Assaying Visual Memory in the Desert Locust
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal Rearing Conditions
2.2. Experimental Arenas

2.3. Teaching Setup
2.3.1. Training Arena as Assessment Arena
2.3.2. Y-Maze as Assessment Arena
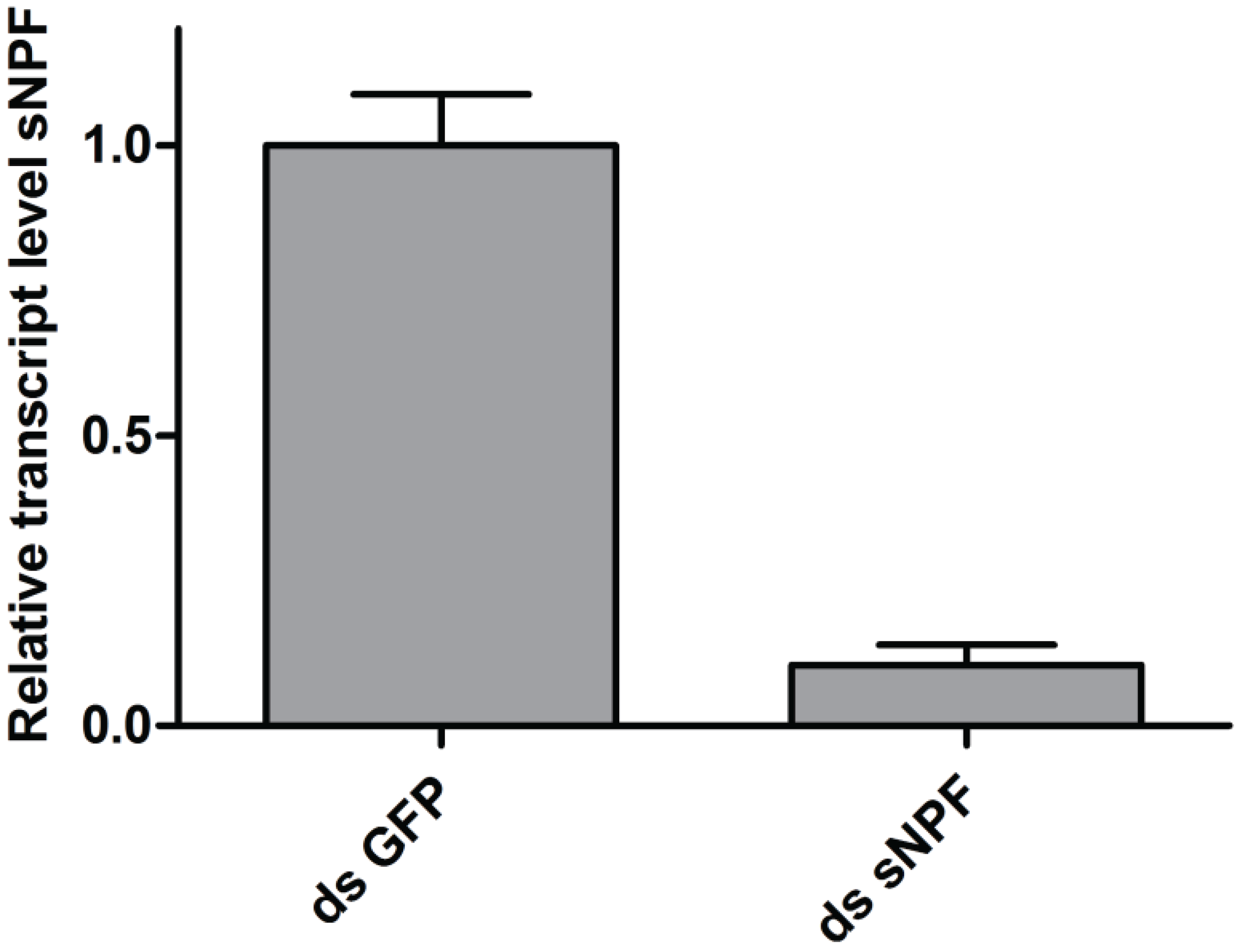
2.4. sNPF Precursor Transcript Knockdown
3. Results
3.1. Assaying Locust Visual Memory

3.2. The Effect of sNPF on Visual Memory

4. Discussion

5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.S.; You, K.H.; Choo, J.K.; Han, Y.M.; Yu, K. Drosophila short neuropeptide F regulates food intake and body size. J. Biol. Chem. 2004, 279, 50781–50789. [Google Scholar] [CrossRef] [PubMed]
- Dillen, S.; Zels, S.; Verlinden, H.; Spit, J.; van Wielendaele, P.; Vanden Broeck, J. Functional characterization of the short neuropeptide F receptor in the desert locust, schistocerca gregaria. PLOS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Dillen, S.; Verdonck, R.; Zels, S.; van Wielendaele, P.; vanden Broeck, J. Identification of the short neuropeptide F precursor in the desert locust: Evidence for an inhibitory role of sNPF in the control of feeding. Peptides 2014, 53, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, P.; Szymczak, M.; Rogalska, L.; Rosinski, G. Developmental and myotropic effects of the Led-NPF-I peptide in tenebrionid beetles. Invertebr. Reprod. Dev. 2013, 57, 1–7. [Google Scholar] [CrossRef]
- Cerstiaens, A.; Benfekih, L.; Zouiten, H.; Verhaert, P.; de Loofa, A.; Schoofs, L. Led-NPF-1 stimulates ovarian development in locusts. Peptides 1999, 20, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Johard, H.A.D.; Yoishii, T.; Dircksen, H.; Cusumano, P.; Rouyer, F.; Helfrich-Forster, C.; Nassel, D.R. Peptidergic clock neurons in Drosophila: Ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J. Comp. Neurol. 2009, 516, 59–73. [Google Scholar] [CrossRef]
- Chen, W.; Shi, W.; Li, L.; Zheng, Z.; Li, T.; Bai, W.; Zhao, Z. Regulation of sleep by the short neuropeptide F (sNPF) in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2013, 43, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Johard, H.A.D.; Enell, L.E.; Gustafsson, E.; Trifilieff, P.; Veenstra, J.A.; Nassel, D.R. Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: Relations to extrinsic neurons expressing different neurotransmitters. J. Comp. Neurol. 2008, 507, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Knapek, S.; Kahsai, L.; Winther, A.M.E.; Tanimoto, H.; Nassel, D.R. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in kenyon cells of Drosophila mushroom bodies. J. Neurosci. 2013, 33, 5340–5345. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L. Traces of Drosophila memory. Neuron 2011, 70, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F.; Lienhard, M.C.; Borst, A.; Fischbach, K.F. Neuronal architecture of the antennal lobe in Drosophila-melanogaster. Cell Tissue Res. 1990, 262, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Nassel, D.R.; Enell, L.E.; Santos, J.G.; Wegener, C.; Johard, H.A.D. A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci. 2008, 9. [Google Scholar] [CrossRef]
- Krashes, M.J.; DasGupta, S.; Vreede, A.; White, B.; Armstrong, J.D.; Waddell, S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 2009, 139, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Root, C.M.; Ko, K.I.; Jafari, A.; Wang, J.W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 2011, 145, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bernays, E.A.; Wrubel, R.P. Learning by grasshoppers—Association of color light-intensity with food. Physiol. Entomol. 1985, 10, 359–369. [Google Scholar] [CrossRef]
- Simoes, P.; Ott, S.R.; Niven, J.E. Associative olfactory learning in the desert locust, Schistocerca gregaria. J. Exp. Biol. 2011, 214, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Tucker, D. Associative learning by locusts: Pairing of visual cues with consumption of protein and carbohydrate. Anim. Behav. 1997, 54, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Holliday, J.L.; Holliday, N.J. Changes in learning-ability and mechanism during development of grasshopper nymphs, melanoplus-bivittatus. Physiol. Entomol. 1995, 20, 109–116. [Google Scholar] [CrossRef]
- Simoes, P.M.V.; Ott, S.R.; Niven, J.E. A long-latency aversive learning mechanism enables locusts to avoid odours associated with the consequences of ingesting toxic food. J. Exp. Biol. 2012, 215, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Brucato, F.H.; Crapo, J.D. Molecular overexpression of extracellular superoxide dismutase increases the dependency of learning and memory performance on motivational state. Behav. Genet. 2000, 30, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Helfrich-Förster, C.; Shafer, O.T.; Wulbeck, C.; Grieshaber, E.; Rieger, D.; Taghert, P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J. Comp. Neurol. 2007, 500, 47–70. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillen, S.; Chen, Z.; Broeck, J.V. Assaying Visual Memory in the Desert Locust. Insects 2015, 6, 409-418. https://doi.org/10.3390/insects6020409
Dillen S, Chen Z, Broeck JV. Assaying Visual Memory in the Desert Locust. Insects. 2015; 6(2):409-418. https://doi.org/10.3390/insects6020409
Chicago/Turabian StyleDillen, Senne, Ziwei Chen, and Jozef Vanden Broeck. 2015. "Assaying Visual Memory in the Desert Locust" Insects 6, no. 2: 409-418. https://doi.org/10.3390/insects6020409
APA StyleDillen, S., Chen, Z., & Broeck, J. V. (2015). Assaying Visual Memory in the Desert Locust. Insects, 6(2), 409-418. https://doi.org/10.3390/insects6020409




