Enzymatic Activity of Soil on the Occurrence of the Endangered Beetle Cheilotoma musciformis (Coleoptera: Chrysomelidae) in Xerothermic Grasslands
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
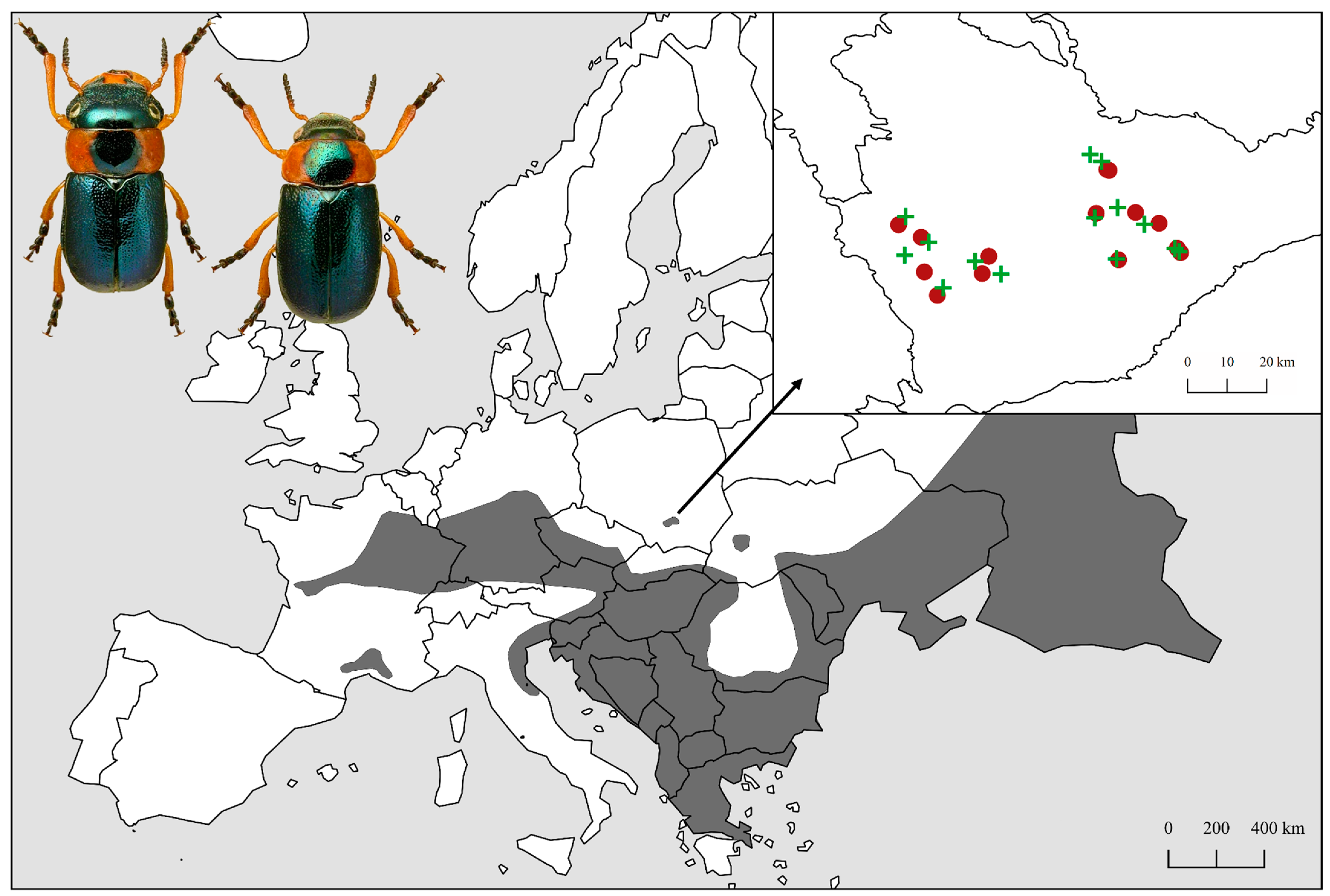
2.1. Study Area
2.2. Beetle Sampling
2.3. Field Study
2.4. Laboratory Tests
2.5. Data Analysis
3. Results
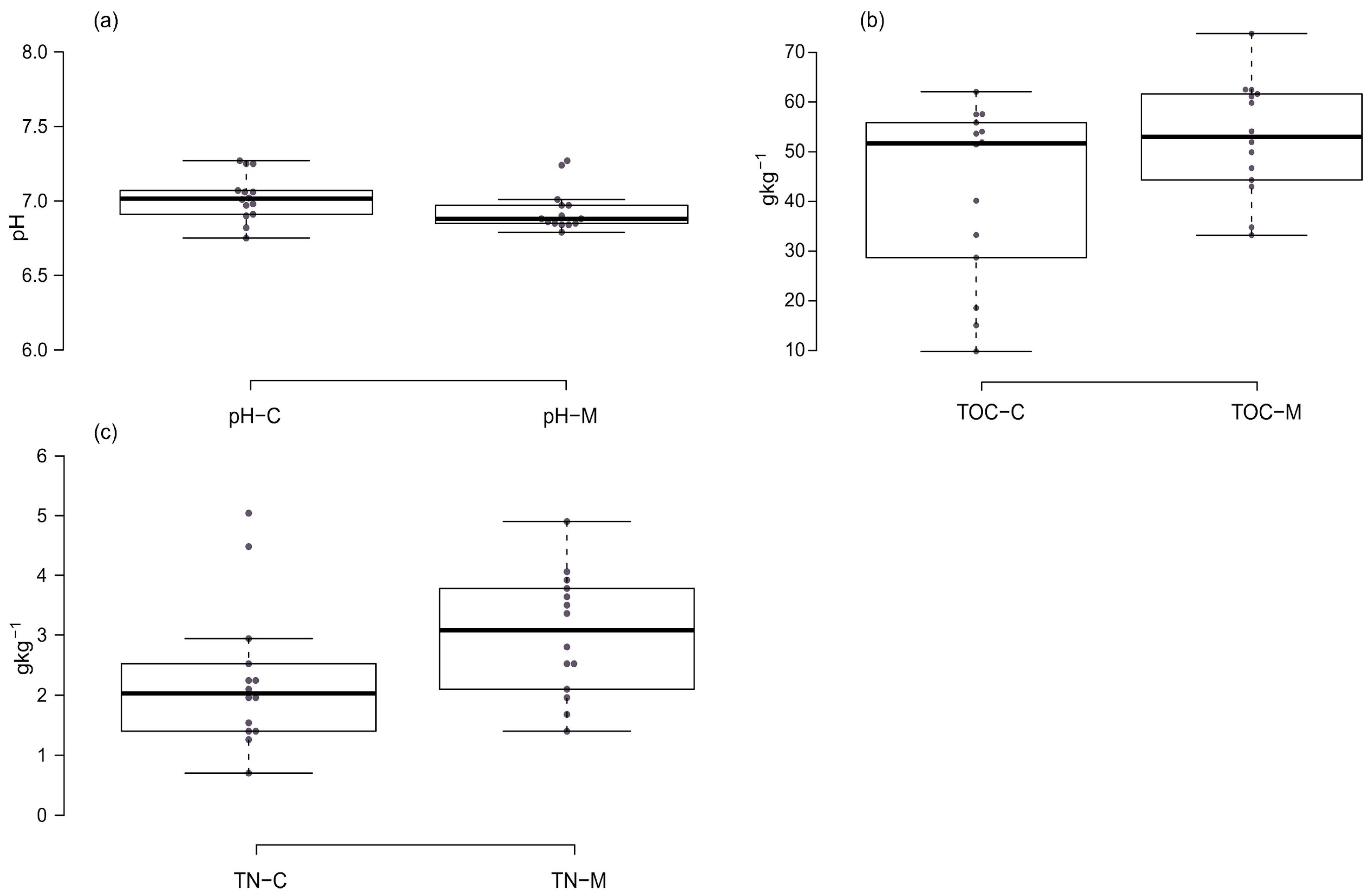
3.1. pHKCl Values of the Tested Soils
3.2. Content of Total Organic Carbon (TOC) and Total Nitrogen (TN) in Tested Soils
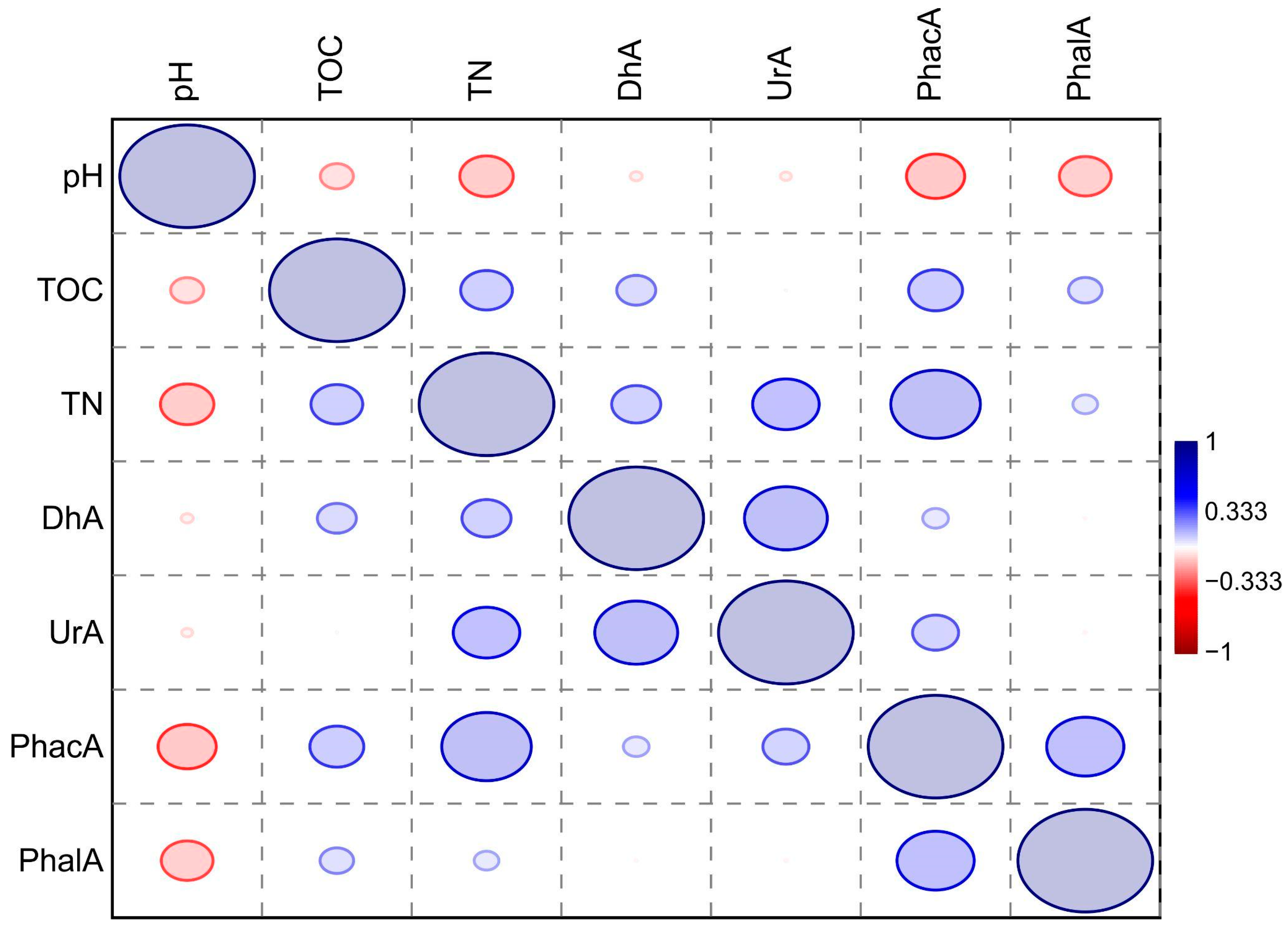
3.3. Activity of Soil Enzymes in Tested Soils
4. Discussion
4.1. The Activity of Enzymes in Soil
4.2. The Physicochemical Properties of Soils
4.3. Soil Condition Consequences for the Occurrence of the Beetle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Löbl, I.; Smetana, A. Catalogue of Palearctic Coleoptera. Vol. 6. Chrysomeloidea; Apollo Books: Stenstrup, Denmark, 2010. [Google Scholar]
- Warchałowski, A. Chrysomelidae. Stonkowate (Insecta: Coleoptera). Część II (Podrodziny: Clytrinae i Cryptocephalinae). Fauna Polski, tom 13; PWN: Warsaw, Poland, 1991. [Google Scholar]
- Warchałowski, A. Chrysomelidae. The Leaf-Beetles of Europe and the Mediterranean Area; Natura Optima Dux Foundation: Warsaw, Poland, 2003. [Google Scholar]
- Lopatin, I.K.; Aleksandrovich, O.R.; Konstantinov, A.S. Check List of Leaf-Beetle Chrysomelidae (Coleoptera) of the Eastern Europe and Northern Asia; Mantis: Olsztyn, Poland, 2004. [Google Scholar]
- Borowiec, L.; Ścibior, R.; Kubisz, D. Critical check-list of the Polish Chrysomeloidea, excluding Cerambycidae (Coleoptera: Phytophaga). Genus 2011, 22, 579–608. [Google Scholar]
- Lopatin, I.; Chikatunov, V.; Pavlíček, T. Catalogue of the beetles (Coleoptera) in Israel and adjacent areas: 3. Chrysomelidae (except Alticinae). Zool. Middle East. 2003, 28, 87–112. [Google Scholar] [CrossRef]
- Mazur, M.A.; Kubisz, D.; Ścibior, R.; Kajtoch, Ł. Zaciętka Cheilotoma musciformis—wymierający relikt stepowej koleopterofauny w Polsce. Chrońmy Przyr. Ojcz. 2015, 71, 336–346. [Google Scholar]
- Kajtoch, Ł.; Kubisz, D.; Lachowska-Cierlik, D.; Mazur, M.A. Conservation genetics of endangered leaf-beetle Cheilotoma musciformis populations in Poland. J. Insect Conser. 2013, 17, 67–77. [Google Scholar] [CrossRef]
- Kirschner, P.; Záveská, E.; Gamisch, A.; Hilpold, A.; Trucchi, E.; Paun, O.; Sanmartín, I.; Schlick-Steiner, B.C.; Frajman, B.; Arthofer, W.; et al. Long-term isolation of European steppe outposts boosts the biome’s conservation value. Nat. Commun. 2020, 11, 1968. [Google Scholar] [CrossRef]
- Burakowski, B.; Mroczkowski, M.; Stefańska, J. Chrząszcze, Coleoptera, Stonkowate—Chrysomelidae. Część 1. Katalog Fauny Polski (Catalogus faunae Poloniae), 23, 16; PWN: Warsaw, Poland, 1990. [Google Scholar]
- Gruev, B.; Tomov, V. Fauna Bulgarica. 13. Coleoptera, Chrysomelidae. Part I. Orsodacninae, Zeugophorinae, Donaciinae, Criocerinae, Clytrinae, Cryptocephalinae, Lamprosomatinae, Eumolpinae; Aedibus Academie Scientiarum Bulgaricae: Sofia, Bulgaria, 1984. [Google Scholar]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Trophic specialization of leaf beetles (Coleoptera, Chrysomelidae) in the Volga Upland. Biol. Bull. Russ. Acad. Sci. 2015, 42, 863–869. [Google Scholar] [CrossRef]
- Heise, W.; Babik, W.; Kubisz, D.; Kajtoch, Ł. A three-marker DNA barcoding approach for ecological studies of xerothermic plants and herbivorous insects from central Europe. Bot. J. Linn. Soc. 2015, 177, 576–592. [Google Scholar] [CrossRef]
- Kajtoch, L.; Kubisz, D.; Heise, W.; Mazur, M.A.; Babik, W. Plant–herbivorous beetle networks: Molecular characterization of trophic ecology within a threatened steppic environment. Mol. Ecol. 2015, 24, 4023–4038. [Google Scholar] [CrossRef] [PubMed]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; WWiP: Warsaw, Poland, 2012. [Google Scholar]
- Ratyńska, H.; Waldon, B. State of preservation of xerothermic grasslands in Kuyavian-Pomeranian region. Ann. UMCS Sec. C Biol. 2011, 66, 63–83. [Google Scholar] [CrossRef]
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Skripchinsky, A.V.; Brygadyrenko, V.V. Range of Pterostichus oblongopunctatus (Coleoptera, Carabidae) in conditions of global climate change. Biosyst. Divers. 2019, 27, 76–84. [Google Scholar] [CrossRef]
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Brygadyrenko, V.V. Modeling the bioclimatic range of Pterostichus melanarius (Coleoptera, Carabidae) in conditions of global climate change. Biosyst. Divers. 2021, 29, 140–150. [Google Scholar] [CrossRef]
- Sakhraoui, A.; Ltaeif, H.B.; Sakhraoui, A.; Rouz, S.; Castillo, J.M. Potential use of wild Onobrychis species for climate change mitigation and adaptation. Crop Sci. 2023, 63, 3153–3174. [Google Scholar] [CrossRef]
- Erber, D. Biology of Camptosomata Clytrinae–Cryptocephalinae–Chlamisinae–Lamprosomatinae. In Biology of Chrysomelidae; Jolivet, P., Petitpierre, E., Hsiao, T.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 513–552. [Google Scholar]
- Medvedev, L.N. Revision of the genus Cheilotoma Chevrolat, 1837 (Coleoptera: Chrysomelidae: Clytrinae). Russ. Entomol. J. 2004, 13, 35–39. [Google Scholar]
- Chaboo, C.S.; Chamorro, M.L.; Schöller, M. Catalogue of Known Immature Stages of Camptosomate Leaf Beetles (Coleoptera, Chrysomelidae, Cryptocephalinae and Lamprosomatinae). Proc. Entomol. Soc. Wash. 2016, 118, 150–217. [Google Scholar] [CrossRef]
- Willis, K.J.; Van Andel, T.A. Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat. Sci. Rev. 2004, 23, 2369–2387. [Google Scholar] [CrossRef]
- Ashcroft, M.B. Identifying refugia from climate change. J Biogeogr. 2010, 37, 1407–1413. [Google Scholar] [CrossRef]
- Pawłowski, J.; Kubisz, D.; Mazur, M. Coleoptera. In Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Polish Academy of Sciences, Institute of Nature Conservation PAS: Cracow, Poland, 2002; pp. 88–110. [Google Scholar]
- Ścibior, R. Cheilotoma musciformis (Goeze, 1777). In Polish Red Data Book of Animals–Invertebrates; Głowaciński, Z., Nowacki, J., Eds.; Institute of Nature Conservation PAS, Cracow, Agriculture University: Poznań, Poland, 1777; pp. 156–157. [Google Scholar]
- Blab, J.; Nowak, E.; Trautmann, W.; Sukopp, H. Rote Liste der Gefährdeten Tiere und Pflanzen in der Bundesrepublik Deutschland; Kilda-Verlag: Greven, Germany, 1984. [Google Scholar]
- Geiser, R. Rote Liste der Käfer (Coleoptera). In Rote Liste Gefährdeter Tiere Deutschlands; Binot, M., Bless, R., Boye, P., Gruttke, H., Pretscher, P., Eds.; Bundesamt für Naturschutz: Bonn, Germany, 1998; Volume 55, pp. 168–230. [Google Scholar]
- Fritzlar, F.; Schöller, M.; Sprick, P. Rote Liste und Gesamtartenliste der Blatt-, Samen- und Resedakäfer (Coleoptera: Chrysomelidae, Bruchidae; Urodontinae) Deutschlands. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 5: Wirbellose Tiere (Teil 3); Ries, M., Balzer, S., Gruttke, H., Haupt, H., Hofbauer, N., Ludwig, G., Matzke-Hajek, G., Eds.; Naturschutz und Biologische Vielfalt: Münster, Germany, 2021; Volume 70, pp. 293–331. [Google Scholar]
- Farkač, J.; Král, D.; Škorpík, M. Red List of Threatened Species in the Czech Republic. Invertebrates; Agentura Ochrany Přírody a Krajiny ČR: Prague, Czech Republic, 2005. [Google Scholar]
- Bidas, M. Rzadkie chrząszcze (Coleoptera) Góry Rzepki w Górach Świętokrzyskich. Naturalia 2012, 1, 133–135. [Google Scholar]
- Sowa, S.; Kulik, M.; Koroluk, A.; Toporowska, J.; Marek, E.; Szewczyk, W.; Szewczyk, M.; Kowalczyk, K.; Paczos-Grzęda, E. Genetic structure of Carlina acanthifolia subsp. utzka populations on the north-western margins of the species range. Glob. Ecol. Conserv. 2020, 24, e01225. [Google Scholar] [CrossRef]
- Kulik, M.; Patkowski, K.; Warda, M.; Lipiec, A.; Bojar, W.; Gruszecki, T.M. Assessment of biomass nutritive value in the context of animal welfare and conservation of selected Natura 2000 habitats (4030, 6120 and 6210) in eastern Poland. Glob. Ecol. Conserv. 2019, 19, e00675. [Google Scholar] [CrossRef]
- Warda, M.; Kulik, M.; Gruszecki, T. The impact of intensive sheep grazing in the spring on the vegetation of xerothermic grasslands in Stawska Góra nature reserve. Ecol. Quest. 2016, 23, 43–50. [Google Scholar] [CrossRef]
- Futa, B.; Patkowski, K.; Bielińska, E.J.; Gruszecki, T.M.; Pluta, M.; Kulik, M.; Chmielewski, S. Sheep and horse grazing in large-scale protection area and its positive impact on chemical and biological soil properties. Pol. J. Soil Sci. 2016, 49, 111–122. [Google Scholar] [CrossRef]
- Futa, B.; Trzcińska, J.; Patkowski, K. Impact of Extensive Sheep Grazing on the Biochemical Status of Soils of the Grassland Habitat of Natura 2000. J. Ecol. Eng. 2024, 25, 256–268. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils. 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Pascual, J.A.; García, G.; Hernández, T.; Moreno, J.L.; Ros, M. Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol. Biochem. 2000, 32, 1877–1883. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and Enzymatic Soil Properties Influenced by Cropping of Primary Wheat under Organic and Conventional Farming Systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Futa, B.; Chmielewski, S.; Patkowski, K.; Gruszecki, T. Quantification of biodiversity related to the active protection of grassland habitats in the eastern Lublin region of Poland based on the activity of soil enzymes. Pol. J. Soil Sci. 2017, 50, 55–62. [Google Scholar] [CrossRef]
- Iuss Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; FAO: Rome, Italy, 2015. [Google Scholar]
- ISO 18400; Soil Quality. In Sampling. International Organization for Standardization: Geneva, Switzerland, 2018.
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenase Aktivit in Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zantua, M.I.; Bremner, J.M. Comparison of methods of assaying urease activity in soils. Soil Biol. Biochem. 1975, 7, 291–295. [Google Scholar] [CrossRef]
- ISO 10390; Soil Quality: Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 14235; Soil Quality. Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 13878; Soil Quality. Determination of Total Nitrogen Content by Dry Combustion. International Organization for Standardization: Geneva, Switzerland, 1998.
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus carbon drives allocation and plant–soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Evans, F.C.; Peterson, C.H. Diversity and pattern in plants and insects. Ecology 1972, 53, 819–829. [Google Scholar] [CrossRef]
- Dugdale, J.S. The insects in relation to plants. In Biogeography and Ecology in New Zealand; Kuschel, G., Ed.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1975; pp. 561–589. [Google Scholar]
- Mulder, C.P.H.; Koricheva, J.; Huss-Danell, K.; Hogberg, P.; Joshi, J. Insects affect relationships between plant species richness and ecosystem processes. Ecol. Lett. 1999, 2, 237–246. [Google Scholar] [CrossRef]
- Uhler, J.; Redlich, S.; Zhang, J.; Hothorn, T.; Tobisch, C.; Ewald, J.; Thorn, S.; Siebold, S.; Mitesser, O.; Morinière, J.; et al. Relationship of insect biomass and richness with land use along a climate gradient. Nat. Commun. 2021, 12, 5946. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in the ecosystem. Sci. Total Environ. 2020, 699, 134181. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, J. Phosphorus content and distribution and the activity of phosphatases in Arenosols in a forest affected by long-term exposure to the effects of the Anwil S.A. nitrogen works in Włocławek. For. Res. Pap. 2015, 76, 250–255. [Google Scholar] [CrossRef]
- Karpiński, L.; Maák, I.E.; Boldgiv, B.; Salata, S.; Gantulga, T.; Mazur, M.A.; Szczepański, W.T. Impact of livestock grazing on the terrestrial arthropod diversity in the arid zone of Mongolia. Eur. Zool. J. 2023, 90, 487–505. [Google Scholar] [CrossRef]
- Olszowska, G. Denoting the intensity of soil biochemical transition according to stand species composition. For. Res. Pap. 2018, 79, 327–334. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E.; Piaszczyk, W. State of soil enzymatic activity in relationship to some chemical properties of Brunic Arenosols. Soil Sci. Ann. 2021, 72, 14064. [Google Scholar] [CrossRef]
- Santos-Silva, J.; dos Santos, G.A.B.; Santos, J.C. Soils and seasonality influence the richness of gall-inducing insects and their host plants in a tropical dry forest. J. Arid Environ. 2022, 196, 104651. [Google Scholar] [CrossRef]
- Kaiser, C.; Koranda, M.; Kitzler, B.; Fuchslueger, L.; Schnecker, J.; Schweiger, P.; Rasche, F.; Zechmeister-Boltenstern, S.; Sessitsch, A.; Richter, A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 2010, 187, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Wolna-Maruwka, A.; Grzyb, A.; Łukowiak, R.; Ceglarek, J.; Niewiadomska, A.; Kayzer, D. Spatial-Temporal Differentiation of Soil Biochemical Parameters and Their Relationship with Nitrogen Resources during the Vegetation Period of Selected Crops. Agriculture 2023, 13, 2034. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.F.; Akhtar, J.; Marcus, S.E.; Fletcher, N.; Field, K.; Knox, P. Cereal Root Exudates Contain Highly Structurally Complex Polysaccharides with Soil-binding Properties. Plant J. 2020, 103, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Weisskopf, L. The modulating effect of bacterial volatiles on plant growth: Current knowledge and future challenges. Plant Signal. Behav. 2012, 7, 79–85. [Google Scholar] [CrossRef]
- Schirawski, J.; Perlin, M.H. Plant–microbe interaction 2017—The good, the bad and the diverse. Int. J. Mol. Sci. 2018, 19, 1374. [Google Scholar] [CrossRef]
- Dick, R.P.; Kandeler, E. Enzymes in soils. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier Ltd.: Oxford, UK, 2005; pp. 448–456. [Google Scholar] [CrossRef]
- Russell, S.; Wyczółkowski, A.I.; Bieganowski, A. Selected Methodological Aspects of Soil Enzyme Activity Tests; Institute of Agrophysics PAS: Lublin, Poland, 2006. [Google Scholar]





| Site | pHKCl | TOC | TN | |||
|---|---|---|---|---|---|---|
| M | C | M | C | M | C | |
| 1 | 6.84 ± 0.01 a | 7.27 ± 0.03 b | 54.11 ± 2.54 a | 15.08 ± 0.24 b | 3.92 ± 0.03 a | 7.27 ± 0.03 b |
| 2 | 6.87 ± 0.03 a | 7.06 ± 0.00 b | 44.31 ± 0.86 a | 51.46 ± 1.53 b | 2.52 ± 0.02 a | 1.40 ± 0.03 b |
| 3 | 6.97 ± 0.01 a | 7.06 ± 0.00 b | 46.71 ± 1.03 a | 51.91 ± 0.55 b | 4.06 ± 0.03 a | 1.40 ± 0.06 b |
| 5 | 6.86 ± 0.02 a | 6.91 ± 0.01 b | 59.83 ± 0.65 a | 62.06 ± 2.28 a | 1.68 ± 0.03 a | 2.94 ± 0.04 b |
| 6 | 6.88 ± 0.01 a | 7.01 ± 0.01 b | 61.14 ± 1.70 a | 54.05 ± 1.58 b | 1.40 ± 0.01 a | 2.52 ± 0.02 b |
| 7 | 6.97 ± 0.00 a | 6.90 ± 0.02 b | 51.93 ± 1.72 a | 53.65 ± 2.83 a | 1.96 ± 0.02 a | 2.24 ± 0.03 b |
| 8 | 7.01 ± 0.01 a | 6.98 ± 0.01 b | 62.45 ± 1.45 a | 40.14 ± 0.50 b | 3.50 ± 0.01 a | 1.54 ± 0.04 b |
| 9 | 6.79 ± 0.02 a | 7.02 ± 0.00 b | 73.78 ± 0.40 a | 57.53 ± 0.46 b | 3.36 ± 0.02 a | 4.48 ± 0.02 b |
| 10 | 6.90 ± 0.01 a | 6.75 ± 0.02 b | 49.91 ± 0.36 a | 57.59 ± 0.76 b | 3.78 ± 0.03 a | 2.24 ± 0.02 b |
| 11 | 6.84 ± 0.00 a | 7.07 ± 0.02 b | 34.79 ± 1.51 a | 28.70 ± 0.39 b | 2.10 ± 0.02 a | 1.96 ± 0.05 a |
| 12 | 6.86 ± 0.00 a | 7.25 ± 0.03 b | 33.2 ± 0.14 a | 9.84 ± 0.16 b | 2.80 ± 0.03 a | 0.70 ± 0.00 b |
| 13 | 7.27 ± 0.02 a | 6.97 ± 0.02 b | 62.51 ± 0.01 a | 18.57 ± 0.07 b | 2.52 ± 0.00 a | 2.10 ± 0.04 b |
| 14 | 7.27 ± 0.02 a | 7.25 ± 0.01 a | 61.63 ± 1.73 a | 33.24 ± 0.33 b | 3.64 ± 0.01 a | 1.96 ± 0.01 b |
| 15 | 6.85 ± 0.01 a | 6.82 ± 0.00 b | 43.00 ± 0.20 a | 55.86 ± 2.32 b | 4.90 ± 0.02 a | 5.04 ± 0.05 a |
| Site | DhA | UrA | PhacA | PhalA | ||||
|---|---|---|---|---|---|---|---|---|
| M | C | M | C | M | C | M | C | |
| 1 | 15.90 ± 0.48 a | 7.07 ± 0.33 b | 28.47 ± 0.33 a | 21.70 ± 0.12 b | 172.27 ± 0.82 a | 59.15 ± 2.54 b | 236.41 ± 0.41 a | 175.13 ± 0.74 b |
| 2 | 1.82 ± 0.40 a | 2.21 ± 0.07 a | 14.94 ± 0.11 a | 14.80 ± 0.64 a | 173.96 ± 3.42 a | 117.85 ± 3.96 b | 245.04 ± 2.22 a | 233.22 ± 0.41 b |
| 3 | 2.55 ± 0.45 a | 3.90 ± 0.41 b | 19.08 ± 0.24 a | 14.61 ± 0.35 b | 186.41 ± 3.08 a | 139.18 ± 2.35 b | 218.64 ± 1.10 a | 234.62 ± 3.30 b |
| 5 | 16.10 ± 0.59 a | 16.80 ± 0.47 a | 16.30 ± 0.17 a | 18.38 ± 0.60 b | 130.04 ± 1.27 a | 164.58 ± 2.12 b | 233.99 ± 1.70 a | 216.23 ± 0.22 b |
| 6 | 19.66 ± 1.69 a | 7.81 ± 0.60 b | 19.93 ± 0.36 a | 15.91 ± 0.35 b | 181.98 ± 3.03 a | 163.47 ± 0.90 b | 239.39 ± 3.84 a | 213.92 ± 0.39 b |
| 7 | 11.84 ± 0.49 a | 7.46 ± 0.34 b | 15.59 ± 0.04 a | 18.29 ± 0.16 b | 166.43 ± 1.28 a | 191.71 ± 0.63 b | 236.61 ± 0.68 a | 242.53 ± 0.30 b |
| 8 | 26.46 ± 2.96 a | 5.47 ± 0.29 b | 20.00 ± 0.17 a | 16.78 ± 0.38 b | 206.07 ± 0.95 a | 160.55 ± 1.81 b | 236.85 ± 0.86 a | 232.93 ± 5.80 a |
| 9 | 24.77 ± 0.39 a | 20.61 ± 0.09 b | 18.31 ± 0.43 a | 21.13 ± 0.17 b | 175.49 ± 8.11 a | 203.28 ± 9.19 b | 217.10 ± 0.71 a | 239.37 ± 0.45 b |
| 10 | 18.77 ± 2.36 a | 14.66 ± 0.20 b | 23.55 ± 0.43 a | 19.07 ± 0.10 b | 138.15 ± 0.38 a | 181.38 ± 0.39 b | 220.67 ± 0.53 a | 246.63 ± 1.04 b |
| 11 | 20.88 ± 2.19 a | 31.83 ± 0.47 b | 15.99 ± 0.51 a | 23.43 ± 0.49 b | 113.44 ± 0.79 a | 141.04 ± 1.06 b | 208.48 ± 1.43 a | 223.09 ± 3.68 b |
| 12 | 18.27 ± 0.39 a | 3.23 ± 0.24 b | 29.32 ± 0.06 a | 17.51 ± 0.11 b | 211.58 ± 1.64 a | 134.50 ± 1.48 b | 224.98 ± 0.48 a | 119.47 ± 0.40 b |
| 13 | 27.12 ± 0.26 a | 25.72 ± 0.35 b | 27.22 ± 0.22 a | 24.02 ± 0.79 b | 148.85 ± 2.26 a | 126.08 ± 1.34 b | 221.79 ± 0.92 a | 220.22 ± 0.62 a |
| 14 | 27.80 ± 0.27 a | 11.94 ± 0.21 b | 20.77 ± 0.04 a | 15.57 ± 0.55 b | 171.34 ± 1.16 a | 124.91 ± 1.11 b | 215.96 ± 1.53 a | 225.74 ± 0.96 b |
| 15 | 30.19 ± 0.06 a | 11.00 ± 0.07 b | 24.41 ± 0.02 a | 20.24 ± 0.62 b | 233.68 ± 2.84 a | 201.86 ± 0.44 b | 254.89 ± 0.71 a | 236.30 ± 1.23 b |
| Model | Variables | d.f. | AIC | Chi2 | p | |||
|---|---|---|---|---|---|---|---|---|
| 1 | PC−1 | TOC | – | – | 2 | 37.9 | 6.9 | 0.032 |
| 2 | PC−1 | – | – | – | 1 | 38.8 | 4.0 | 0.046 |
| 3 | TOC | – | – | – | 1 | 39.2 | 3.6 | 0.058 |
| 4 | PC−2 | PC−1 | – | – | 2 | 39.3 | 5.5 | 0.065 |
| 5 | PC−2 | PC−1 | TOC | – | 3 | 39.7 | 7.1 | 0.068 |
| 6 | PC−1 | TOC | PhalA | – | 3 | 39.9 | 6.9 | 0.075 |
| 7 | PC−1 | PhalA | – | – | 2 | 39.9 | 4.9 | 0.087 |
| 8 | PC−2 | – | – | – | 1 | 40.2 | 2.6 | 0.107 |
| 9 | PC−2 | TOC | – | – | 2 | 40.5 | 4.3 | 0.114 |
| 10 | TOC | PhalA | – | – | 2 | 41.2 | 3.6 | 0.162 |
| 11 | PC−2 | PC−1 | PhalA | – | 3 | 41.2 | 5.6 | 0.133 |
| 12 | PhalA | – | – | – | 1 | 41.4 | 1.4 | 0.230 |
| 13 | PC−2 | PC−1 | TOC | PhalA | 4 | 41.7 | 7.1 | 0.129 |
| 14 | PC−2 | PhalA | – | – | 2 | 42.0 | 2.8 | 0.250 |
| 15 | PC−2 | TOC | PhalA | – | 3 | 42.5 | 4.4 | 0.226 |
| Intercept | – | – | – | – | 40.8 | – | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futa, B.; Kulik, M.; Kajtoch, Ł.; Mazur, M.A.; Jaźwa, M.; Ścibior, R.; Wielgos, J. Enzymatic Activity of Soil on the Occurrence of the Endangered Beetle Cheilotoma musciformis (Coleoptera: Chrysomelidae) in Xerothermic Grasslands. Insects 2024, 15, 307. https://doi.org/10.3390/insects15050307
Futa B, Kulik M, Kajtoch Ł, Mazur MA, Jaźwa M, Ścibior R, Wielgos J. Enzymatic Activity of Soil on the Occurrence of the Endangered Beetle Cheilotoma musciformis (Coleoptera: Chrysomelidae) in Xerothermic Grasslands. Insects. 2024; 15(5):307. https://doi.org/10.3390/insects15050307
Chicago/Turabian StyleFuta, Barbara, Mariusz Kulik, Łukasz Kajtoch, Miłosz A. Mazur, Małgorzata Jaźwa, Radosław Ścibior, and Justyna Wielgos. 2024. "Enzymatic Activity of Soil on the Occurrence of the Endangered Beetle Cheilotoma musciformis (Coleoptera: Chrysomelidae) in Xerothermic Grasslands" Insects 15, no. 5: 307. https://doi.org/10.3390/insects15050307






