Gut Microbiota Affects Host Fitness of Fall Armyworm Feeding on Different Food Types
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Food Types
2.2. Performance of FAWs Feeding on Different Food Types
2.3. Determining the Diversity of FAW Gut Microbial Communities after Feeding on Different Food Types
2.3.1. Sample Collection and DNA Extraction
2.3.2. PCR Amplification and 16S rDNA Sequencing
2.4. Data Analysis
3. Results
3.1. Performances of FAWs Feeding on Different Food Types
3.2. Diversity of FAW Gut Microbial Communities after Feeding on Different Food Types
3.3. Correlations between Gut Microbes and FAW Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Guo, J.F.; Zhang, M.D.; Gao, Z.P.; Wang, D.J.; He, K.L.; Wang, Z.Y. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. 2021, 28, 602–610. [Google Scholar] [CrossRef]
- He, L.M.; Wu, Q.L.; Gao, X.W.; Wu, K.M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Wu, Z.W.; Shi, P.Q.; Zeng, Y.H.; Huang, W.F.; Huang, Q.Z.; Ma, X.H.; Guo, L.Z. Population life tables of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on three host plants. Plant Prot. 2019, 45, 59–64. [Google Scholar] [CrossRef]
- Xu, P.J.; Zhang, D.D.; Wang, J.; Wu, K.M.; Wang, X.W.; Wang, X.F.; Ren, G.W. The host perference of Spodoptera frugiperda on maize and tobacco. Plant Prot. 2019, 45, 61–64. [Google Scholar] [CrossRef]
- Yang, X.M.; Sun, X.X.; Zhao, S.Y.; Li, J.Y.; Chi, X.C.; Jiang, Y.Y.; Wu, K.M. Population occurrence, spatial distribution and sampling technique of fall armyworm Spodoptera frugiperda in wheat fields. Plant Prot. 2020, 46, 10–16. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, M.; Liu, H.H.; Xiao, Q.; Li, X.Y. Occurrence and control of Spodoptera frugiperda in early sowing wheat field in northern Jiangsu province. Plant Prot. 2020, 46, 212–215. [Google Scholar] [CrossRef]
- Xu, L.N.; Chen, Y.T.; Xu, T.T.; Bi, S.J.; Tong, Q.; Hu, B.J.; Hu, F.; Wang, Z.Y. Fall armyworm damaging rice seedlings in Anhui province. Plant Prot. 2022, 48, 310–313. [Google Scholar] [CrossRef]
- Yang, J.J.; Tao, Y.Q.; LIu, Q.; Zheng, Z.W.; Zhou, H.Z. Rice seedlings were damaged by fall armyworm in Wuxue of Hubei province. China Plant Prot. 2020, 40, 44–45. (In Chinese) [Google Scholar]
- Zhang, H. Preliminary report of rice seedlings affected by fall armyworm and biotype identification in Yunxiao of Fujian province. China Plant Prot. 2020, 40, 41–43+53. (In Chinese) [Google Scholar]
- Zhang, L.; Jin, M.H.; Zhang, D.D.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular identification of invasive fall armyworm Spodoptera frugiperda in Yunnan Province. Plant Prot. 2019, 45, 19–24. (In Chinese) [Google Scholar] [CrossRef]
- Xu, L.N.; Hu, B.J.; Su, J.Y.; Qi, R.D.; Su, W.H.; Qiu, K.; Zhou, Z.Y.; Zheng, Z.Y.; Zhang, Q.Y.; Hu, F.; et al. Genetic analysis of the fall armyworm Spodoptera frugiperda invaded in Anhui province. Plant Prot. 2019, 45, 47–53. [Google Scholar] [CrossRef]
- Ansari, M.S.; Hasan, F.; Ahmad, N. Influence of various host plants on the consumption and utilization of food by Pieris brassicae (Linn.). Bull. Entomol. Res. 2012, 102, 231–237. [Google Scholar] [CrossRef]
- Truzi, C.C.; Holzhausen, H.G.; Alvaro, J.C.; De Laurentis, V.L.; Vieira, N.F.; Vacari, A.M.; De Bortoli, S.A. Food consumption utilization, and life history parameters of Helicoverpa armigera (Lepidoptera: Noctuidae) reared on diets of varying protein level. J. Insect Sci. 2019, 19, 12. [Google Scholar] [CrossRef]
- Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. Host-plant adaptation in fall armyworm host strains: Comparison of food consumption, utilization, and detoxication enzyme activities. Ann. Entomol. Soc. Am. 1995, 88, 80–91. [Google Scholar] [CrossRef]
- Silva-Brandão, K.L.; Horikoshi, R.J.; Bernardi, D.; Omoto, C.; Figueira, A.; Brandao, M.M. Transcript expression plasticity as a response to alternative larval host plants in the speciation process of corn and rice strains of Spodoptera frugiperda. BMC Genom. 2017, 18, 792. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.W.; Zhang, J.M.; Zhang, Z.J.; Huang, J.; Wang, L.K.; Khan, M.M.; Shah, S.; Fernandez-Grandon, G.M.; Lu, Y.B. Role of digestive protease enzymes and related genes in host plant adaptation of a polyphagous pest, Spodoptera frugiperda. Insect Sci. 2021, 28, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, G.P. The consumption and utilization of food by insects. In Advances in Insect Physiology; Beament, J.W.L., Treherne, J.E., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 5, pp. 229–288. [Google Scholar] [CrossRef]
- Parra, J.R.P.; Panizzi, A.R.; Haddad, M.L. Nutritional indices for measuring insect food intake and utilization. In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 13–49. [Google Scholar] [CrossRef]
- Biere, A.; Bennett, A.E.; Fox, C. Three-way interactions between plants, microbes and insects. Funct. Ecol. 2013, 27, 567–573. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Peiffer, M.; Tan, C.W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D.; et al. Fall armyworm-associated gut bacteria modulate plant defense responses. Mol. Plant-Microbe Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 20. [Google Scholar] [CrossRef]
- Biere, A.; Tack, A.J.M.; Bennett, A. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct. Ecol. 2013, 27, 646–660. [Google Scholar] [CrossRef]
- Ge, S.X.; Shi, F.M.; Pei, J.H.; Hou, Z.H.; Zong, S.X.; Ren, L.L. Gut bacteria associated with Monochamus saltuarius (Coleoptera: Cerambycidae) and their possible roles in host plant adaptations. Front. Microbiol. 2021, 12, 687211. [Google Scholar] [CrossRef]
- Bost, A.; Martinson, V.G.; Franzenburg, S.; Adair, K.L.; Albasi, A.; Wells, M.T.; Douglas, A.E. Functional variation in the gut microbiome of wild Drosophila populations. Mol. Ecol. 2018, 27, 2834–2845. [Google Scholar] [CrossRef]
- Schretter, C.E.; Vielmetter, J.; Bartos, I.; Marka, Z.; Marka, S.; Argade, S.; Mazmanian, S.K. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature 2018, 563, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Gut microbes alter the walking activity of fruit flies. Nature 2018, 563, 2. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef] [PubMed]
- Gichuhi, J.; Sevgan, S.; Khamis, F.; Van den Berg, J.; du Plessis, H.; Ekesi, S.; Herren, J.K. Diversity of fall armyworm, Spodoptera frugiperda and their gut bacterial community in Kenya. PeerJ 2020, 8, e8701. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, R.N. Improvements in the identification of strains facilitate population studies of fall armyworm subgroups. Ann. Entomol. Soc. Am. 2012, 105, 351–358. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2023. [Google Scholar]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.; Zaneveld, J.; Caporaso, J.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Couture, J.J.; Mason, C.J.; Habeck, C.W.; Lindroth, R.L. Behavioral and morphological responses of an insect herbivore to low nutrient quality are inhibited by plant chemical defenses. Arthropod-Plant Interact. 2016, 10, 341–349. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Zhou, C.; Long, G.Y.; Yang, X.B.; Wei, Z.Y.; Liao, Y.J.; Yang, H.; Hu, C.X. Fitness of fall armyworm, Spodoptera frugiperda to three solanaceous vegetables. J. Integr. Agric. 2021, 20, 755–763. [Google Scholar] [CrossRef]
- Moran, N.A.; Ochman, H.; Hammer, T.J. Evolutionary and ecological consequences of gut microbial communities. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Mikaelyan, A.; Dietrich, C.; Kohler, T.; Poulsen, M.; Sillam-Dusses, D.; Brune, A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef]
- Benjamino, J.; Lincoln, S.; Srivastava, R.; Graf, J. Low-abundant bacteria drive compositional changes in the gut microbiota after dietary alteration. Microbiome 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, L.; Ikonen, S.; Makkonen, H.; Hanski, I. Larval growth rate is associated with the composition of the gut microbiota in the Glanville fritillary butterfly. Oecologia 2016, 181, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Graf, J. Lessons from digestive-tract symbioses between bacteria and invertebrates. Annu. Rev. Microbiol. 2016, 70, 375–393. [Google Scholar] [CrossRef]
- Jing, T.Z.; Qi, F.H.; Wang, Z.Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, X.; De Mandal, S.; Shakeel, M.; Hua, Y.; Shoukat, R.F.; Fu, D.; Jin, F. Gut microbiota mediate Plutella xylostella susceptibility to Bt Cry1Ac protoxin is associated with host immune response. Environ. Pollut. 2021, 271, 116271. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Wang, H.; Liao, L.; Feng, Y.; Fan, X.; Zhang, L.; Chen, S. Kinetics and novel degradation pathway of permethrin in Acinetobacter baumannii ZH-14. Front. Microbiol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Saati-Santamaria, Z.; Rivas, R.; Kolarik, M.; Garcia-Fraile, P. A new perspective of Pseudomonas-Host Interactions: Distribution and potential ecological functions of the genus Pseudomonas within the bark beetle holobiont. Biology 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ling, X.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. Serratia symbiotica enhances fatty acid metabolism of pea aphid to promote host development. Int. J. Mol. Sci. 2021, 22, 5951. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.L.; Zhang, V.; Lamberti, L.; Jones, E.W.; Obadia, B.; Korasidis, N.; Gavryushkin, A.; Carlson, J.M.; Beerenwinkel, N.; Ludington, W.B. Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. USA 2018, 115, E11951–E11960. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.; Kostiuk, B.; Foley, E.; Pukatzki, S. Commensal pathogen competition impacts host viability. Proc. Natl. Acad. Sci. USA 2018, 115, 7099–7104. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, Y.; Guo, L.; Wang, A.; Lu, M.; Xu, L. Variation of gut microbiota caused by an imbalance diet is detrimental to bugs’ survival. Sci. Total Environ. 2021, 771, 144880. [Google Scholar] [CrossRef]

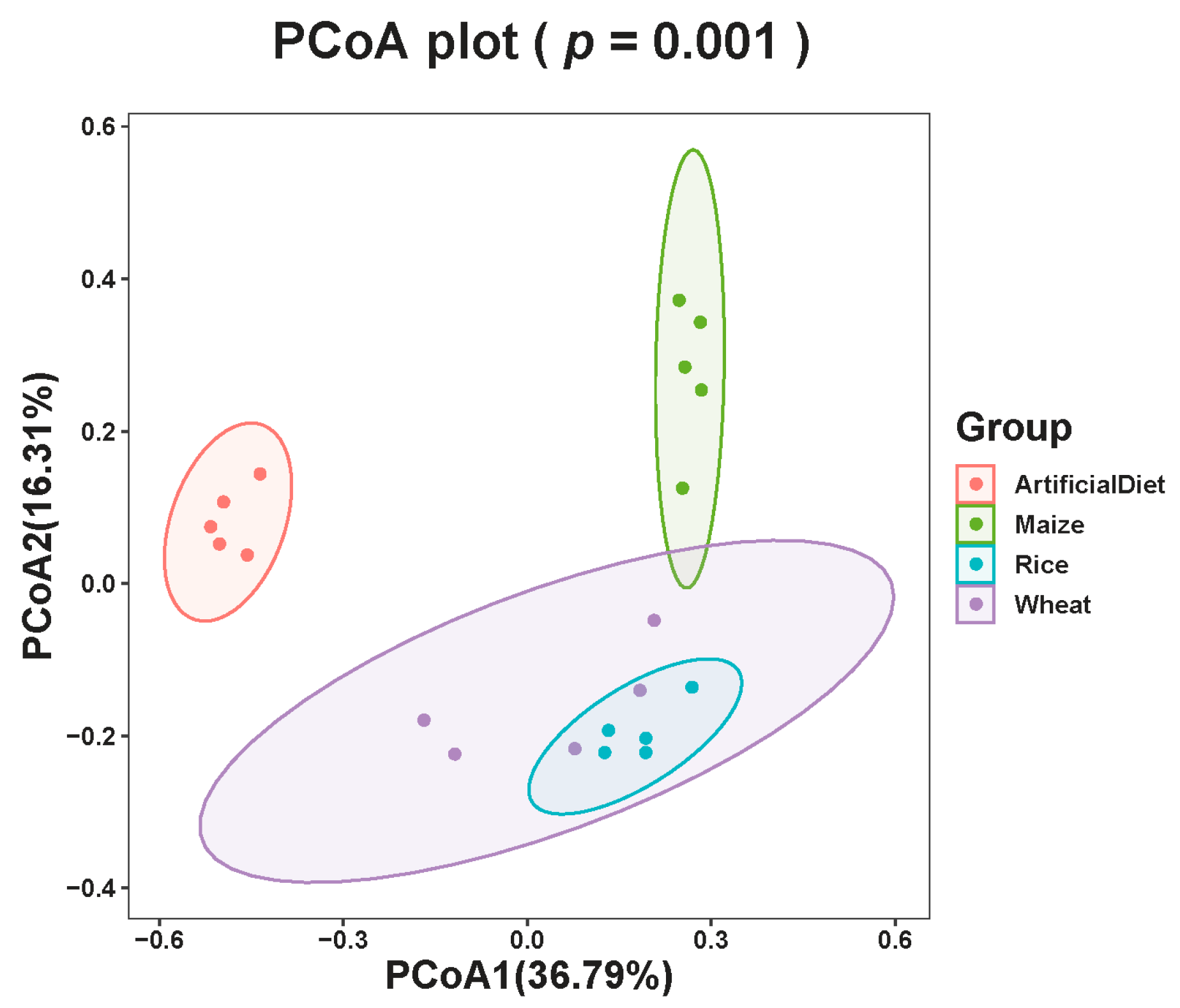


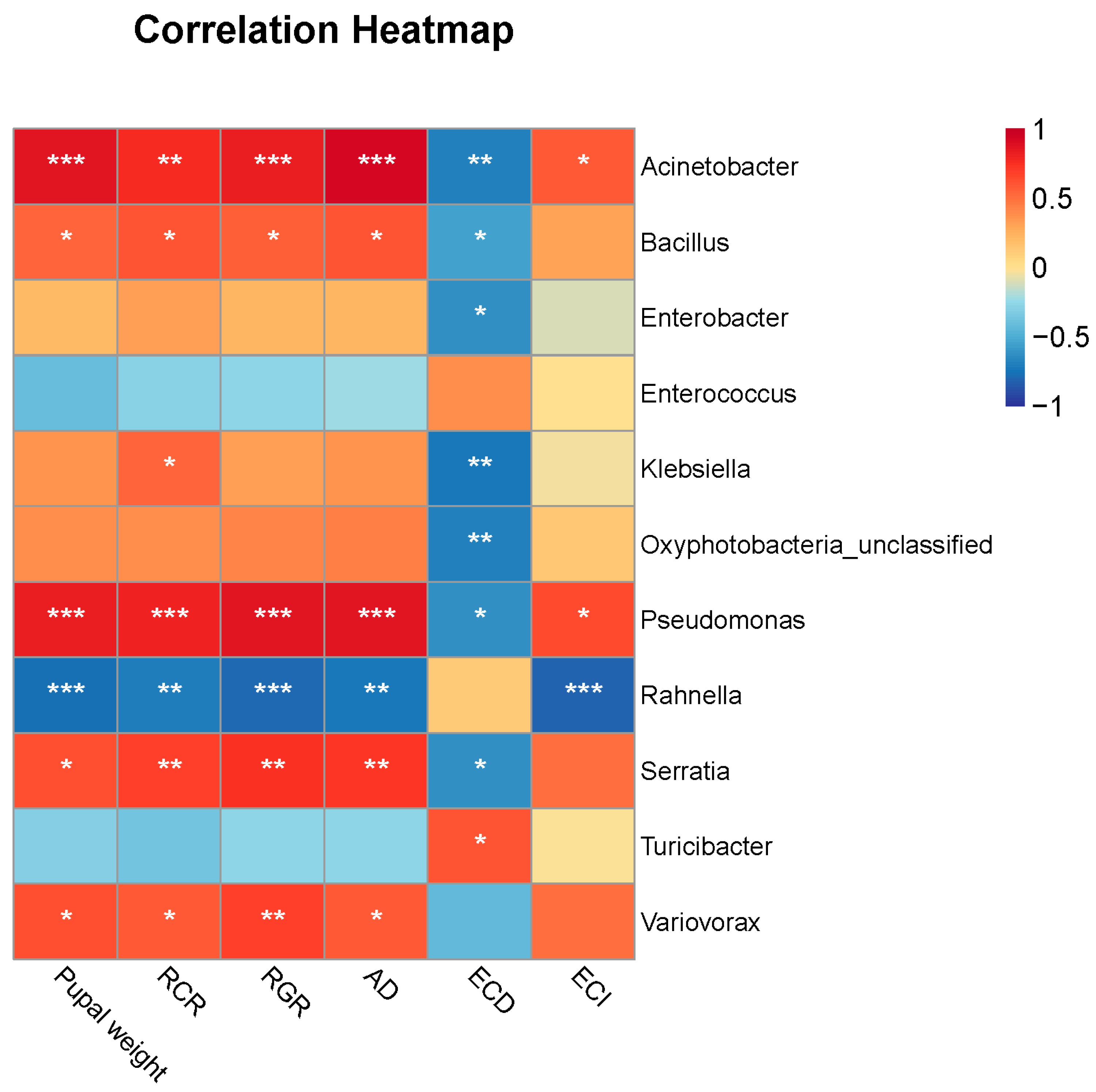
| Parameter | Artificial Diet | Maize | Wheat | Rice | ||||
|---|---|---|---|---|---|---|---|---|
| Intrinsic rate of increase, r (d−1) | 0.19 ± 0.00 a | 0.18 ± 0.00 a | 0.19 ± 0.00 a | 0.15 ± 0.01 b | ||||
| Finite rate of increase, λ (d−1) | 1.21 ± 0.00 a | 1.20 ± 0.00 a | 1.21 ± 0.00 a | 1.17 ± 0.01 b | ||||
| Net reproductive rate, R0 (offspring) | 573.01 ± 95.19 a | 306.99 ± 65.71 bc | 321.83 ± 61.13 b | 162.15 ± 39.31 c | ||||
| Mean generation time, T (d) | 32.79 ± 0.28 a | 30.79 ± 0.31 b | 30.08 ± 0.38 b | 32.98 ± 0.69 a | ||||
| APOP (for female) | n | days | n | days | n | days | n | days |
| 28 | 4.03 ± 0.17 ab | 20 | 3.80 ± 0.22 ab | 25 | 3.60 ± 0.21 b | 16 | 4.50 ± 0.35 a | |
| Oviposition days (for female) | n | days | n | days | n | days | n | days |
| 37 | 6.82 ± 0.22 a | 21 | 6.70 ± 0.46 a | 35 | 7.04 ± 0.67 a | 21 | 7.81 ± 0.59 a | |
| Mean fecundity (for reproductive female) | n | offspring/ individual | n | Offspring/ individual | n | offspring/ individual | n | offspring/ individual |
| 37 | 1548.68 ± 161.33 a | 21 | 1461.86 ± 134.09 a | 35 | 919.51 ± 122.08 b | 21 | 772.14 ± 114.69 b | |
| Indexes | Food Types | |||
|---|---|---|---|---|
| Artificial Diet | Maize | Wheat | Rice | |
| Dry/fresh weight ratio of food (%) | 21.32 ± 0.10 a | 8.24 ± 0.08 d | 14.19 ± 0.08 c | 14.97 ± 0.18 b |
| Fresh weight of FAW larvae after feeding for 24 h (mg) | 322.48 ± 8.64 a | 258.55 ± 4.79 b | 262.77 ± 7.74 b | 221.86 ± 5.86 c |
| Dry weight of FAW larvae after feeding for 24 h (mg) | 58.14 ± 1.97 a | 43.80 ± 1.27 b | 40.12 ± 1.14 b | 24.75 ± 0.60 c |
| RCR (%) | 194.83 ± 5.35 c | 381.10 ± 6.58 a | 294.33 ± 11.34 b | 271.94 ± 5.61 b |
| RGR (%) | 74.13 ± 1.24 a | 76.94 ± 1.07 a | 56.87 ± 2.64 b | 38.25 ± 1.06 c |
| AD (%) | 68.39 ± 1.28 a | 45.04 ± 1.16 b | 31.87 ± 0.73 c | 26.51 ± 1.24 d |
| ECD (%) | 56.87 ± 2.29 a | 45.95 ± 2.06 b | 61.16 ± 1.99 a | 55.66 ± 3.15 a |
| ECI (%) | 38.51 ± 1.08 a | 20.29 ± 0.44 b | 19.36 ± 0.55 b | 14.14 ± 0.43 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, D.; Ren, Q.; Sun, J.; Zhang, L.; Cheng, Y.; Jiang, X. Gut Microbiota Affects Host Fitness of Fall Armyworm Feeding on Different Food Types. Insects 2024, 15, 304. https://doi.org/10.3390/insects15050304
Ma L, Wang D, Ren Q, Sun J, Zhang L, Cheng Y, Jiang X. Gut Microbiota Affects Host Fitness of Fall Armyworm Feeding on Different Food Types. Insects. 2024; 15(5):304. https://doi.org/10.3390/insects15050304
Chicago/Turabian StyleMa, Lin, Daotong Wang, Qilin Ren, Jiaqi Sun, Lei Zhang, Yunxia Cheng, and Xingfu Jiang. 2024. "Gut Microbiota Affects Host Fitness of Fall Armyworm Feeding on Different Food Types" Insects 15, no. 5: 304. https://doi.org/10.3390/insects15050304






