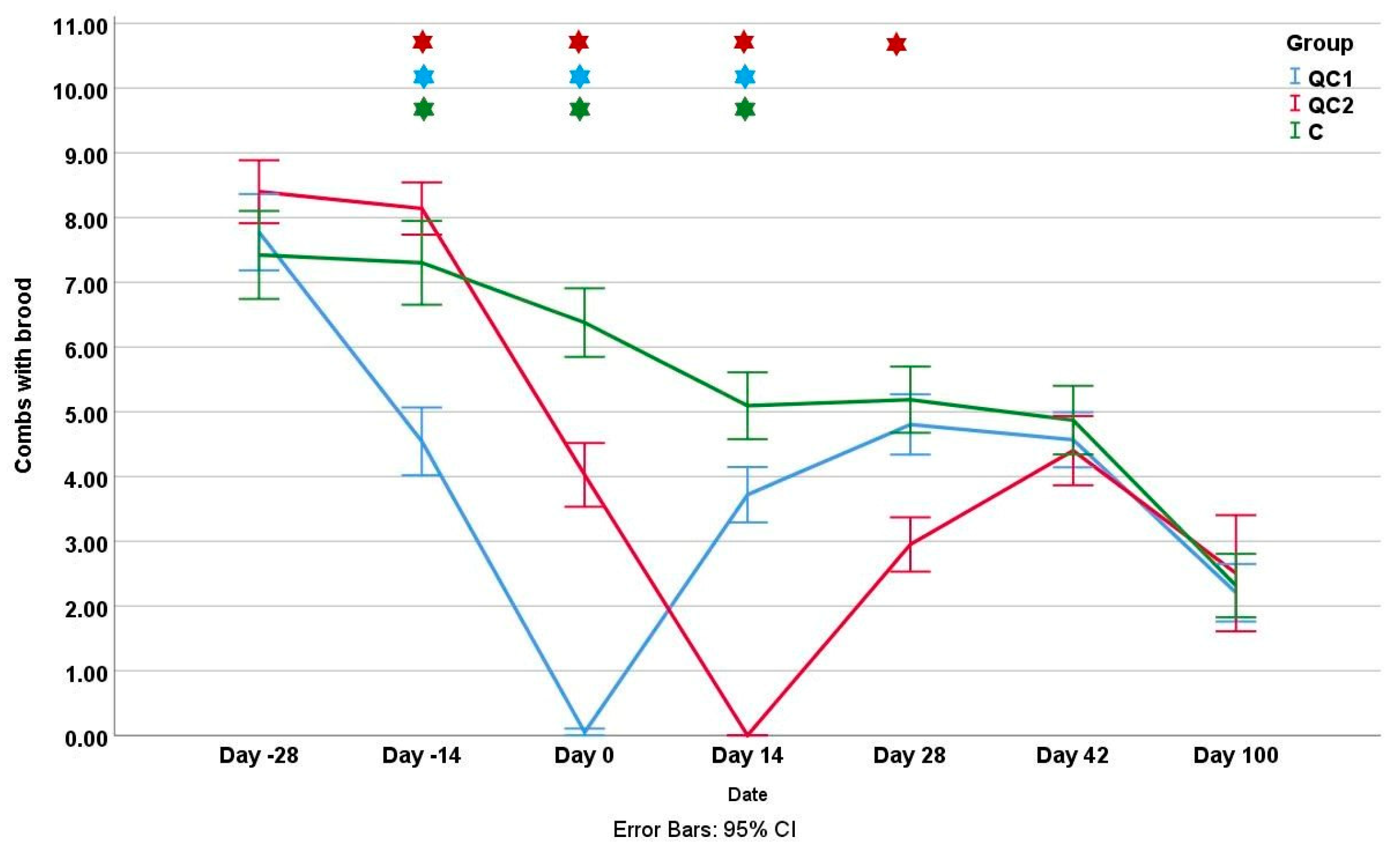
3.1. Colony Strength
3.2. Honey Production
3.3. V. destructor Infestation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dooremalen, C.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.J.M.; van Langevelde, F.; Blacquière, T. Winter Survival of Individual Honey Bees and Honey Bee Colonies Depends on Level of Varroa destructor Infestation. PLoS ONE 2012, 7, e36285. [Google Scholar] [CrossRef]
- Thoms, C.A.; Nelson, K.C.; Kubas, A.; Steinhauer, N.; Wilson, M.E.; vanEngelsdorp, D. Beekeeper Stewardship, Colony Loss, and Varroa destructor Management. Ambio 2019, 48, 1209–1218. [Google Scholar] [CrossRef]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, Conservation and Current Threats to European Honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef]
- Martel, A.C.; Zeggane, S.; Aurières, C.; Drajnudel, P.; Faucon, J.P.; Aubert, M. Acaricide Residues in Honey and Wax after Treatment of Honey Bee Colonies with Apivar® or Asuntol® 50. Apidologie 2007, 38, 534–544. [Google Scholar] [CrossRef]
- Jamal, M.; Aziz, M.A.; Naeem, M.; Iqbal, Z.; Khalid, A.; Siddique, F.; Khan, K.A.; Ghramh, H.A. Detection of Flumethrin Acaricide Residues from Honey and Beeswax Using High Performance Liquid Chromatography (HPLC) Technique. J. King Saud Univ. Sci. 2020, 32, 2229–2235. [Google Scholar] [CrossRef]
- Almecija, G.; Poirot, B.; Cochard, P.; Suppo, C. Inventory of Varroa destructor Susceptibility to Amitraz and Tau-Fluvalinate in France. Exp. Appl. Acarol. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Assessing the Resistance to Acaricides in Varroa destructor from several Spanish locations. Parasitol. Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, F.D. Detection of Amitraz Resistance and Reduced Treatment Efficacy in the Varroa Mite, Varroa destructor, within Commercial Beekeeping Operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Büchler, R.; Uzunov, A.; Kovačić, M.; Prešern, J.; Pietropaoli, M.; Hatjina, F.; Pavlov, B.; Charistos, L.; Formato, G.; Galarza, E.; et al. Summer Brood Interruption as Integrated Management Strategy for Effective Varroa Control in Europe. J. Apic. Res. 2020, 59, 764–773. [Google Scholar] [CrossRef]
- Pietropaoli, M.; Giacomelli, A.; Milito, M.; Pizzariello, M.; Gobbi, C.; Scholl, F.; Formato, G. Integrated Pest Management Strategies against Varroa destructor: The Use of Oxalic Acid Combined with Innovative Cages to Obtain the Absence of Brood. Eur. J. Integr. Med. 2012, 15, 93. [Google Scholar]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef]
- Uzunov, A.; Büchler, R. Alternative Methods for Varroa Control in Honey Bee Colonie; Swiss Agency for Development and Cooperation—SDC: Skopje, North Macedonia, 2020.
- Imdorf, A.; Charrière, J.D.; Bachofen, B. Efficiency Checking of the Varroa jacobsoni Control Methods by Means of Oxalic Acid. Apiacta 1997, 32, 89–91. [Google Scholar]
- Gregorc, A.; Planinc, I. The Control of Varroa destructor Using Oxalic Acid. Vet. J. 2002, 163, 306–310. [Google Scholar] [CrossRef]
- Nanetti, A.; Büchler, R.; Charrière, J.-D.; Friesd, I.; Helland, S.; Imdorf, A.; Korpela, S.; Kristiansen, P. Oxalic Acid Treatments for Varroa control (Review). Apiacta 2003, 38, 81–87. [Google Scholar]
- Al Toufailia, H.; Scandian, L.; Ratnieks, F.L.W. Towards Integrated Control of Varroa: 2) Comparing Application Methods and Doses of Oxalic Acid on the Mortality of Phoretic Varroa destructor Mites and Their Honey Bee Hosts. J. Apic. Res. 2015, 54, 108–120. [Google Scholar] [CrossRef]
- Berry, J.A.; Bartlett, L.J.; Bruckner, S.; Baker, C.; Braman, S.K.; Delaplane, K.S.; Williams, G.R. Assessing Repeated Oxalic Acid Vaporization in Honey Bee (Hymenoptera: Apidae) Colonies for Control of the Ectoparasitic Mite Varroa destructor. J. Insect Sci. 2022, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, K.; Hopkins, B.K.; Sheppard, W.S. Comparison of Oxalic Acid Drip and HopGuard for Pre-Winter Varroa destructor Control in Honey Bee (Apis mellifera) Colonies. J. Apic. Res. 2022. [Google Scholar] [CrossRef]
- Bogdanov, S.; Charrière, J.D.; Imdorf, A.; Kilchenmann, V.; Fluri, P. Determination of Residues in Honey after Treatments with Formic and Oxalic Acid under Field Conditions. Apidologie 2002, 33, 399–409. [Google Scholar] [CrossRef]
- Rademacher, E.; Harz, M. Oxalic Acid for the Control of Varroosis in Honey Bee Colonies—A Review. Apidologie 2006, 37, 98–120. [Google Scholar] [CrossRef]
- Maggi, M.D.; Damiani, N.; Ruffinengo, S.R.; Brasesco, M.C.; Szawarski, N.; Mitton, G.; Mariani, F.; Sammataro, D.; Quintana, S.; Eguaras, M.J. The Susceptibility of Varroa destructor against Oxalic Acid: A Study Case. Bull. Insectol. 2017, 70, 39–44. [Google Scholar]
- Domatsky, A.N.; Domatskaya, T.F. Effectiveness of Oxalic Acid in Varroatosis in the Apiaries of Tyumen Region, Russia. Ukr. J. Ecol. 2018, 8, 143–147. [Google Scholar]
- Toomemaa, K. The Synergistic Effect of Weak Oxalic Acid and Thymol Aqueous Solutions on Varroa Mites and Honey Bees. J. Apic. Res. 2018, 58, 37–52. [Google Scholar] [CrossRef]
- Gregorc, A.; Adamczyk, J.; Kapun, S.; Planinc, I. Integrated Varroa Control in Honey Bee (Apis mellifera carnica) Colonies with or without Brood. J. Apic. Res. 2016, 55, 253–258. [Google Scholar] [CrossRef]
- Gregorc, A.; Alburaki, M.; Werle, C.; Knight, P.R.; Adamczyk, J. Brood Removal or Queen Caging Combined with Oxalic Acid Treatment to Control Varroa Mites (Varroa destructor) in Honey Bee Colonies (Apis mellifera). Apidologie 2017, 48, 821–832. [Google Scholar] [CrossRef]
- Kovačić, M.; Puškadija, Z.; Charistos, L.; Hatjina, F.; Pietropaoli, M.; Formato, G.; Wilde, J.; Uzunov, A.; Büchler, R. Effect of Timing of Queen Caging on Honey Production. In Proceedings of the Coloss Varroa Task Force Spring 2020 Workshop Proceedings, Pulawy, Poland, 18–21 February 2020. [Google Scholar]
- Evans, K.C.; Underwood, R.M.; López-Uribe, M.M. Combined Effects of Oxalic Acid Sublimation and Brood Breaks on Varroa Mite (Varroa destructor) and Deformed Wing Virus Levels in Newly Established Honey Bee (Apis mellifera) Colonies. J. Apic. Res. 2022, 61, 197–205. [Google Scholar] [CrossRef]
- Büchler, R. Vital Colonies Thanks to Complete Brood Removal. ADIZ/db/IF 2009, 10–12. Available online: brood-removal-web.pdf (accessed on 3 September 2023).
- Lodesani, M.; Costa, C.; Besana, A.; Dall’Olio, R.; Franceschetti, S.; Tesoriero, D.; Vaccari, G. Impact of Control Strategies for Varroa destructor on Colony Survival and Health in Northern and Central Regions of Italy. J. Apic. Res. 2014, 53, 155–164. [Google Scholar] [CrossRef]
- Lodesani, M.; Franceschetti, S.; Dall’Ollio, R. Evaluation of Early Spring Bio-Technical Management Techniques to Control Varroosis in Apis mellifera. Apidologie 2019, 50, 131–140. [Google Scholar] [CrossRef]
- Hatjina, F.; Haristos, L. Indirect Effects of Oxalic Acid Administered by Trickling Method on Honey Bee Brood. J. Apic. Res. 2005, 44, 172–174. [Google Scholar] [CrossRef]
- Cabbri, R.; Ferlizza, E.; Nanetti, A.; Monari, E.; Andreani, G.; Galuppi, R.; Isani, G. Biomarkers of Nutritional Status in Honeybee Haemolymph: Effects of Different Biotechnical Approaches for Varroa destructor Treatment and Wintering Phase. Apidologie 2018, 49, 606–618. [Google Scholar] [CrossRef]
- Uzunov, A.; Büchler, R.; Costa, C.; Mondet, F.; Bienkowska, M.; Hatjina, F.; Meixner, M.; Andonov, S.; Kovačić, M.; Dall’Olio, R.; et al. Book of Methods-Performance Testers; EURBEST Project (AGRI-2017-0346); Bee Institute in Kirchhain: Kirchhain, Germany, 2021. [Google Scholar]
- Pietropaoli, M.; Tlak Gajger, I.; Costa, C.; Gerula, D.; Wilde, J.; Adjlane, N.; Sánchez, P.A.; Škerl, M.I.S.; Bubnič, J.; Formato, G. Evaluation of Two Commonly Used Field Tests to Assess Varroa destructor Infestation on Honey Bee (Apis mellifera) Colonies. Appl. Sci. 2021, 11, 4458. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard Methods for Varroa Research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Fries, I.; Imdorf, A.; Rosenkranz, P. Survival of Mite Infested (Varroa destructor) Honey Bee (Apis mellifera) Colonies in a Nordic Climate. Apidologie 2006, 37, 564–570. [Google Scholar] [CrossRef]
- Hatjina, F.; Costa, C.; Büchler, R.; Uzuno, A.; Drazic, M.; Filipi, J.; Charistos, L.; Ruottinen, L.; Andonov, S.; Meixner, M.D.; et al. Population Dynamics of European Honey Bee Genotypes under Different Environmental Conditions. J. Apic. Res. 2014, 53, 233–247. [Google Scholar] [CrossRef]
- Fluri, P.; Lüscher, M.; Wille, H.; Gerig, L. Changes in Weight of the Pharyngeal Gland and Haemolymph Titres of Juvenile Hormone, Protein and Vitellogenin in Worker Honey Bees. J. Insect Physiol. 1982, 28, 61–68. [Google Scholar] [CrossRef]
- Greenberg, J.K.; Xia, J.; Zhou, X.; Thatcher, S.R.; Gu, X.; Ament, S.A.; Newman, T.C.; Green, P.J.; Zhang, W.; Robinson, G.E.; et al. Behavioral Plasticity in Honey Bees Is Associated with Differences in Brain MicroRNA Transcriptome. Genes Brain Behav. 2012, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Rangel, J.; Grozinger, C.M. Honey Bee (Apis mellifera) Larval Pheromones May Regulate Gene Expression Related to Foraging Task Specialization. BMC Genom. 2019, 20, 592. [Google Scholar] [CrossRef]
- Fries, I.; Hansen, H.; Imdorf, A.; Rosenkranz, P. Swarming in Honey Bees (Apis mellifera) and Varroa destructor Population Development in Sweden. Apidologie 2003, 34, 389–397. [Google Scholar] [CrossRef]
- Rangel, J.; Seeley, T.D. Colony Fissioning in Honey Bees: Size and Significance of the Swarm Fraction. Insectes Sociaux 2012, 59, 453–462. [Google Scholar] [CrossRef]
- Lait, C.G.; Borden, J.H.; Kovacs, E.; Moeri, O.E.; Campbell, M.; MacHial, C.M. Treatment with Synthetic Brood Pheromone (SuperBoost) Enhances Honey Production and Improves Overwintering Survival of Package Honey Bee (Hymenoptera: Apidae) Colonies. J. Econ. Entomol. 2012, 105, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Amdam, G.V. The Curious Case of Aging Plasticity in Honey Bees. FEBS Lett. 2010, 584, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.J.; Van Santen, E.; Ellis, J.D.; Johnson, R. Evaluating the Efficacy of Oxalic Acid Vaporization and Brood Interruption in Controlling the Honey Bee Pest Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 2020, 113, 582–588. [Google Scholar] [CrossRef]
- Roberts, J.M.K.; Schouten, C.N.; Sengere, R.W.; Jave, J.; Lloyd, D. Effectiveness of Control Strategies for Varroa jacobsoni and Tropilaelaps mercedesae in Papua New Guinea. Exp. Appl. Acarol. 2020, 80, 399–407. [Google Scholar] [CrossRef]
- Chantawannakul, P.; Ramsey, S.; vanEngelsdorp, D.; Khongphinitbunjong, K.; Phokasem, P. Tropilaelaps Mite: An Emerging Threat to European Honey Bee. Curr. Opin. Insect Sci. 2018, 26, 69–75. [Google Scholar] [CrossRef]
- Popovska Stojanov, D.; Dimitrov, L.; Danihlík, J.; Uzunov, A.; Golubovski, M.; Andonov, S.; Brodschneider, R. Direct Economic Impact Assessment of Winter Honeybee Colony Losses in Three European Countries. Agriculture 2021, 11, 398. [Google Scholar] [CrossRef]
- Milani, N. The Resistance of Varroa jacobsoni Oud. to Acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Sammataro, D.; Untalan, P.; Guerrero, F.; Finley, J. The Resistance of Varroa Mites (Acari: Varroidae) to Acaricides and the Presence of Esterase. Int. J. Acarol. 2005, 31, 67–74. [Google Scholar] [CrossRef]
- Fisher, A.; Rangel, J. Exposure to Pesticides during Development Negatively Affects Honey Bee (Apis mellifera) Drone Sperm Viability. PLoS ONE 2018, 13, e0208630. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for Resistance to Varroa Destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Guichard, M.; Dietemann, V.; Neuditschko, M.; Dainat, B. Advances and Perspectives in Selecting Resistance Traits against the Parasitic Mite Varroa destructor in Honey Bees. Genet. Sel. Evol. 2020, 52, 71. [Google Scholar] [CrossRef] [PubMed]
- Büchler, R.; Kovačić, M.; Buchegger, M.; Puškadija, Z.; Hoppe, A.; Brascamp, E.W. Evaluation of Traits for the Selection of Apis mellifera for Resistance against Varroa destructor. Insects 2020, 11, 618. [Google Scholar] [CrossRef] [PubMed]

| Number of Combs with Bees | Number of Brood Combs | |||||
|---|---|---|---|---|---|---|
| Source | df | Mean Square | F | df | Mean Square | F |
| Model | 26 | 1504.739 | 359.315 ** | 26 | 447.303 | 260.864 ** |
| Apiary | 8 | 570.201 | 136.158 ** | 8 | 80.272 | 46.814 ** |
| Group | 2 | 1.172 | 0.411 | 2 | 2.017 | 1.176 |
| Apiary × Group | 15 | 3.020 | 0.721 | 15 | 3.263 | 1.903 * |
| Error | 152 | 4.188 | 152 | 1.715 | ||
| Total | 178 | 178 | ||||
| R2 = 0.984 (adjusted R2 = 0.981) | R2 = 0.978 (adjusted R2 = 0.974) | |||||
| Number of Combs with Bees | Number of Brood Combs | |||||
|---|---|---|---|---|---|---|
| Source | df | Mean Square | F | df | Mean Square | F |
| Model | 26 | 297.319 | 123.159 ** | 26 | 43.323 | 12.773 ** |
| Apiary | 8 | 84.157 | 34.976 ** | 8 | 29.556 | 8.714 ** |
| Group | 2 | 0.538 | 0.224 | 2 | 2.786 | 0.821 |
| Apiary × Group | 15 | 2.978 | 1.237 | 15 | 1.805 | 0.532 |
| Error | 133 | 2.406 | 133 | 3.392 | ||
| Total | 159 | 159 | ||||
| R2 = 0.960 (adjusted R2 = 0.952) | R2 = 0.714 (adjusted R2 = 0.658) | |||||
| Source of Variation | df | Mean Square | F |
|---|---|---|---|
| Model | 26 | 2087.885 | 67.980 ** |
| Apiary | 8 | 2146.907 | 69.901 ** |
| Group | 2 | 199.852 | 6.507 ** |
| Apiary ×Group | 15 | 80.000 | 2.605 ** |
| Error | 151 | 30.713 | |
| Total | 177 |
| Group | Mean | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| QC1 | 12.091 a | 0.729 | 10.650 | 13.532 |
| QC2 | 16.009 b | 0.803 | 14.423 | 17.596 |
| C | 15.142 b | 0.705 | 13.749 | 16.535 |
| Infestation with V. destructor on day −28 | Infestation with V. destructor on day 42 | |||||
|---|---|---|---|---|---|---|
| Source | df | Mean Square | F | df | Mean Square | F |
| Model | 11 | 61.277 | 25.277 ** | 11 | 15.185 | 15.186 ** |
| Apiary | 8 | 30.962 | 12.772 ** | 8 | 5.170 | 5.171 ** |
| Group | 2 | 0.417 | 0.172 | 2 | 8.894 | 8.894 ** |
| Error | 167 | 2.424 | 152 | 1.000 | ||
| Total | 178 | 163 | ||||
| R2 = 0.625 (adjusted R2= 0.600) | R2 = 0.542 (adjusted R2= 0.511) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, M.; Uzunov, A.; Tlak Gajger, I.; Pietropaoli, M.; Soroker, V.; Adjlane, N.; Benko, V.; Charistos, L.; Dall’Olio, R.; Formato, G.; et al. Honey vs. Mite—A Trade-Off Strategy by Applying Summer Brood Interruption for Varroa destructor Control in the Mediterranean Region. Insects 2023, 14, 751. https://doi.org/10.3390/insects14090751
Kovačić M, Uzunov A, Tlak Gajger I, Pietropaoli M, Soroker V, Adjlane N, Benko V, Charistos L, Dall’Olio R, Formato G, et al. Honey vs. Mite—A Trade-Off Strategy by Applying Summer Brood Interruption for Varroa destructor Control in the Mediterranean Region. Insects. 2023; 14(9):751. https://doi.org/10.3390/insects14090751
Chicago/Turabian StyleKovačić, Marin, Aleksandar Uzunov, Ivana Tlak Gajger, Marco Pietropaoli, Victoria Soroker, Noureddine Adjlane, Valerija Benko, Leonidas Charistos, Raffaele Dall’Olio, Giovanni Formato, and et al. 2023. "Honey vs. Mite—A Trade-Off Strategy by Applying Summer Brood Interruption for Varroa destructor Control in the Mediterranean Region" Insects 14, no. 9: 751. https://doi.org/10.3390/insects14090751





