Superparasitism in the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the Implications for Mass Rearing and Augmentative Release
Abstract
:1. Introduction
2. Superparasitism and Biological Control
2.1. The Case of Diachasmimorpha longicaudata
| Anastrepha ludens | Diachasmimorpha longicaudata | |||
|---|---|---|---|---|
| Time of daily exposure | Number of exposed host larvae/unit | * Number of females per cage | Duration of exposure (h) | Obtained sex ratio ♀:♂ |
| (1) 08:00 | 3,100 | 2,600 | 1 | 4:7 |
| (2) 12:00 | 2,400 | 2,600 | 1 | 3:5 |
| (3) 16:00 | 2,400 | 2,600 | 1:45 | 2:8 |
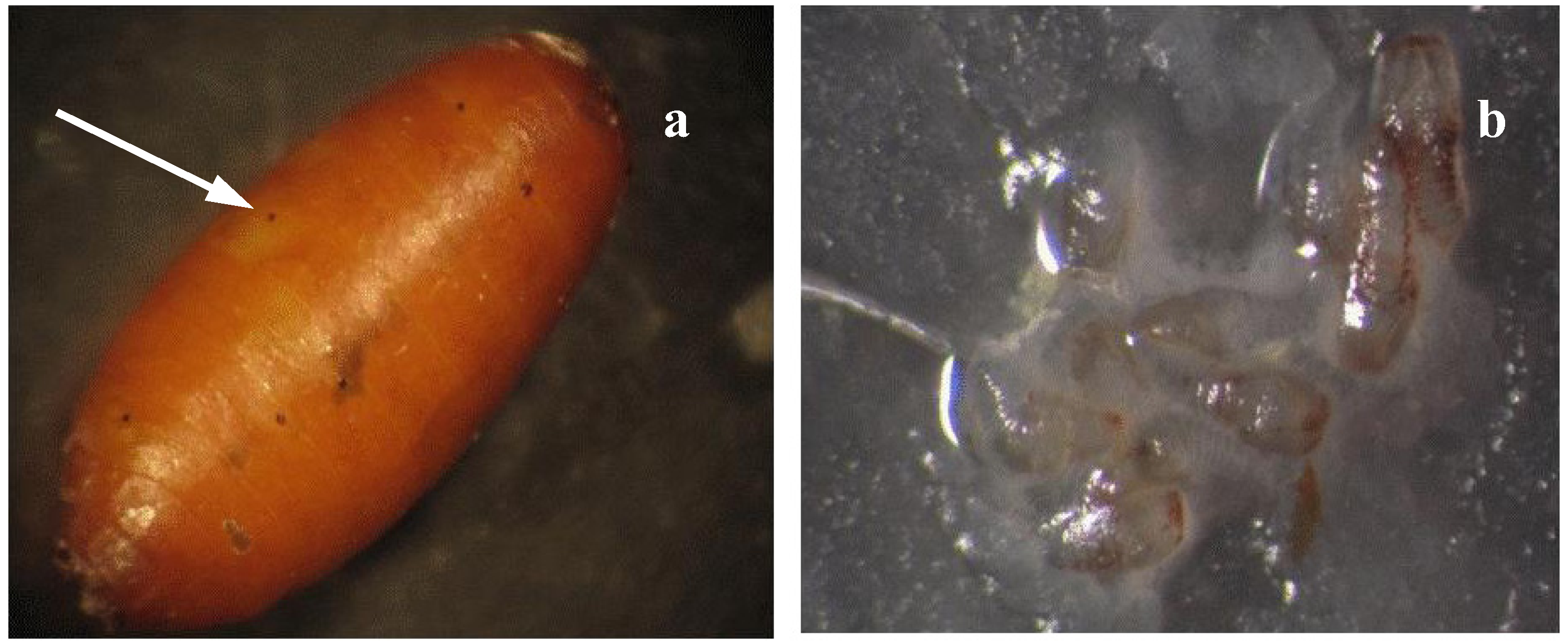
3. Manipulating Mass-Rearing Conditions
4. Superparasitism in Other Fruit Fly Parasitoid Species
5. Conclusions and Future Perspectives
Acknowledgments
References
- Godfray, H.C.J. Parasitoids,Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; p. 473. [Google Scholar]
- van Alphen, J.J.M.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Annu. Rev. Entomol. 1990, 35, 59–79. [Google Scholar] [CrossRef]
- van Alphen, J.J.M.; Jervis, M.A. Foraging behaviour. In Insect Natural Enemies. Practical Approaches to Their Study and Evaluation; Jervis, M., Kid, N., Eds.; Chapman & Hall: London, UK, 1996; pp. 1–62. [Google Scholar]
- Rosenheim, J.A.; Hongkham, D. Clutch size in an obligately siblicidal parasitoid wasp. Anim. Behav. 1996, 51, 841–852. [Google Scholar] [CrossRef]
- White, J.A.; Andow, D.A. Benefits of self-superparasitism in a polyembryonic parasitoid. Biol. Control 2008, 46, 133–139. [Google Scholar]
- Visser, M.E.; van Alphen, J.J.M.; Nell, H.W. Adaptive superparasitism and time allocation in solitary parasitoids: The influence of the number of parasitoids depleting the patch. Behaviour 1990, 114, 214–227. [Google Scholar]
- Visser, M.E.; Luyckx, B.; Nell, H.W.; Boskamp, G.J.F. Adaptive superparasitism and patch time allocation in solitary parasitoids: Marking of parasitized hosts in relation to the pay-off from superparasitism. Ecol. Entomol. 1992, 17, 76–62. [Google Scholar] [CrossRef]
- Wylie, H.G. Delayed development in Microctomus vittatae (Hymenoptera: Braconidae) in superparasitized adults of Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Can. Entomol. 1983, 115, 441–442. [Google Scholar] [CrossRef]
- Iwasa, Y.; Higashi, M.; Matsuda, H. Theory of oviposition strategy in parasitoids. I. Effect of mortality and limited egg number. Theor. Popul. Biol. 1984, 26, 205–227. [Google Scholar] [CrossRef]
- Roitberg, B.D.; Mangel, M.; Lalonde, R.G. Seasonal dynamic shifts in patch exploitation by parasitic wasps. Behav. Ecol. 1992, 3, 156–165. [Google Scholar] [CrossRef]
- Roitberg, B.; Sircom, J.; van Alphen, J.J.M.; Mangel, M. Life expectancy and reproduction. Nature 1993, 364, 108. [Google Scholar]
- Rosenheim, J.A.; Mangel, M. Patch-leaving rules for parasitoids with imperfect host discrimination. Ecol. Entomol. 1994, 19, 374–380. [Google Scholar] [CrossRef]
- Waage, J.K. Family planning in parasitoids: Adaptive patterns of progeny and sex allocation. In Insect Parasitoids; Waage, J.K., Greathead, D., Eds.; Academic Press: London, UK, 1986; pp. 63–95. [Google Scholar]
- Mackauer, M.; Chau, A. Adaptive self superparasitism in a solitary parasitoid wasp: The influence of clutch size on offspring size. Funct. Ecol. 2001, 15, 335–343. [Google Scholar] [CrossRef]
- Darrouzet, E.; Imbert, E.; Chevier, C. Self-superparasitism consequences for offspring sex ratio in the solitary ectoparasitoid Eupelmus vuilleti. Entomol. Exp. Appl. 2003, 109, 167–171. [Google Scholar] [CrossRef]
- Sirot, E.; Ploye, H.; Bernstein, C. State dependent superparasitism in a solitary parasitoid: Egg load and survival. Behav. Ecol. 1997, 8, 226–232. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Perez-Lachaud, G.; Liedo, P. Host size, superparasitism and sex ratio in mass-reared Diachasmimorpha longicaudata, a fruit fly parasitoid. Biocontrol 2011, 56, 11–17. [Google Scholar] [CrossRef]
- Montoya, P.; Ruiz, L.; Perez-Lachaud, G.; Cancino, J.; Liedo, P. Field superparasitism by Diachasmimorpha longicaudata attacking Anastrepha spp. larvae on mango fruits. Biol. Control 2012. submitted for publication. [Google Scholar]
- van Lenteren, J.C.; Bakker, K.; van Alphen, J.J.M. How to analyze host discrimination. Ecol. Entomol. 1978, 3, 71–75. [Google Scholar] [CrossRef]
- van Lenteren, J.C. Host discrimination by parasitoids. In Semiochemicals. Their Role in Pest Control; Nordlun, A.D., Jones, R.L., Lewis, W.J., Eds.; John Wiley & Sons: New York, NY, USA, 1981; pp. 153–179. [Google Scholar]
- Mackauer, M. Host discrimination and larval competition in solitary endoparasitoids. In Critical Issues in Biological Control; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept/VHC Publishers: Andover, UK, 1990; pp. 41–62. [Google Scholar]
- Eller, F.J.; Tumlinson, J.H.; Lewis, W.J. Intraspecific competition in Microplitis croceipes (Hymenoptera: Braconidae), a parasitoid of Heliothis species (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Amer. 1990, 83, 504–508. [Google Scholar]
- Harvey, J.A.; Harvey, I.F.; Thompson, D.J. The effect of superparasitism on development of the solitary parasitoid wasp, Venturia canescens (Hymenoptera: Ichneumonidae). Ecol. Entomol. 1993, 18, 203–208. [Google Scholar] [CrossRef]
- Bai, B.; Mackauer, M. Influence of superparasitism on development rate and adult size in a solitary parasitoid Aphidius ervi. Funct. Ecol. 1992, 6, 302–307. [Google Scholar] [CrossRef]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their us. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef]
- Wanjberg, E.; Pizzol, J.; Babault, M. Genetic variation in progeny allocation in Trichogramma maidis. Entomol. Exp. App. 1989, 53, 177–187. [Google Scholar] [CrossRef]
- Gonzalez, P.; Montoya, P.; Perez-Lachaud, G.; Cancino, J.; Liedo, P. Superparasitism in mass reared Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae), a parasitoid of fruit flies (Diptera: Tephritidae). Biol. Control 2007, 40, 320–326. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Lundgren, J.G. Sex ratios of commercially reared biological control agents. Biol. Control 2000, 19, 77–93. [Google Scholar] [CrossRef]
- King, B.H. Sex ratio manipulation by parasitoid wasps. In Evolution and Diversity of Sex Ratio in Insects and Mites; Wrensch, D.L., Ebberte, M., Eds.; Chapman and Hall: New York, NY, USA, 1993; pp. 418–441. [Google Scholar]
- Ode, P.J.; Hardy, I.W.C. Parasitoid sex ratios and biological control. In Behavioural Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Wajnberg, E., Bernstein, C., van Alphen, J.J.M., Eds.; Blackwell Publishing: Oxford, UK, 2008; pp. 253–291. [Google Scholar]
- Wylie, H.G. Sex ratio variability of Muscidifurax zaraptor (Hymenoptera: Pteromalidae). Can. Entomol. 1979, 111, 105–109. [Google Scholar] [CrossRef]
- Sivinski, J. The past and potential of biological control of fruit flies. In Fruit Flies Pests. A World Assessment of Their Biology and Management; McPheron, B.A., Stek, G.J., Eds.; St. Louis Press: Delray Beach, FL, USA, 1996; pp. 369–375. [Google Scholar]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 212–224. [Google Scholar]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The Augmentative biological control component in the Mexican campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. [Google Scholar]
- Montoya, P.; Cancino, J.; Zenil, M.; Gomez, E.; Villaseñor, A. Parasitoid releases in the control of Ceratitis capitata (Diptera: Tephritidae) outbreaks, in coffee growing zones of Chiapas, Mexico. Vedalia 2005, 12, 85–89. [Google Scholar]
- Sivinski, J.M.; Calkins, C.O.; Baranowski, R.M.; Harris, D.; Brambila, J.; Diaz, J.; Bums, R.E.; Holler, T.; Dodson, D. Suppression of Caribbean fruit fly (Anastrepha suspensa (Loew) Diptera: Tephritidae) population through releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Wharton, R.A.; Marsh, P. New world Opiinae (Hymenoptera: Braconidae) parasitic on Tephritidae (Diptera). J. Wash. Acad. Sci. 1978, 68, 147–167. [Google Scholar]
- Ovruski, S.; Aluja, M.; Sivinski, J.; Wharton, R. Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the southern United States: Diversity, distribution, taxonomic status and their use in fruit fly biological control. Int. Pest Mgmt Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]
- Lopez, M.; Aluja, M.; Sivinski, J. Hymenopterous larval-pupal and pupal parasitoids of Anastrepha flies (Diptera: Tephritidae) in Mexico. Biol. Control 1999, 15, 119–129. [Google Scholar] [CrossRef]
- Garcia-Medel, D.; Sivinski, J.; Diaz-Fleischer, F.; Ramirez-Romero, R.; Aluja, M. Foraging behavior by six fruit fly parasitoids (Hymenoptera: Braconidae) released as single- or multiple- species cohorts in field cages: Influence of fruit location and host density. Biol. Control 2007, 43, 12–22. [Google Scholar] [CrossRef]
- Purcell, M.F.; Jackson, C.G.; Long, J. P.; Batchelor, M.A. Influence of guava ripening on parasitism levels by Diachasmimorpha longicaudata (Ashmead) and other parasitoids of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Biol. Control 1994, 4, 396–403. [Google Scholar] [CrossRef]
- Cancino, J.; Ruiz, L.; Gomez, Y.; Toledo, J. Irradiación de larvas de Anastrepha ludens (Loew) (Diptera: Tephritidae) para inhibir la emergencia de moscas en la cría del parasitoide Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) (in Spanish). Folia Entomol. Mex. 2002, 41, 195–208. [Google Scholar]
- Sivinski, J.; Aluja, M.; Holler, T. Food sources for adult Diachasmimorpha longicaudata, a parasitoid of tephritid fruit flies: Effects on longevity and fecundity. Ent. Exp. Appl. 2006, 118, 193–202. [Google Scholar] [CrossRef]
- Cancino, J.; Ruiz, L.; Lopez, P.; Moreno, F. Cría masiva de parasitoides. In Moscas de la Fruta: Fundamentos y Procedimientos para su Manejo (in Spanish); Montoya, P., Toledo, J., Hernández, E., Eds.; S y G Editores: Mexico, D.F., Mexico, 2010; pp. 291–306. [Google Scholar]
- Cancino, J.; Montoya, P. Advances and Perspectives in the mass rearing of fruit fly parasitoids in Mexico. In Fruit Flies of Economic Importance: From Basic to Applied Knowledge; Sugayama, R., Zucchi, R.A., Ovruski, S.M., Sivinski, J., Eds.; Press Color: Bahia, Brazil, 2008; pp. 133–142. [Google Scholar]
- Lawrence, P.O.; Greany, P.D.; Nation, J.L.; Baranowski, R.M. Oviposition behavior of Biosteres longicaudatus, a parasite of the Caribbean fruit fly Anastrepha suspensa. Ann. Entomol. Soc. Amer. 1978, 71, 253–256. [Google Scholar]
- Montoya, P.; Benrey, B.; Barrera, J.F.; Zenil, M.; Ruiz, L.; Liedo, P. Oviposition behavior and conspecific host discrimination in Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a fruit fly parasitoid. Biocontrol Sci. Technol. 2003, 13, 683–690. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Aluja, M. Functional response and superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a parasitoid of fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Amer. 2000, 93, 47–54. [Google Scholar] [CrossRef]
- Gonzalez, P.; Montoya, P.; Perez-Lachaud, G.; Cancino, J.; Liedo, P. Host discrimination and superparasitism in wild and mass-reared Diachasmimorpha longicaudata (Hym.: Braconidae) females. Biocontrol Sci. Technol. 2010, 20, 137–148. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Cordero, R.A. Superparasitism and sex ratio adjustment in a wasp parasitoid: results at variance with Local Mate Competition? Oecologia 2003, 136, 365–373. [Google Scholar] [CrossRef]
- van Dijken, M.J.; Waage, J.K. Self and conspecific self superparasitism by the egg parasitoid Trichogramma evanescens. Entomol. Exp. Appl. 1987, 43, 183–192. [Google Scholar] [CrossRef]
- van Dijken, M.J.; van Stratum, P.; van Alphen, J.J.M. Superparasitism and sex ratio in the solitary parasitoid Epidinocarsis lopezi. Entomol. Exp. Appl. 1993, 68, 51–58. [Google Scholar] [CrossRef]
- Quicke, D.L.J. Parasitic Wasps; Chapman and Hall: London, UK, 1997; p. 470. [Google Scholar]
- Carabajal-Paladino, L.Z.; Papeschi, A.G.; Cladera, J.L. Immature stages of development in the parasitoid wasp Diachasmimorpha longicaudata. J. Insect Sci. 2010, 10, 56. [Google Scholar]
- Ibrahim, A.G.; Palacio, I.P.; Rohani, L. Biology of Diachasmimorpha longicaudata, a parasitoid of Carambola fruit fly, (Diptera: Tephritidae). Pertanika J. Trop. Agric. Sci. 1994, 17, 139–143. [Google Scholar]
- Wang, X.; Messing, R.H.; Bautista, R.C. Competitive superiority of early acting species: A case study of Opiine fruit fly parasitoids. Biocontrol Sci. Technol. 2003, 13, 391–402. [Google Scholar] [CrossRef]
- Ode, P.J.; Heinz, K.M. Host-size-dependent sex ratio theory and improving mass-reared parasitoids sex ratios. Biol. Control 2002, 24, 31–41. [Google Scholar] [CrossRef]
- Cancino, J.; Montoya, P.; López, P.; Moreno, F. Estrategias de exposición larvaria en la cría masiva de Diachasmimorpha longicaudata para optimizar la emergencia de adultos (in Spanish). Report of Finished Products 2001, Moscafrut Program SAGARPA-IICA, Tapachula, Chiapas, Mexico. Unpublished data, 2011; 1–9. [Google Scholar]
- Planta Moscafrut. Annual Report of Quality Control, Moscafrut Program SAGARPA-IICA, Metapa, Chiapas, Mexico. 2011; 33. [Google Scholar]
- Yamada, Y.Y.; Sugaura, K. Evidence for adaptive self-superparasitism in the dryinid parasitoid Haplogonatopus atratus when conspecifics are present. Oikos 2003, 103, 175–181. [Google Scholar] [CrossRef]
- Duan, J.J.; Messing, R.H. Host specificity of Diachasmimorpha kraussii (Hymenoptera: Braconidae), a newly introduced Opiine fruit fly parasitoid with four nontarget tephritids in Hawaii. Biol. Control 2000, 19, 28–34. [Google Scholar] [CrossRef]
- Nuñez-Campero, S.R.; Ovruski, S.M.; Aluja, M. Survival analysis and demographic parameters of the pupal parasitoid Coptera haywardi (Hymenoptera: Diapriidae), reared on Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2012, 61, 40–46. [Google Scholar]
- Cancino, J.; Liedo, P.; Ruiz, L.; Lopez, G.; Montoya, P.; Barrera, J.F.; Sivinski, J.; Aluja, M. Discrimination by Coptera haywardi (Hymenoptera: Diapriidae) of hosts previously attacked by conspecifics or by the larval parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biocontrol Sci. Technol. 2012, in press. [Google Scholar]
- Kazimírová, M.; Vallo, V. Larval morphology and development of Coptera occidentalis. BioControl 1999, 44, 263–280. [Google Scholar] [CrossRef]
- Montoya, P.; Suarez, A.; Lopez, F.; Cancino, J. Fopius arisanus Oviposition in four Anastrepha fruit fly species of economic importance in Mexico. BioControl 2009, 54, 437–444. [Google Scholar] [CrossRef]
- Rousse, P.; Gourdon, F.; Quilici, S. Host specificity of the egg pupal parasitoid Fopius arisanus (Hymenoptera: Braconidae) in La Reunion. Biol. Control 2006, 37, 284–290. [Google Scholar] [CrossRef]
- Quimio, G.H.; Walter, G.H. Host preference and host suitability in an egg-pupal fruit-fly parasitoid, Fopius arisanus (Sonan) (Hymenoptera: Braconidae). J. Appl. Entomol. 2001, 125, 135–140. [Google Scholar]
- Ayala-Ayala, A.P. Estrategias de superparasitismo de parasitoides nativos y exóticos sobre la mosca Mexicana de la fruta Anastrepha ludens Loew (Diptera: Tephritidae). M.Sc. thesis, Michoacana University of San Nicolás de Hidalgo, Morelia, Michoacán, Mexico, 2012; p. 43. [Google Scholar]
- Lawrence, P.O. Morphogenesis and cytopathic effects of the Diachasmimorpha longicaudata entomopoxvirus in host haemocytes. J. Insect Physiol. 2005, 51, 221–233. [Google Scholar] [CrossRef]
- Mackauer, M.; Bai, B.; Chow, A.; Danyk, T. Asymmetric larval competition between two species of solitary parasitoid wasps: the influence of superparasitism. Ecol. Entomol. 1992, 17, 233–236. [Google Scholar] [CrossRef]
- Ueno, T. Host feeding and acceptance by a parasitic wasp (Hymenoptera: Ichneumonidae) as influenced by egg load and experience in a patch. Evol. Ecol. 1999, 13, 33–44. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the Implications for Mass Rearing and Augmentative Release. Insects 2012, 3, 900-911. https://doi.org/10.3390/insects3040900
Montoya P, Pérez-Lachaud G, Liedo P. Superparasitism in the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the Implications for Mass Rearing and Augmentative Release. Insects. 2012; 3(4):900-911. https://doi.org/10.3390/insects3040900
Chicago/Turabian StyleMontoya, Pablo, Gabriela Pérez-Lachaud, and Pablo Liedo. 2012. "Superparasitism in the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the Implications for Mass Rearing and Augmentative Release" Insects 3, no. 4: 900-911. https://doi.org/10.3390/insects3040900




