Combinations of Lemongrass and Star Anise Essential Oils and Their Main Constituent: Synergistic Housefly Repellency and Safety against Non-Target Organisms
Abstract
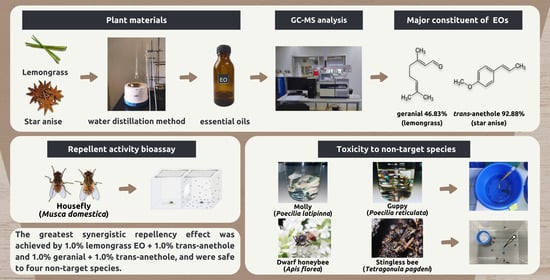
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction of Two Plant Essential Oils
2.2. Chemicals
2.3. The Tested Formulations
2.4. Housefly Rearing
2.5. Repellent Activity Bioassay
2.6. Safety Bioassay of Four Non-Target Species
2.6.1. Bioassay of Dwarf Honeybee and Stingless Bee
2.6.2. Bioassay of Guppy and Molly
2.7. Ethics and Guidelines for Bioassays
2.8. Morphological Alteration
2.9. Statistical Analysis
3. Results
3.1. GC–MS Analysis of Two Plant Essential Oils
3.2. Repellent Activity
3.3. Toxicity to Non-Target Species
3.4. Morphological Alterations after Repellent Bioassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R., IV; Gerry, A.C.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Cortinhas, L.B.; Mendonca, P.M.; Braga, M.V.; Queiroz, M.M.C. Ultrastructure of the immature stages of Musca domestica (Diptera: Muscidae: Muscinae). J. Med. Entomol. 2020, 57, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Issa, R. Musca domestica acts as transport vector hosts. Bull. Natl. Res. Cent. 2019, 43, 73. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Hoharvar, B.; Kenti, T.E. A systemic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Dzialo, M.C.; Spaepen, S.; Nsabimana, D.; Gielens, K.; Devriese, H.; Crauwels, S.; Tito, R.Y.; Raes, J.; Lievens, B.; et al. Microbial communities of the house fly Musca domestica vary with geographical location and habital. Microbiome 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Haselton, A.T.; Acevedo, A.; Kuruvilla, J.; Werner, E.; Kiernan, J.; Dhar, P. Repellency of α-pinene against the house fly, Musca domestica. Phytochemistry 2015, 117, 469–475. [Google Scholar] [CrossRef]
- Chintalchere, J.M.; Dar, M.A.; Raut, K.D.; Pandit, R.S. Bioefficacy of Lemongrass and tea tree essential oils against house fly, Musca domestica. Proc. Natl. Acad. Sci. USA 2021, 91, 307–318. [Google Scholar] [CrossRef]
- Weeks, J.A.; Guiney, P.D.; Nikiforov, A.I. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag. 2012, 8, 120–134. [Google Scholar] [CrossRef]
- Shrestha, B.; Lee, Y. Cellular and molecular mechanisms of DEET toxicity and disase-carrying insect vectors: A review. Genes Genom. 2020, 42, 1131–1144. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- El Zayyat, E.A.; Soliman, M.I.; Elleboudy, N.A.; Ofaa, S.E. Musca domestica laboratory susceptibility to three ethnobotanical culinary plants. Environ. Sci. Pollut. Res. 2015, 22, 15844–15852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Vet. Entomol. 2011, 25, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, A.K. Repellent and insecticidal properties of essential oils against housefly, Musca domestica L. Int. J. Trop. Insect Sci. 1991, 12, 487–491. [Google Scholar] [CrossRef]
- Nteziyaremye, P.; Cherutoi, J.; Makatiani, J.; Muhizi, T. Insecticidal potential of essential oils from Cupressus lusitanica growing in ecological zones of Rwanda against adult housefly, Musca domestica L. Int. J. Trop. Sci. 2023, 43, 895–907. [Google Scholar] [CrossRef]
- Chauhan, N.; Malik, A.; Sharma, S. Repellency potential of essential oils against housefly, Musca domestica L. Environ. Sci. Pollut. Res. 2018, 25, 4707–4714. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verun Hook.f. and their major active constitutes. Ind. Crops Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Takawirapat, W.; Sittichok, S. Ovicidal and repellent activities of several plant essential oils against Periplneta americana L. and enhanced activities from their combined formulation. Sci. Rep. 2022, 12, 12070. [Google Scholar] [CrossRef]
- Soonwera, M.; Phasomkusolsil, S. Efficacy of Thai herbal essential oils as green repellent against mosquito vectors. Acta Trop. 2015, 142, 127–130. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef] [PubMed]
- Sawatthum, A. Role of stingless bee, Tetragonula pegdeni and European honey bee, Apis mellifera in the pollination of confectionery sunflower. Thai J. Sci. Technol. 2020, 9, 368–377. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Crop.: Carol Stream, IL, USA, 2007; ISBN 979-1932633214. [Google Scholar]
- NIST 17. The NIST 17 Mass spectral library (NIST2017/EPA/NIH). In National Standards and Technology; National Institute of Standards and Technology Standard Reference Data Program: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Zellner, B.A.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review. Flavour Fragr. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Soonwera, M. Adulticidal activity against housefly (Musca domestica L.; Muscidae: Diptera) of combinations of Cymbopogon citratus and Eucalyptus globulus essential oils and their major constituents. Int. J. Agric. Technol. 2023, 19, 1127–1134. [Google Scholar]
- Thai Industrial Standards Institute Ministry of Industry. Herbal Repellent Products or Liquid Insect Protection (TISI841/2553). 2023. Available online: http://www.agriman.doae.go.th/home/Research/Herb57/5.pdf (accessed on 8 January 2024).
- Pashte, V.V.; Patil, C.S. Toxicity and poisoning symptoms of selected insecticides to honey bees (Apis mellifera mellifera L.). Arch. Biol. Sci. 2018, 70, 5–12. [Google Scholar] [CrossRef]
- Chibee, G.U.; Ojelabi, O.; Fajana, H.; Akinpelu, B.; Kehinde, T.; Awodriran, M.U.; Obuotor, E.M.; Owojori, O.J. Effects of cypermethrin as a model chemical on life cycle and biochemical responses of the tropical stingless bee Meliponula bocandei Spinola, 1853. Environ. Adv. 2018, 5, 100074. [Google Scholar] [CrossRef]
- The National Research Council of Thailand. Ethical Principles and Guidelines for the Use of Animals. 2023. Available online: https://labanimals.nrct.go.th./ACT/ (accessed on 5 January 2024).
- ARRIVE. The Animal Research: Reporting of In Vivo Experiment Guidelines. 2023. Available online: https://arriveguidelines.org/arrive-guidelines (accessed on 5 January 2024).
- Rutledge, L.C.; Wirtz, R.A.; Buescher, M.D.; Mehr, Z.A. Mathematical models of the effectiveness and persistence of mosquito repellents. J. Am. Mosq. Control Assoc. 1985, 1, 56–62. [Google Scholar] [PubMed]
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef]
- Atkovska, K.; Kuvendziev, S.; Mustafa, E.; Marinkovski, M.; Ghaffari, P.; Lisichkov, K. Essential oils as green repellents against mosquito vectors. Qual. Life 2021, 12, 51–60. [Google Scholar] [CrossRef]
- Nararak, J.; Sanguanpong, U.; Sukkanon, C.; Manguin, S.; Chareonviriyaphap, T. Synergistic repellent and irritant effects of a mixture of β-caryophyllene oxide and vetiver oil against mosquito vectors. Insects 2023, 14, 773. [Google Scholar] [CrossRef]
- Verma, B.K.; Verma, R.S.; Chuahan, A.; Bisht, A. Evaluation of essential oil yield and chemical composition of eight lemongrass (Cymbopogon spp.) cultivars under Himalayan region. J. Essent. Oil Res. 2015, 27, 197–203. [Google Scholar] [CrossRef]
- Ranitha, M.; Abdurahman, H.N.; Ziad, A.S.; Azhari, H.N.; Thana, R.S. A comparative study of lemongrass (Cymbopogon citratus) essential oil extracted by microwave- assisted hydro distillation (MAHD) and conventional hydrodistillation (HD) method. Int. J. Chem. Eng. Appl. 2014, 5, 104–108. [Google Scholar] [CrossRef]
- Thanh, N.D.B.; Duc, T.H.; Dung, N.T. Kinetics and modeling of oil extraction from Vietnam lemongrass by steam distillation. Vietnam J. Sci. Technol. 2017, 55, 58–65. [Google Scholar] [CrossRef]
- Madi, Y.F.; Choucry, M.A.; Meselhy, M.R.; El-Kashoury, E.S.A. Essential oil of Cymbopogon citratus cultivation in Egypt: Seasonl variation in chemical composition and anticholinesterase activity. Nat. Prod. Res. 2021, 35, 4063–4067. [Google Scholar] [CrossRef]
- Bossou, A.D.; Mangelinckx, S.; Yedomonhan, H.; Boko, P.M.; Akogbeto, M.C.; Kimpe, N.D.; Avlessi, F.; Sohounhloue, D.C.K. Chemical composition and insecticidal activity of plant essential oil from Benin against Anopheles gambiae (Giles). Parasites Vectors 2013, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Rahimi-Nasrabadi, M.; Chalabi, H. Determination of essential oil components of star anise (Illicium verum) using simultaneous hydro distillation-static headspace liquid-phase micro extraction-gas chromatography mass spectrometry. Anal. Lett. 2009, 42, 1382–1397. [Google Scholar] [CrossRef]
- Outemsaa, B.; Oubihi, A.; Jaber, H.; Kenfaeoui, I.; Ihamdan, R.; Azhari, H.E.; Ouhssine, M. Chemical composition, antioxidant and antimicrobial activities of the essential oil of Illicium verum. Int. Congr. Health Vigil. 2021, 319, 01052. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef] [PubMed]
- Koba, K.; Sanda, K.; Guyon, C.; Raynaud, C.; Chaumont, J.P.; Nicod, L. In vitro cytotoxic activity of Cymbopogon citratus L. and Cymbopogon nardus L. essential oils from Togo. Bangladesh J. Pharmacol. 2009, 4, 29–34. [Google Scholar] [CrossRef]
- Mohamed Hanaa, A.R.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemongrass (Cymbopogon citratus) essential oil as a potential anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Awad, M.; Amer, A.; Hassan, N.N.; Ibarhin, E.S.; Ali, H.M.; Akrami, M.; Salem, M.Z.M. Insecticidal activity of lemongrass essential oil as an eco-friendly agent against the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 2021, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Z.T.; Sanchez, F.F.; Santos, A.R.d.; Amara, A.C.F.; Ferreira, J.L.P.; Escalona-Arranz, J.C.; Queiroz, M.M.d.C. Chemical composition and insecticidal activity of Cymbopogon citratus essential oil from Cuba and Brazil against housefly. Rev. Bras. Paraitol. Vet. 2015, 24, 36–44. [Google Scholar] [CrossRef]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A comprehensive review of the pharmacology, chemistry, traditional uses and quality control of star anise (Illicium verum Hook. F.): An aromatic medicinal plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef] [PubMed]
- Tajidin, N.E.; Ahmad, S.H.; Rosenani, A.B.; Azimah, H.; Munirah, M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stage. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef]
- Rocha, R.P.; de Melo, E.C.; Barbosa, L.C.A.; dos Santos, R.H.S.; Cecon, P.R.; Dallacort, R.; Sati, A. Influence of plant age on the content and composition of essential oil of Cymbopogon citratus (D.C.) Staff. J. Med. Plant Res. 2014, 8, 1121–1126. [Google Scholar] [CrossRef]
- Saby, B.A.; Farouk, A.; Badr, A.N. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Helliyon 2021, 7, e07721. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jnanesha, A.C.; Lal, R.K. Coppicing impact on the essential oil yield and its chemical composition of lemongrass cultivars of the genus Cymbopogon under–arid region of South India. Acta Ecol. Sinica 2023, 43, 20–26. [Google Scholar] [CrossRef]
- Enriquez-Estrella, M.A.; Poveda-Diaz, S.E.; Alvarado-Huatatoca, G.I. Bioactives of lemongrass used in the industry. Rev. Mex. Cienc. Agric. 2023, 14, 1–11. [Google Scholar] [CrossRef]
- Das, N.G.; Dhiman, S.; Talukdar, P.K.; Rabha, B.; Goswami, D.; Veer, V. Synergistic mosquito-repellent activity of Curcuma longa, Pogostemon heyneanus and Zanthoxylum limonella essential oils. J. Infect. Public Health 2015, 8, 323–328. [Google Scholar] [CrossRef]
- Soonwera, M.; Sittichok, S. Adulticidal activities of Cymbopogon citratus (Stapf.) and Eucalyptus globulus (Labill.) essential oils and their synergistic combination against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. 2020, 27, 20201–20214. [Google Scholar] [CrossRef]
- Lachance, S.; Grange, G. Repellent effectiveness of seven plant essential oils, sunflower oil and natural insecticides against horn flies on pastured dairy cows and heifers. Med. Vet. Entomol. 2014, 28, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Phasomkusolsil, S.; Soonwera, M. Insect repellent activity of medicinal plant oils against Aedes aegypti (Linn.), Anopheles minimus (Theobald) and Culex quinquefasciatus Say base on protection time and biting rate. Southeast Asian J. Trop. Med. Public. Health 2010, 41, 831–840. [Google Scholar] [PubMed]
- Sritabutra, D.; Soonwera, M. Repellent activity of herbal essential oils against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say). Asian Pac. J. Trop. Dis. 2013, 3, 271–276. [Google Scholar] [CrossRef]
- Pavela, R. Acute and synergistic effects of some monoterpenoid essential oil compound on the housefly (Musca domestica). J. Essent. Oil-Bear. Plants 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B.; et al. Essential oils for a sustainable control of honeybee varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Ethnobotabical knowledge on botanical repellents employed in the African region against mosquito vectors-A review. Exp. Parasitol. 2016, 167, 103–108. [Google Scholar] [CrossRef]
- Baldacchino, F.; Tramut, C.; Salem, A.; Lienard, E.; Deletre, E.; Franc, M.; Martin, T.; Duavallet, G.; Jay-Robert, P. The repellency of lemongrass oil against stable flies, tested using video tracking. Parasite 2013, 20, 21. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Elumalai, K.; Devanesan, S.; Govindarajan, M.; Krishnappa, K.; Maggi, F. The aromatic ginger Kaemferia galangal L. (Zingiberaceae) essential oil and its main composition are effective larvicidal agents against Aedes vittatus and Anopheles maculatus without toxicity on the non-target aquatic fauna. Ind. Crops Prod. 2020, 158, 113012. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Larvicidal activity of the essential oil from Amomum subulatum Roxb. (Zingiberaceae) against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae), and non-target impact on four mosquito natural enemies. Physiol. Mol. Plant Pathol. 2018, 101, 219–224. [Google Scholar] [CrossRef]
- Rajeswary, M.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Zingiber cernuum (Zingiberaceae) essential oil as effective larvicide and oviposition deterrent on six mosquito vectors, with little non-target toxicity on four aquatic mosquito predators. Environ. Sci. Pollut. Res. 2018, 25, 10307–10316. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Mujchariyakul, W.; Laksanavilat, N.; Junhirun, P. Acute toxicity of essential oil compound (thymol and 1,8-cineole) to insectivorous guppy, Poecilia reticulata Peters, 1859. Agric. Nat. Resour. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Sabahi, Q.; Hamiduzzaman, M.M.; Barajas-Perez, J.S.; Tapia-Gonzalez, J.M.; Guzman-Novoa, E. Toxicity of anethole and the essential oils of lemongrass and sweet marigold to parasitic mite Varroa destructor and their selective for honey bee (Apis mellifera) workers and larvae. Psyche 2018, 2018, 6196289. [Google Scholar] [CrossRef]
- Burgger, B.P.; Martinez, L.C.; Plata-Rueda, A.; de Castro e Castro, B.M.; Soares, M.A.; Wilcken, C.F.; Carvalho, A.G.; Serrao, J.E.; Zanuncio, J.C. Bioactive of the Cymbopogon citratus (Poaceae) essential oil and its terpenoids constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2019, 9, 8358. [Google Scholar] [CrossRef]
- Zenobio, J.E.; Sanchez, B.C.; Archuleta, L.C.; Sepulveda, M.S. Effects of triclocarban, N,N,-diethyl-meta-toluamide, and a mixture of pharmaceuticals and personal care products of fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2014, 33, 910–919. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Li, J.; Ai, S.; Fu, X.; Fan, B.; Li, W.; Liu, Z. Aquatic life criteria derivation and ecological risk assessment of DEET in China. Ecotoxicol. Environ. Saf. 2020, 188, 109881. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Narlioglou, M.; Hatzis, T. Toxic encephalopathy associated with use of DEET insect repellents: A case analysis of its toxicity in children. Hum. Exp. Toxicol. 2001, 20, 8–14. [Google Scholar] [CrossRef]
- Swale, D.R.; Bloomquist, J.R. Is DEET a dangerous neurotoxicant? Pest. Manag. Sci. 2019, 75, 2068–2070. [Google Scholar] [CrossRef] [PubMed]
- Chen-Hussey, V.; Behrens, R.; Logan, L.G. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasites Vectors 2014, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Monkonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Shnha, S.; Jothiramajayam, M.; Ghosh, M.; Mukherjee, A. Evaluation of toxicity of essential oils palmarasa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 2014, 68, 71–77. [Google Scholar] [CrossRef]
- Asif, M.; Yehya, A.H.S.; Al-Mansoub, M.A.; Revadigar, V.; Ezzat, M.O.; Ahamed, M.B.K.; Oon, C.E.; Murugaiyah, V.; Majid, A.S.A.; Majid, A.M.S.A. Anticancer attributes of Illicium verum essential oil against colon cancer. S. Afr. J. Bot. 2016, 103, 156–161. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soonwera, M.; Sinthusiri, J.; Passara, H.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Murata, K. Combinations of Lemongrass and Star Anise Essential Oils and Their Main Constituent: Synergistic Housefly Repellency and Safety against Non-Target Organisms. Insects 2024, 15, 210. https://doi.org/10.3390/insects15030210
Soonwera M, Sinthusiri J, Passara H, Moungthipmalai T, Puwanard C, Sittichok S, Murata K. Combinations of Lemongrass and Star Anise Essential Oils and Their Main Constituent: Synergistic Housefly Repellency and Safety against Non-Target Organisms. Insects. 2024; 15(3):210. https://doi.org/10.3390/insects15030210
Chicago/Turabian StyleSoonwera, Mayura, Jirisuda Sinthusiri, Hataichanok Passara, Tanapoom Moungthipmalai, Cheepchanok Puwanard, Sirawut Sittichok, and Kouhei Murata. 2024. "Combinations of Lemongrass and Star Anise Essential Oils and Their Main Constituent: Synergistic Housefly Repellency and Safety against Non-Target Organisms" Insects 15, no. 3: 210. https://doi.org/10.3390/insects15030210