Live Detection of Intracellular Chitin in Butterfly Wing Epithelial Cells In Vivo Using Fluorescent Brightener 28: Implications for the Development of Scales and Color Patterns
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Butterfly Rearing
2.2. Experimental Operations
2.3. Fluorescent Probes
2.4. Confocal Imaging
3. Results
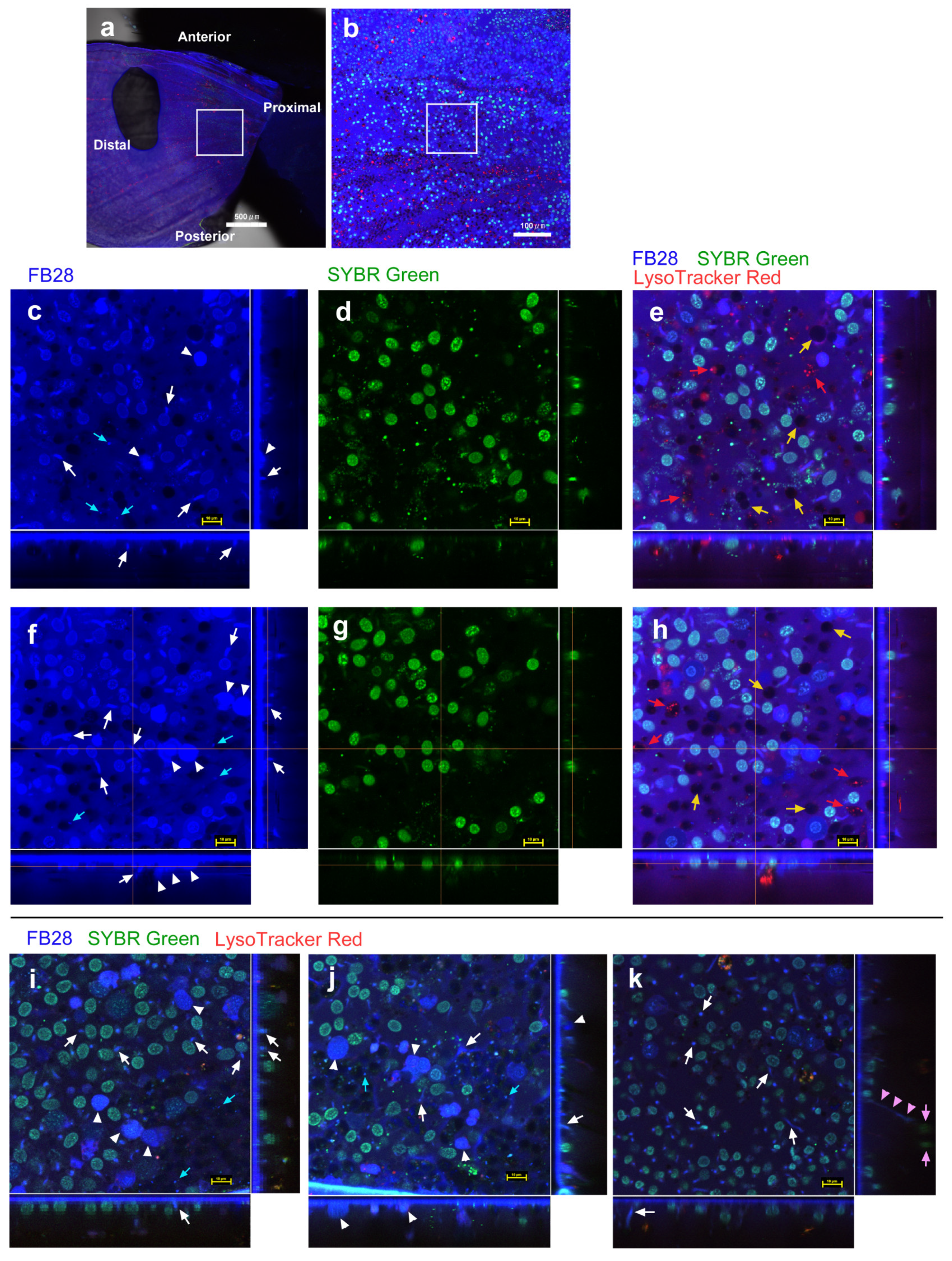
3.1. FB28 and SYBR Green I: Proximal Regions
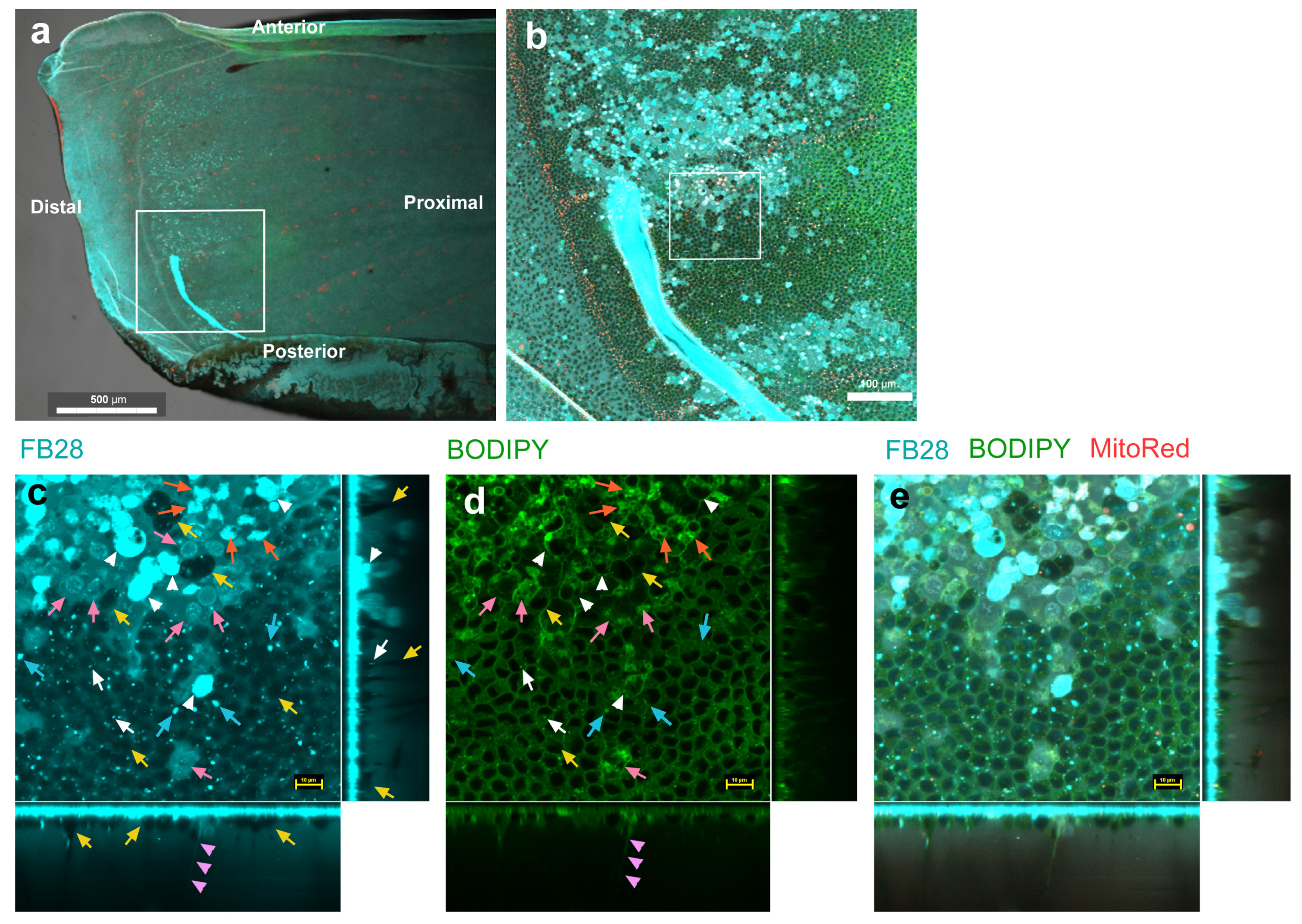
3.2. FB28 and BODIPY: Distal Regions
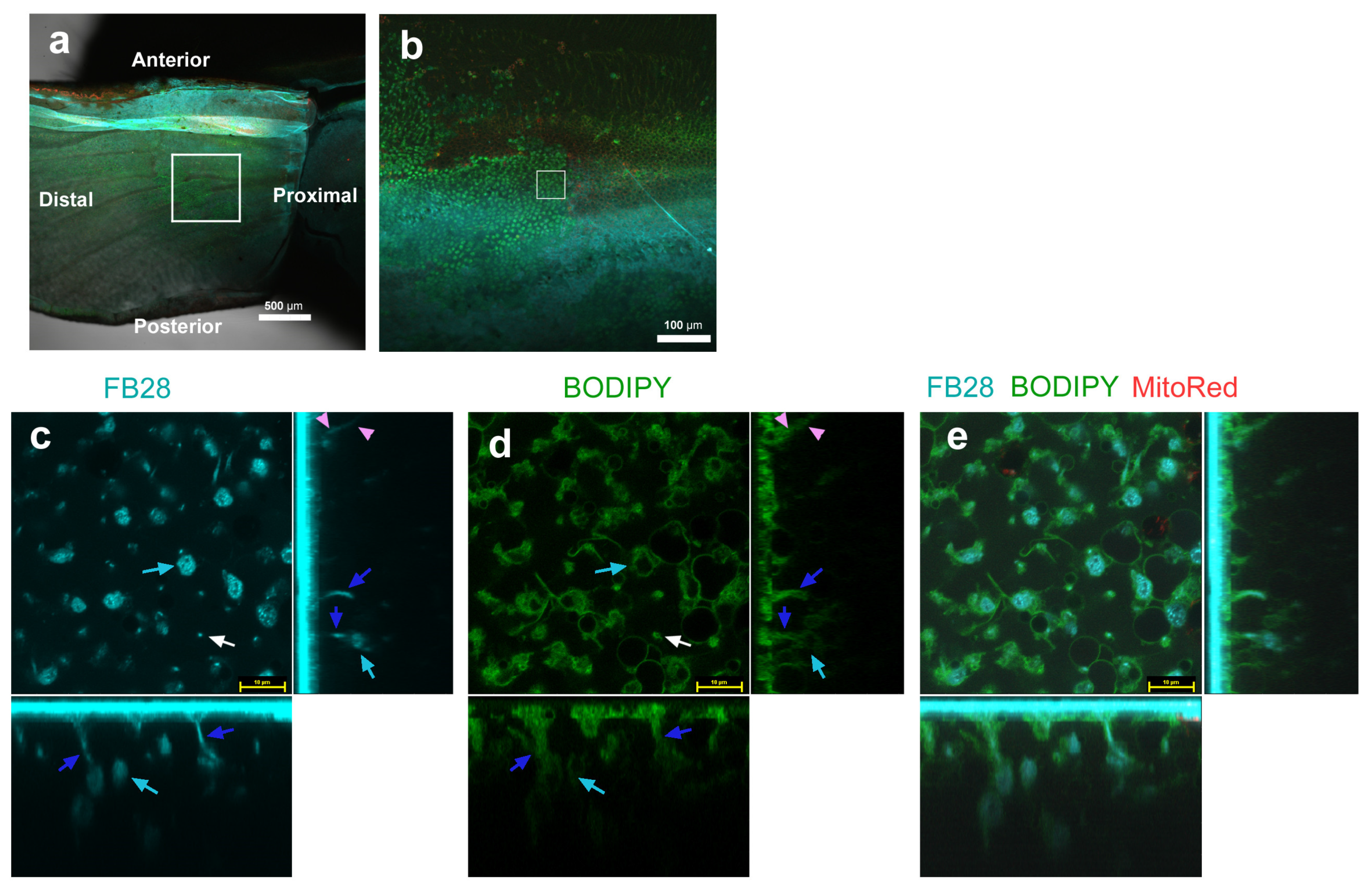
3.3. FB28 and BODIPY: Middle Regions
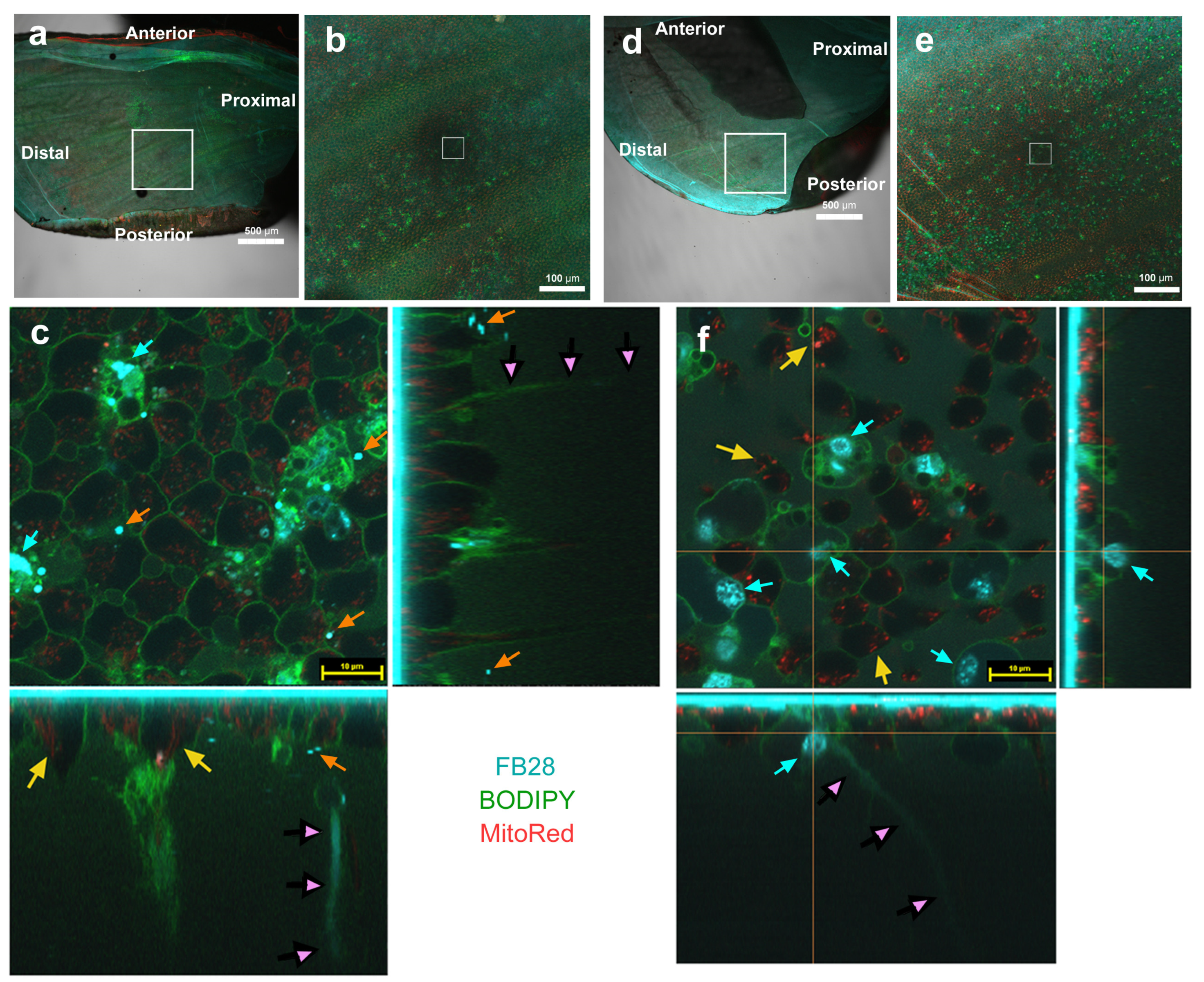
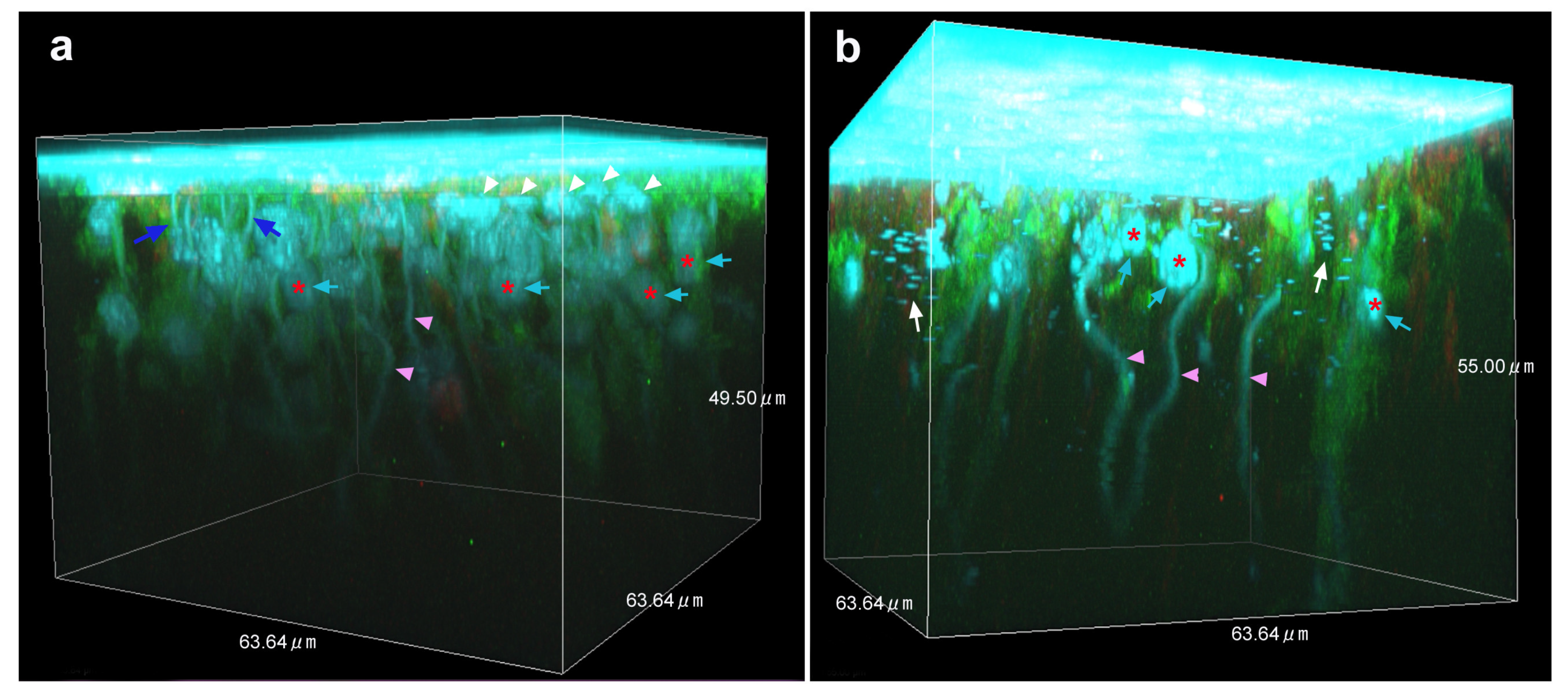
3.4. Three-Dimensional Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stricker, S.A.; Whitaker, M. Confocal laser scanning microscopy of calcium dynamics in living cells. Microsc. Res. Technol. 1999, 46, 356–369. [Google Scholar] [CrossRef]
- Tovey, S.C.; Brighton, P.J.; Willars, G.B. Confocal microscopy: Theory and applications for cellular signaling. Methods Mol. Biol. 2005, 312, 57–85. [Google Scholar] [CrossRef]
- Hanson, G.T.; Hanson, B.J. Fluorescent probes for cellular assays. Comb. Chem. High Throughput Screen. 2008, 11, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A.; Nagai, T.; Mizuno, H. Engineering fluorescent proteins. Adv. Biochem. Eng. Biotechnol. 2005, 95, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Nagano, T. Small-molecule fluorophores and fluorescent probes for bioimaging. Pflug. Arch. Eur. J. Physiol. 2013, 465, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. New calcium indicators and buffers with high selectivity against magnesium and protons: Design, synthesis, and properties of prototype structures. Biochemistry 1980, 19, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 1981, 290, 527–528. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent probes for sensing and imaging within specific cellular organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef]
- Choi, N.-E.; Lee, J.-Y.; Park, E.-C.; Lee, J.-H.; Lee, J. Recent advances in organelle-targeted fluorescent probes. Molecules 2021, 26, 217. [Google Scholar] [CrossRef]
- Lin, J.; Yang, K.; New, E.J. Strategies for organelle targeting of fluorescent probes. Org. Biomol. Chem. 2021, 19, 9339–9357. [Google Scholar] [CrossRef]
- Lu, S.; Dai, Z.; Cui, Y.; Kong, D.-M. Recent development of advanced fluorescent molecular probes for organelle-targeted cell imaging. Biosensors 2023, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J.; Pallai, S.R. Integumentary system. In Physiological Systems in Insects, 4th ed.; Academic Press: Burlington, NJ, USA, 2022; pp. 87–142. [Google Scholar]
- Andersen, S.O. Cuticle sclerotization and tanning. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: New York, NY, USA, 2012; pp. 167–192. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Kramer, K.J. Chitin metabolism in insects. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: New York, NY, USA, 2012; pp. 193–235. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, chemistry and biology. In Targeting Chitin-Containing Organisms—Advances in Experimental Medicine and Biology; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; Volume 1142, pp. 5–18. [Google Scholar] [CrossRef]
- Moussian, B. The apical plasma membrane of chitin-synthesizing epithelia. Insect Sci. 2013, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B.; Schwarz, H.; Bartoszewski, S.; Nüsslein-Volhard, C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 2005, 264, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Yang, Q. Chitin organizing and modifying enzymes and proteins involved in remodeling of the insect cuticle. In Targeting Chitin-Containing Organisms—Advances in Experimental Medicine and Biology; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; Volume 1142, pp. 83–114. [Google Scholar] [CrossRef]
- Sobala, L.F.; Adler, P.N. The gene expression program for the formation of wing cuticle in Drosophila. PLoS Genet. 2016, 12, e1006100. [Google Scholar] [CrossRef]
- Moussian, B.; Tång, E.; Tonning, A.; Helms, S.; Schwarz, H.; Nüsslein-Volhard, C.; Uv, A.E. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 2006, 133, 163–171. [Google Scholar] [CrossRef]
- Devine, W.P.; Lubarsky, B.; Shaw, K.; Luschnig, S.; Messina, L.; Krasnow, M.A. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 17014–17019. [Google Scholar] [CrossRef]
- Harrington, B.J.; Raper, K.B. Use of a fluorescent brightener to demonstrate cellulose in the cellular slime molds. Appl. Microbiol. 1968, 16, 106–113. [Google Scholar] [CrossRef]
- Hughes, J.; McCully, M.E. The use of an optical brightener in the study of plant structure. Stain Technol. 1975, 50, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Herth, W.; Schnepf, E. The fluorochrome, Calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Fischer, J.M.C.; Peterson, C.A.; Bols, N.C. A new fluorescent test for cell vitality using Calcofluor white M2R. Stain Technol. 1985, 60, 69–79. [Google Scholar] [CrossRef]
- Hoch, H.C.; Galvani, C.D.; Szarowski, D.H.; Turner, J.N. Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia 2005, 97, 580–588. [Google Scholar] [CrossRef]
- Sviben, S.; Spaeker, O.; Bennet, M.; Albéric, M.; Dirks, J.-H.; Moussian, B.; Fratzl, P.; Bertinetti, L.; Politi, Y. Epidermal cell surface structure and chitin–protein co-assembly determine fiber architecture in the locust cuticle. ACS Appl. Mater. Interfaces 2020, 12, 25581–25590. [Google Scholar] [CrossRef] [PubMed]
- Sobala, L.F.; Wang, Y.; Adler, P.N. ChtVis-Tomato, a genetic reporter for in vivo visualization of chitin deposition in Drosophila. Development 2015, 142, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Dinwiddie, A.; Null, R.; Pizzano, M.; Chuong, L.; Krup, A.L.; Tan, H.E.; Patel, N.H. Dynamics of F-actin prefigure the structure of butterfly wing scales. Dev. Biol. 2014, 392, 404–418. [Google Scholar] [CrossRef]
- Day, C.R.; Hanly, J.J.; Ren, A.; Martin, A. Sub-micrometer insights into the cytoskeletal dynamics and ultrastructural diversity of butterfly wing scales. Dev. Dyn. 2019, 248, 657–670. [Google Scholar] [CrossRef]
- Lloyd, V.J.; Burg, S.L.; Harizanova, J.; Hill, O.; Enciso-Romero, J.; Cooper, R.L.; Flenner, S.; Longo, E.; Greving, I.; Nadeau, N.J.; et al. The actin cytoskeleton plays multiple roles in structural color formation in butterfly wing scales. bioRxiv 2023. bioRxiv:2023.06.01.542791. [Google Scholar] [CrossRef]
- Ghiradella, H. Insect cuticular surface modifications: Scales and other structural formations. Adv. Insect Physiol. 2010, 38, 135–180. [Google Scholar] [CrossRef]
- Kazama, M.; Ichinei, M.; Endo, S.; Iwata, M.; Hino, A.; Otaki, J.M. Species-dependent microarchitectural traits of iridescent scales in the triad taxa of Ornithoptera birdwing butterflies. Entomol. Sci. 2017, 20, 255–269. [Google Scholar] [CrossRef]
- Thayer, R.C.; Allen, F.I.; Patel, N.H. Structural color in Junonia butterflies evolves by tuning scale lamina thickness. eLife 2020, 9, e52187. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, V.J.; Nadeau, N.J. The evolution of structural colour in butterflies. Curr. Opin. Genet. Dev. 2021, 69, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Finet, C.; Banerjee, T.D.; Saranathan, V.; Monteiro, A. Antennapedia and optix regulate metallic silver wing scale development and cell shape in Bicyclus anynana butterflies. Cell Rep. 2022, 40, 111052. [Google Scholar] [CrossRef] [PubMed]
- Flaven-Pouchon, J.; Moussian, B. Fluorescent microscopy-based detection of chitin in intact Drosophila melanogaster. Front. Physiol. 2022, 13, 856369. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Nakazato, Y. Butterfly wing color pattern modification inducers may act on chitin in the apical extracellular site: Implications in morphogenic signals for color pattern determination. Biology 2022, 11, 1620. [Google Scholar] [CrossRef]
- Nijhout, H.F. The Development and Evolution of Butterfly Wing Patterns; Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Kusaba, K.; Otaki, J.M. Positional dependence of scale size and shape in butterfly wings: Wing-wide phenotypic coordination of color-pattern elements and background. J. Insect Physiol. 2009, 55, 174–182. [Google Scholar] [CrossRef]
- Iwata, M.; Ohno, Y.; Otaki, J.M. Real-time in vivo imaging of butterfly wing development: Revealing the cellular dynamics of the pupal wing tissue. PLoS ONE 2014, 9, e89500. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Live cell imaging of butterfly pupal and larval wings in vivo. PLoS ONE 2015, 10, e0128332. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Spontaneous long-range calcium waves in developing butterfly wings. BMC Dev. Biol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Iwasaki, M.; Ohno, Y.; Otaki, J.M. Butterfly eyespot organiser: In Vivo imaging of the prospective focal cells in pupal wing tissues. Sci. Rep. 2017, 7, 40705. [Google Scholar] [CrossRef]
- Iwata, M.; Tsutsumi, M.; Otaki, J.M. Developmental dynamics of butterfly wings: Real-time in vivo whole-wing imaging of twelve butterfly species. Sci. Rep. 2018, 8, 16848. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Otaki, J.M. Real-time in vivo imaging of the developing pupal wing tissues in the pale grass blue butterfly Zizeeria maha: Establishing the lycaenid system for multiscale bioimaging. J. Imaging 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, Y.; Otaki, J.M. Protein delivery to insect epithelial cells in vivo: Potential application to functional molecular analysis of proteins in butterfly wing development. BioTech 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Butterfly eyespot color pattern formation requires physical contact of the pupal wing epithelium with extracellular materials for morphogenic signal propagation. BMC Dev. Biol. 2020, 20, 6. [Google Scholar] [CrossRef]
- De Giorgio, E.; Giannios, P.; Espinàs, M.L.; Llimargas, M. A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023, 21, e3001978. [Google Scholar] [CrossRef]
- Palazzo, R.E.; Vogel, J.M.; Schnackenberg, B.J.; Hull, D.R.; Wu, X. Centrosome maturation. Curr. Top. Dev. Biol. 1999, 49, 449–470. [Google Scholar] [CrossRef]
- Bornens, M. The centrosome in cells and organisms. Science 2012, 335, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell. Endocrinol. 2020, 518, 110987. [Google Scholar] [CrossRef]
- Regolini, M. The centrosome as a geometry organizer. In The Golgi Apparatus and Centriole—Results and Problems in Cell Differentiation; Kloc, M., Ed.; Springer: Cham, Switzerland, 2019; Volume 67, pp. 253–276. [Google Scholar] [CrossRef]
- Adler, P.N. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2002, 2, 525–535. [Google Scholar] [CrossRef]
- Eaton, S. Cell biology of planar polarity transmission in the Drosophila wing. Mech. Dev. 2003, 120, 1257–1264. [Google Scholar] [CrossRef]
- McNeill, H. Planar cell polarity: Keeping hairs straight is not so simple. Cold Spring Harb. Perspect. Biol. 2010, 2, a003376. [Google Scholar] [CrossRef]
- Adler, P.N. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr. Top. Dev. Biol. 2012, 101, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.; Adler, P.N. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development 2012, 139, 906–916. [Google Scholar] [CrossRef]
- Adler, P.N.; Sobala, L.F.; Thom, D.; Nagaraj, R. Dusky-like is required to maintain the integrity and planar cell polarity of hairs during the development of the Drosophila wing. Dev. Biol. 2013, 379, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Lu, Q.; Liu, J.; Ling, X.; Wang, W.; Liu, P.; Chen, H. Foldable units and wing expansion of the oakleaf butterfly during eclosion. J. Bionic Eng. 2022, 19, 724–736. [Google Scholar] [CrossRef]
- Locke, M.; Huie, P. Epidermal feet in insect morphogenesis. Nature 1981, 293, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Nardi, J.B. Tracheole migration in an insect wing. Evidence for guidance by epithelial processes. Wilhelm Roux’s Arch. Dev. Biol. 1984, 194, 1–8. [Google Scholar] [CrossRef]
- Locke, M. The structure of epidermal feet during their development. Tissue Cell 1985, 17, 901–921. [Google Scholar] [CrossRef]
- Nardi, J.; Magee-Adams, S.M. Formation of scale spacing patterns in a moth wing: I. Epithelial feet may mediate cell rearrangement. Dev. Biol. 1986, 116, 278–290. [Google Scholar] [CrossRef]
- Delhanty, P.; Locke, M. The development of epidermal feet in preparation for metamorphosis in an insect. Tissue Cell 1989, 21, 891–909. [Google Scholar] [CrossRef]
- Ramírez-Weber, F.-A.; Kornberg, T.B. Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 1999, 97, 599–607. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Kornberg, T.B. Cytonemes and the dispersion of morphogens. WIREs Dev. Biol. 2014, 3, 445–463. [Google Scholar] [CrossRef]
- Kornberg, T.B.; Roy, S. Cytonemes as specialized signaling filopodia. Development 2014, 141, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Scholpp, S. Cytonemes in development. Curr. Opin. Genet. Dev. 2019, 57, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell Stress 2020, 4, 30–43. [Google Scholar] [CrossRef] [PubMed]
- McDougal, A.D.; Kang, S.; Yaqoob, Z.; So, P.T.C.; Kolle, M. In vivo visualization of butterfly scale cell morphogenesis in Vanessa cardui. Proc. Natl. Acad. Sci. USA 2021, 118, e2112009118. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakazato, Y.; Otaki, J.M. Live Detection of Intracellular Chitin in Butterfly Wing Epithelial Cells In Vivo Using Fluorescent Brightener 28: Implications for the Development of Scales and Color Patterns. Insects 2023, 14, 753. https://doi.org/10.3390/insects14090753
Nakazato Y, Otaki JM. Live Detection of Intracellular Chitin in Butterfly Wing Epithelial Cells In Vivo Using Fluorescent Brightener 28: Implications for the Development of Scales and Color Patterns. Insects. 2023; 14(9):753. https://doi.org/10.3390/insects14090753
Chicago/Turabian StyleNakazato, Yugo, and Joji M. Otaki. 2023. "Live Detection of Intracellular Chitin in Butterfly Wing Epithelial Cells In Vivo Using Fluorescent Brightener 28: Implications for the Development of Scales and Color Patterns" Insects 14, no. 9: 753. https://doi.org/10.3390/insects14090753




