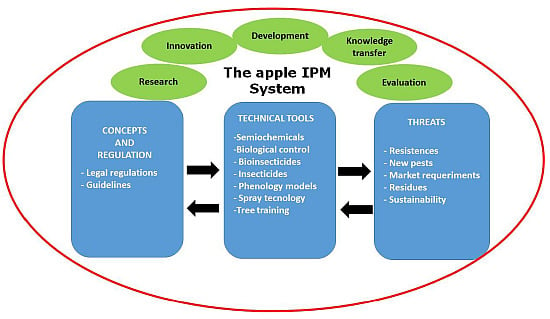
Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept?
Abstract
:1. Introduction
2. Current IPM Tools and Techniques Used in Integrated Fruit Production
2.1. Pesticides
2.2. Bio-Pesticides
2.3. Semiochemicals, Mating Disruption and Mass Trapping
2.4. Biological Control
2.5. Phenology Models, Economic Thresholds and Decision Support Systems
2.6. Improvements in Spray Technology
2.7 Innovation in the Tree Training
3. Integrated Fruit Production and Technical Guidelines


4. Present and Future Threats to IPM
4.1. Pesticide Resistance
4.2. New Pest Threats
5. What Has Been Changed in Market Requirements and Residues?
| Year | |||||||
|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |
| N | |||||||
| Residue analysis | 560 | 725 | 584 | 603 | 607 | 617 | 575 |
| % of sample with residue up to 30% of MRL | 96.3 | 95.4 | 96.6 | 95.2 | 97.2 | 99.6 | 99.0 |
| % of sample with residue between 30% - 50% of MRL | 3.0 | 2.9 | 1.5 | 2.7 | 2.0 | 0.2 | 0.0 |
| % of sample with residue between 50 and 100% of MRL | 0.7 | 1.4 | 1.2 | 1.2 | 0.6 | 0.0 | 0.5 |
| % of sample with residue more than 100% of MRL | 0.0 | 0.3 | 0.7 | 0.9 | 0.2 | 0.2 | 0.5 |

| Pest /Desease | Plant, Pest or Disease Phenological Stage | Control Method |
|---|---|---|
| Apple scab | From 07 (BBCH* scale) | Models to decide the right time to spray fungicides |
| San José scale | From 07 (BBCH scale) | Paraffinic oils and insecticides in pre-bloom. |
| Powdery mildew | From 10 (BBCH scale) | Fungicides |
| Fire blight | Pre-bloom Bloom | Bactericides based on copper in pre-bloom and microbial control during bloom |
| Aphids (Rosy apple aphid, Green aphid) | Pre- and/or postbloom treatments | Biological control + Insecticides |
| Woody Apple aphid | Pre- and/or postbloom | Biological control + Insecticides |
| Leafrollers | Pre- and/or postbloom treatments | Insecticides based on visual inspections (pre-bloom) and on captures in monitoring traps (post-bloom) |
| Codling moth | 64 (BBCH scale) | Mating disruption (as a base system) plus CpGv or common insecticides based on captures in monitoring traps |
| Leopard moth | 64 (BBCH scale) | Mating disruption (as a base system) |
| Mediterranean Fruit Fly | At the beginning of the flight | Mass trapping or attract and kill |
| European Red Mite | Pre- and/or postbloom treatments | Biological control. Miticides, only in case of not Phytoseiidae |
| Oriental Fruit Moth | Mating disruption (as a base system) plus insecticides based on captures in monitoring traps | |
| Storage diseases | No fungicides |
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Stetter, J.; Folker, L. Innovation in crop protection: trends in research (review). Angew. Chem. Int. Ed. 2000, 39, 1724–1744. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US-EPA). Available online: http://www.epa.gov/pesticides/factsheets/ipm.htm (accessed on 10 June 2015).
- New York State Integrated Pest Management program. Available online: http://nysipm.cornell.edu/publications/apple_man/default.asp (accessed on 10 June 2015).
- Hodgson, E. Pesticides: Past, present and future. In Pesticides and the Future: Toxicological Studies of Risks and Benefits; Hodgson, E., Roe, R.M., Motoyama, N., Eds.; North Carolina State University: Raleigh, NC, USA, 1991; pp. 3–12. [Google Scholar]
- McHardy, W.E. Current Status of IPM in Apple orchards. Crop Prot. 2000, 19, 801–806. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology and the Search for a Truly Sustainable Agriculture; United Nations Environment Programme Environmental Training Network for Latin America and the Caribbean: Mexico City, Mexico, 2005; pp. 13–27. [Google Scholar]
- Pickett, J.H.; Putman, W.L.; LeRoux, E.J. Progress in harmonizing biological and chemical control of orchard pests in eastern Canada. Proc. 10th Int. Congr. Entomol. 1958, 3, 169–174. [Google Scholar]
- DeBach, P.; Bartlett, B. Effects of insecticides on biological control of insect pests of citrus. J. Econ. Entomol. 1951, 44, 372–383. [Google Scholar] [CrossRef]
- Smith, R.F.; Allen, W.W. Insect control and the balance of nature. Sci. Am. 1954, 190, 38–42. [Google Scholar] [CrossRef]
- Smith, R.F.; Hagen, K.S. Integrated control programs in the future of biological control. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Stern, V.M.; Smith, R.F.; van den Bosch, R.; Hagen, K.S. The integration of chemical and biological control of the spotted alfalfa aphid. The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Boller, E.F.; van Lenteren, J.C.; Delucchi, V. International Organization for Biological Control of Noxious Animals and Plants; History of the First 50 Years (1956–2006); Boller, E.F., van Lenteren, J.C., Delucchi, V., Eds.; IOBC: Zurich, Switzerland, 2006; p. 275. [Google Scholar]
- van den Bosch, R.; Stern, V.M. The integration of chemical and biological control in arthropod pests. Annu. Rev. Entomol. 1962, 7, 367–387. [Google Scholar] [CrossRef] [PubMed]
- FAO. 1966a. Proceedings of the FAO Symposium on Integrated Pest Control Rome, Rome, Italy, 11–15 October 1965.
- FAO. 1966b. Proceedings of FAO Symposium Integrated Pest Control Rome, Rome, Italy, 11–15 October 1965; pp. Part 1, p. 91; Part 2, p. 186; Part 3, p. 129.
- Kogan, M.; Bajava, W.I. Integrated Pest Management: a global reality? Annais de Sociedade Entológica do Brasil. Available online: http://dx.doi.org/10.1590/S0301-80591999000100001 (accessed on 10 June 2015).
- Boller, E.F.; Avilla, J.; Jörg, E.; Malavolta, C.; Wijnands, F.; Esbjerg, P. Integrated Production: Principles and Technical Guidelines, 3rd ed.; c/ Swiss Federal Research Station of Horticulture: Wädenswil, Switzerland, 2004. [Google Scholar]
- Cross, J.V.; Dickler, E. Guidelines for Integrated Production of pome fruits in Europe: IOBC Technical Guideline III. IOBC-WPRS Bull. 1994, 17, 1–8. [Google Scholar]
- Freier, B.; Boller, E.F. Integrated pest management in Europe—History, policy, achievements and implementation. In Integrated Pest Management: Dissemination and Impact; Peschin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherland, 2009; Volume 2, pp. 435–454. [Google Scholar]
- Maredia, K.M. Integrated Pest management in the Global Arena; CABI: Cambridge, MA, USA, 2003. [Google Scholar]
- Bazoche, P.; Combris, P.; Giraud-Heraud, E.; Seabra Pinto, A.; Bunte, F.; Tsakiridou, E. Willingness to pay for pesticide reduction in the EU: nothing but organic? Eur. Rev. Agric. Econ. 2014, 41, 87–109. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Adamson, D.; Furlong, M.J. The future of IPM: whither or wither? Aust. J. Entomol. 2009, 48, 85–96. [Google Scholar] [CrossRef]
- Weddle, P.W.; Welter, S.C.; Thomson, D. History of IPM in California pears 50 years of pesticide use and the transition to biologically intensive IPM. Pest Manag. Sci. 2009, 65, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Damos, P. Current issues in integrated pest management of Lepidoptera pest threats in Industrial crop models. In Lepidoptera: Ecology, Behavior and Management; Guerritore, E., DeSare, J., Eds.; Nova Science Publications: New York, NY, USA, 2013; pp. 45–86. [Google Scholar]
- Damos, P.; Savopoulou-Soultani, M. Microlepidoptera of Economic Significance in Fruit Production: Challenges, Constrains and Future Perspectives of Integrated Pest Management. In Moths: Types, Ecological Significance and Control; Cauterruccio, L., Ed.; Nova Science Publications: New York, NY, USA, 2011; pp. 75–113. [Google Scholar]
- Pesticides-European Commission. Available online: http://ec.europa.eu/food/plant/pesticides/index_en.htm (accessed on 10 June 2015).
- Strategy on the sustainable use of pesticides. Available online: http://ec.europa.eu/environment/index_en.htm (accessed on 10 June 2015).
- Schuster, D.J.; Stanley, P.A. Biorational Insecticides for integrated pest management in tomatos. Biol. Control. 2007, 41, 99–109. [Google Scholar]
- Science for Environmental Policy, EU. 2008. Available online: http://ec.europa.eu/environment/integration/research/newsalert/pdf/134na5.pdf (accessed on 10 June 2015).
- Lacey, L.A.; Shapiro-Ilan, D.I. Microbial Control of Insect Pests in Temperate Orchard Systems: Potential for Incorporation into IPM. Annu. Rev. Entomol. 2008, 53, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Andermatt, M.; Mani, E.; Wildbolz, T.H.; Lüthy, P. Susceptibility of Cydia pomonella to Bacillus thuringiensis under laboratory and field conditions. Entomol. Exp. Appl. 1988, 49, 291–295. [Google Scholar] [CrossRef]
- Ioriatti, C.; Pasqualini, E.; Delaiti, M. Effectiveness of Bacillus thuringiensis Berliner on three species of apple leafrollers. Boll. Ist. Entomol. Guido Grandi' Stud Bologna 1996, 50, 73–93. [Google Scholar]
- Cross, J.V.; Solomon, M.G.; Chandler, D.; Jarrett, P.; Richardson, P.N.; Winstanley, D.; Bathon, H.; Huber, J.; Keller, B.; Langenbruch, G.A.; Zimmermann, G. Biocontrol of pest of apples and pears in northern and central Europe: 1. microbial agents and nematodes. Biocontrol Sci. Technol. 1999, 9, 125–149. [Google Scholar] [CrossRef]
- Tanada, J. A granulosis-virus of the codling moth, Carpocapsa pomonella L. (Oleuthreutidae, Lepidoptera). J. Insect Pathol. 1964, 6, 378–380. [Google Scholar]
- Falcon, L.A.; Huber, J. Biological control of the codling moth. In Tortricid Pests, Their Biology, Natural Enemies and Control; van Der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers: Amsterdam, The Netherland, 1991; pp. 355–369. [Google Scholar]
- Lacey, L.A.; Thomson, D.; Vincent, C.; Arhturs, S.P. Codling moth granulovirus: A comprehensive review. Biocontrol Sci. Technol. 2008, 18, 639–663. [Google Scholar] [CrossRef]
- Schmidt, S.; Tomasi, C.; Pasqualini, E.; Ioriatti, C. The biological efficacy of pear ester on the activity of granulosis virus for codling moth. J. Pest. Sci. 2008, 81, 29–34. [Google Scholar] [CrossRef]
- Wua, Z.-W.; Fana, J.-B.; Yua, H.; Wanga, D.; Zhanga, Y.-L. Ultraviolet protection of the Cydia pomonella granulovirus using zinc oxide and titanium dioxide. Biocontrol Sci. Technol. 2015, 25, 97–107. [Google Scholar] [CrossRef]
- Knight, A.; Witzgall, P. Combining mutualistic yeast and pathogenic virus—A novel method for codling moth control. J. Chem. Ecol. 2013, 39, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G.O., Jr. Nematode parasites. In Tortricid Pests, their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhius, H.H., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 273–281. [Google Scholar]
- Berkvens, N.; van Vaerenbergh, J.; Maes, M.; Beliën, T.; Viaene, N. Entomopathogenic nematodes fail to parasitize the woolly apple aphid Eriosoma lanigerum as their symbiotic bacteria are suppressed. J. Appl. Entomol. 2014, 138, 644–655. [Google Scholar] [CrossRef]
- Cossentine, J.E.; Judd, G.J.R.; Bissett, J.D.; Lacey, L.A. Susceptibility of apple clearwing moth larvae, Synanthedon myopaeformis (Lepidoptera: Sesiidae) to Beauveria bassiana and Metarhizium brunneum. Biocontrol Sci. Technol. 2010, 20, 703–707. [Google Scholar] [CrossRef]
- Knight, A.L.; Howell, J.F.; McDonough, L.M.; Weiss, M. Mating disruption of codling moth (Lepidoptera: Tortricidae) with polyethylene tube dispensers: determining emission rates and the distribution of fruit injuries. J. Agric. Entomol. 1995, 12, 85–100. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J Chem Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P. Pheromones—Future techniques for insect control? IOBC-WPRS Bull. 2001, 24, 114–122. [Google Scholar]
- Cardé, R.T. Using pheromones to disrupt mating of moth pests. In Perspectives in Ecological Theory and Integrated Pest Management; Kogan, M., Jepson, P., Eds.; Cambridge University Press: Cambridge, MA, USA, 2007; pp. 122–169. [Google Scholar]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Hebert, V.R.; Brunner, J.F.; Jones, V.P.; Doerr, M.; Hilton, R. Evaluation of pheromone release from commercial mating disruption dispensers. J. Agric. Food Chem. 2005, 53, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Bohneblust, E.; Hull, L.A.; Krawczyk, G. A comparison of various mating disruption technologies for control of two internally feeding Lepidoptera in apples. Entomol. Exp. Appl. 2011, 138, 202–211. [Google Scholar] [CrossRef]
- Baldessari, M.; Ioriatti, C.; Angeli, G. Evaluation of Puffer® CM, a release device of pheromone to control codling moth on apple in Italy. IOBC-WPRS Bull. 2013, 91, 199–204. [Google Scholar]
- McGhee, P.S.; Gut, L.J.; Miller, J.R. Aerosol emitters disrupt codling moth, Cydia pomonella, competitively. Pest Manag Sci. 2014, 70, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Gut, L.J.; Stelinski, L.L.; Thompson, D.R.; Miller, J.R. Behavior modifying chemicals: prospects and constraints in IPM. In Integrated Pest Management: Potential, Constraints, and Challenges; Koul, O., Dhaliwal, G.S., Cuperus, G., Eds.; CABI Press: Wallingford, UK, 2004; pp. 73–121. [Google Scholar]
- Waldner, W. Three years of large-scale control of codling moth by mating disruption in the South Tyrol, Italy. IOBC-WPRS Bull. 1997, 20, 35–44. [Google Scholar]
- Knight, A. Codling moth area wide integrated pest management. In Area Wide Pest Management: Theory and Implementation; Koul, O., Cuperus, G.W., Elliott, N., Eds.; CABI. Press: Wallingford, UK, 2008; pp. 159–190. [Google Scholar]
- Kovanci, O.B.; Kumral, N.A.; Larsen, T.E. High versus ultra-low volume spraying of a microencapsulated pheromone formulation for codling moth control in two apple cultivars. J. Pest Manag. 2010, 56, 1–7. [Google Scholar] [CrossRef]
- Angeli, G.; Anfora, G.; Baldessari, M.; Germinara, G.S.; Rama, F.; Cristofaro, A.D.; Ioriatti, C. Mating disruption of codling moth Cydia pomonella with high densities of Ecodian sex pheromone dispensers. J. App. Ent. 2007, 131, 311–318. [Google Scholar] [CrossRef]
- Pluciennik, Z. The control of Codling moth (Cydia pomonella L.) population using mating disruption method. Jour. Hort. Res. 2013, 21, 65–70. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Mcghee, P.; Haas, M.; Il’ichev, A.L.; Gut, L.J. Sprayable Microencapsulated Sex Pheromone Formulations for Mating Disruption of Four Tortricid Species: Effects of Application Height, Rate, Frequency, and Sticker Adjuvant. J. Econ. Entomol. 2007, 100, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Stelinski, L.L.; Gut, L.J.; Haas, M.; McGhee, P.; Epstein, D. Evaluation of aerosol devices for simultaneous disruption of sex pheromone communication in Cydia pomonella and Grapholita molesta (Lepidoptera: Tortricidae). J. Pest Sci. 2007, 80, 225–233. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Il’ichev, A.L.; Gut, L.J. Efficacy and release rate of reservoir pheromone dispensers for simultaneous mating disruption of codling moth and oriental fruit moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2009, 102, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.F. Fruit Fly control in Hawaii with poison-bait sprays containing protein hydrolysates. J. Econ. Entomol. 1952, 45, 838–843. [Google Scholar] [CrossRef]
- Escudero, A.; Vilajeliu, M.; Batllori, L. Captura masiva para el control de la mosca mediterránea de la fruta (Ceratitis capitata Wied.) en manzano. Phytoma España 2005, 171, 26–31. [Google Scholar]
- Faccioli, G.; Pasqualini, E.; Baronio, P. Optimal trap density in Cossus-cossus (Lepidoptera, Cossidae) Mass-Trapping. J. Econ. Entomol. 1993, 86, 850–853. [Google Scholar] [CrossRef]
- Bosch, D.; Sarasua, M.J.; Avilla, J. Mass trapping of Synanthedon myopaeformis (Borkhausen) in Lleida (Spain) with pheromone traps. IOBC-WPRS Bull. 2001, 24, 167–171. [Google Scholar]
- Alemany, A.; Miranda, M.A.; Alonso, R.; Escorza, C.M. Efectividad del trampeo masivo de hembras de Ceratitis capitata (Diptera:Tephritidae) a base de atrayentes alimentarios. “Efecto-borde” y papel de los frutales abandonados como potenciadores de la plaga. Boletín de Sanidad vegetal Plagas 2004, 30, 255–264. [Google Scholar]
- Sastre, C.; Melo, J.C.; Borreli, G. La captura de hembras: una posible salida en el control de mosca de la fruta (Ceratitis capitata, Wied.) en melocotonero. Phytoma España 1999, 113, 42–47. [Google Scholar]
- Batllori, L.; Escudero, A.; Vilajeliu, M.; Garcia, F.; Benejam, J. Area-wide mass trapping to control Ceratitis capitata (Wied.) on stone fruits in Girona, NE of Spain. IOBC-WPRS Bull 2008, 37, 73–82. [Google Scholar]
- Cohen, H.; Yuval, B. Perimeter Trapping Strategy to Reduce Mediterranean Fruit Fly (Diptera: Tephritidae) Damage on Different Host Species in Israel. J. Econ. Entomol. 2000, 93, 721–725. [Google Scholar] [CrossRef]
- Batllori, J.L.; Vilajeliu, M; Vilardell, P.; Creixell, A.; Carbó, M.; Esteba, G.; Raset, F.; Vayreda, F.; Giné, M.; Curós, D. Área piloto de reducción de insecticidas en plantaciones comerciales de manzano. Fruticultura Profesional 2003, 136, 49–54. [Google Scholar]
- Epsky, N.D.; Heath, R.R.; Guzman, A.; Meyer, W.L. Visual cue and chemical cue interactions in a dry trap with food-based synthetic attractant for Ceratitis capitata and Anastrepha ludens (Diptera: Tephritidae). Environ. Entomol. 1995, 24, 1387–1395. [Google Scholar] [CrossRef]
- Peñarrubia-María, E.; Quilici, S.; Schmitt, C.; Escudero-Colomar, L.A. Evaluation of candidate systems for mass trapping against Ceratitis spp. on La Réunion island. Pest Manag. Sci. 2014, 70, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Colomar, L.A.; Vilajeliu, M.; IRTA, Girona, Spain. Unpublished work. 2015.
- Batllori, L; Vilajeliu, M.; Escudero-Colomar, L.A.; Vilardell, P.; Usall, J. Guia técnica Fruit.Net per a la producción de Poma. Available online: http://www.ruralcat.net/c/document_library/get_file?uuid=0e20413d-d0ca-43c6-81f2-31390c44efcd&groupId=10136 (accessed on 16 June 2015).
- Adams, R.G., Jr.; Prokopy, R.J. Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae): An effective predator of the apple aphid (Homoptera: Aphididae) in Massachusetts. Prot. Ecol. 1980, 2, 27–39. [Google Scholar]
- Carroll, D.P.; Hoyt, S.C. Augmentation of European Earwigs (Dermaptera: Forficulidae) for Biological Control of Apple Aphid (Homoptera: Aphididae) in an Apple Orchard. J. Econ. Entomol. 1984, 77, 738–740. [Google Scholar] [CrossRef]
- Costa-Comelles, J.; García-Marí, F.; Laborda, R.; Marzal, C. Integrated control of the european red mite by phytoseidid predators in Lerida apple orchards. Int. Conf. Pests Agricultura 1987, 3, 17–24. [Google Scholar]
- Brown, MW. Role of the aphid predator guild in controlling spirea aphid populations on apple in West Virginia, USA. Biological Control 2004, 29, 189–198. [Google Scholar] [CrossRef]
- Mathews, C.R.; Bottrell, D.G.; Brown, M.W. Habitat manipulation of the apple orchard floor to increase ground-dwelling predators and predation of Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biol. Control 2004, 30, 265–273. [Google Scholar] [CrossRef]
- Avilla, J.; Bosch, D.; Escudero-Colomar, A.; Sarasua, M.J. Manzano, peral y melocotonero. In Control Biológico de Plagas Agrícolas; Jacas, J., Urbaneja, A., Eds.; Phytoma-España: Valencia, Spain, 2008; pp. 348–365. [Google Scholar]
- Brown, M.W.; Mathrews, C.R. Conservation biological control of spiraea aphid, Aphis spiraecola (Hemiptera: Aphididae) on apple by providing natural alternative food resources. Eur. J. Entomol. 2008, 105, 537–540. [Google Scholar] [CrossRef]
- Boreau de Roincé, C.; Lavigne, C.; Ricard, J.-M.; Franck, P.; Bouvier, J.-C.; Gracin, A.; Sydmondson, W.O.C. Predation by generalist predators on the codling moth versus a closely-related emerging pest the oriental fruit moth: a molecular analysis. Agric. For. Entomol. 2012, 14, 260–269. [Google Scholar] [CrossRef]
- Dib, H.; Simon, S.; Sauphanor, B.; Capowiez, Y. The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south-eastern France. Biol. Control 2010, 55, 97–109. [Google Scholar] [CrossRef]
- Desneaux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial asthropods. Ann. Rev. Ent. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Tricoire-Leignel, H.; Thany, S.H.; Gadenne, C.; Anton, P. Pest insect olfaction in an insecticide-contaminated environment: Info-disruption or hormesis effect. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roubos, C.R.; Rodriguez-Saona, C.; Isaacs, R. Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol Control 2014, 75, 28–38. [Google Scholar] [CrossRef]
- Maalouly, M.; Franck, P.; Bouvier, J.C.; Toubon, J.F.; Lavigne, C. Codling moth parasitism is affected by semi-natural habitats and agricultural practices at orchard and landscape levels. Agric. Ecosyst. Environ. 2013, 169, 33–42. [Google Scholar] [CrossRef]
- Malagnoux, L.; Marliac, G.; Simon, S.; Rault, M.; Capowiez, Y. Management strategies in apple orchards influence earwig community. Chemosphere 2015, 124, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wearing, C.H.; de Boer, J.A. Mortality of San Jose scale (Diaspidiotus perniciosus Hemiptera: Diaspididae) on an apple tree. N. Z. Entomol. 2014, 37, 107–124. [Google Scholar] [CrossRef]
- Agnello, A.; Reissing, W.H.; Harris, T. Management of summer populations of European red mite (Acari:Tetranychidae) on apple with horticultural oil. J. Econ. Entomol. 1994, 87, 148–161. [Google Scholar] [CrossRef]
- Hall, F.R. Effects of synthetic pyerethroids on major insects and mite pests of apple. J. Econ. Entomol. 1979, 72, 441–446. [Google Scholar] [CrossRef]
- Sentenac, G.; Bonafos, R.; Ruelle, B.; Coulon, T.; Escaffre, P.; Auger, P.; Kreiter, S. Effects non intenntionnels de certain produits phyopharmaceutiques sur Typhlodromus pyri, Kampimodromus aberrans et Phytoseius plumifer. Phytoma 2002, 555, 50–55. [Google Scholar]
- Hoy, M.; Westigard, P.H.; Hoyt, S.C. Release and evaluation of a laboratory-selected, pyrethroid-resistant strain of the predaceous mite Metaseiulus occidentalis (Acari:Phytoseiidae) in Southern Oregon Pear orchards and a Washington apple orchard. J. Econ. Entomol. 1983, 76, 383–388. [Google Scholar] [CrossRef]
- Markwick, N.P.; Wearing, C.H.; Shaw, P.W. Pyrethroid insecticides for apple pest control: 1. Development of pyrethroid-resistant predatory mites. In Proceedings of the Forty Third New Zealand Weed and Pest Control Conference, Dunedin, New Zealand, 14–16 August 1990; pp. 296–300.
- Hardan, J.M.; Rogers, M.L.; Gaul, S.O.; Bent, E.D. Insectary rearing and initial testing in Canada of an organophosphate/pyrethroid-resistant strain of the predatos mite Typhlodromus pyri (Acari: Phytoseiidae) from New Zealand. Environ Entomol. 1997, 26, 1424–1436. [Google Scholar] [CrossRef]
- Bonafos, R.; Serrano, E.; Auger, P.; Kreiter, S. Resistance to deltamethrin, lambda-cyhalothrin and chlorpyriphos-ethyl in some populations of Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari:Phytoseiidae) from vineyards in the south-west of France. Crop Prot. 2007, 26, 169–172. [Google Scholar] [CrossRef]
- Szabó, A.; Pènses, B.; Sipos, P.; Hegyi, T.; Hajdú, Z.; Markó, V. Pest Management systems affect composition but not abundance of phytosiid mites (Acari: Phytoseiidae) in apple orchards. Exp. Appl. Acarol. 2014, 62, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Duso, C.; Pasini, M. Distribution of the predatory mite Amblyseius andersoni (Acari:Phytoseiidae) on different apple cultivars. J. Pest Sci. 2003, 76, 33–40. [Google Scholar]
- Cuthberson, A.; Qiu, B.-L.; Murchie, A.K. Anystis baccarum: an important generalist predatory mite to be considered in apple orchard pest management strategies. Insects 2014, 5, 615–628. [Google Scholar] [CrossRef]
- Baker, R.T. Predation of leafroler larvae by spiders and mites. New Zeal. Entomol. 1983, 6, 22–23. [Google Scholar]
- Wearing, C.H.; Attfield, B.A.; Colhoun, K. Biological control of woolly apple aphid, Eriosoma lanigerum (Hausmann), during transition to integrated fruit production for pipfruit in Central Otago, New Zealand. N. Z. J. Crop Hortic. Sci. 2010, 38, 255–273. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Weber, D.C.; Hull, L.A. Coccinellidae as predators of mites: Stethorini in biological control. Biol. Control 2009, 51, 268–283. [Google Scholar] [CrossRef]
- Sciarretta, A.; Trematerra, P. Geostatistical tools for the study of insect spatial distribution: Practical implications in the integrated management of orchard and vineyard pests. Plant Protect. Sci. 2014, 50, 97–110. [Google Scholar]
- Boller, E.F.; Hani, F.; Poehling, H.M. Ecological Infrastructures: Ideabook on Functional Biodiversity at the Farm Level; LBL: Lindau, Switzerland, 2004. [Google Scholar]
- Simon, S.; Bouvier, J.C.; Debras, J.F.; Sauphanor, B. Biodiversity and pest management in orchard systems. A review. Agron. Sustainable Dev. 2010, 30, 139–152. [Google Scholar] [CrossRef]
- Garcia-Salazar, C.; Gut, L.J.; Whalon, M.E. Hedgerow barriers and other reduced-risk controls for managing Oriental fruit moth, Grapholitha molesta (Busck) (Lepidoptera: Tortricidae) in apples. Renew. Agr. Food Syst. 2007, 22, 181–188. [Google Scholar] [CrossRef]
- Miñarro, M.; Prida, E. Hedgerows surroundings organic apple orchards in north-west Spain: potential to conserve beneficial insects. Agric. For. Entomol. 2013, 15, 382–390. [Google Scholar] [CrossRef]
- Markò, V.; Jenser, G.; Mihàlyi, K.; Hegyi, T.; Balàzs, K. Flowers for better pest control? Effects of apple orchard groundcover management on mites (Acari), leafminers (Lepidoptera, Scitellidae), and fruit pests. Biocontrol Sci. Technol. 2012, 22, 39–60. [Google Scholar] [CrossRef]
- Song, B.; Jiao, H.; Tang, G.; Yao, Y. Combining repellent and attractive aromatic plants to enhance biological control of three tortricids species (Lepidoptera:Tortricidae) in an apple orchard. Fla. Entomol. 2014, 97, 1679–1689. [Google Scholar] [CrossRef]
- Brown, M.W.; Tworkoski, T. Pest management benefits of compost mulch in apple orchards. Agric. Ecosyst. Environ. 2004, 103, 465–472. [Google Scholar] [CrossRef]
- Croft, B.A.; McRae, I.V. Persistence of Typhlodromus pyri and Metaseiulus occidentalis (Acari: Phytoseiidae) on apple after inoculative release and competition with Zetzelia mali (Acari: Stigmaeidae). Environ. Entomol. 1992, 21, 1168–1177. [Google Scholar] [CrossRef]
- Grasswitz, T.R.; Burts, E.C. Effect of native natural enemies and augmentative releases of Chrysoperla rufilabris Burmeister and Aphidoletes aphidimyza (Rondani) on the population dynamics of the green apple aphid, Aphis pomi De Geer. Int. J. Pest Manag. 1995, 41, 176–183. [Google Scholar] [CrossRef]
- Ahmad, S.; Pozzebon, A.; Duso, C. Augmentative releases of the predatory mite Kampimodromus aberrans in organic and conventional apple orchards. Crop Prot. 2013, 52, 47–56. [Google Scholar] [CrossRef]
- Damos, P.; Savopoulou-Soultani, M. Development and statistical evaluation of models in forecasting major lepidopterous peach pest complex for integrated pest management programs. Crop Prot. 2010, 29, 1190–1199. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Hutchins, S.H.; Higley, L.G. Economic injury levels in theory and practice. Annu. Rev. Entomol. 1986, 31, 341–368. [Google Scholar] [CrossRef]
- Damos, P.; Savopoulou-Soultani, M. Population dynamics of Anarsia lineatella (Lep: Gelechiidae) in relation to crop damage and development of Economic Injury Levels. J. Appl. Entomol. 2009, 134, 105–115. [Google Scholar] [CrossRef]
- Damos, P.; Karabatakis, S. Real time pest modelling through the World Wide Web: Decision making from theory to praxis. Integrated protection of fruit crops. IOBC-WPRS Bull. 2013, 91, 253–258. [Google Scholar]
- Karabatakis, S.; Damos, P. Supporting integrated pest management using open data networks and information technology through the World Wide Web. In Proceedings of ESA 60th Annual meeting, Knoxville, TN, USA, 11–14 November 2013.
- Balsari, P.; Marucco, P. The New EU Directive Requirements and the Innovation in Pesticide Application Techniques. J. ASTM Int. 2011, 8, 1–21. [Google Scholar]
- Polveche, V.; Crete, X.; Chapuis, G.; Douzals, J.P. Effects of nozzle types, windbreak and vegetation stages on drift performances issued from an orchard sprayer. In Proceedings of Suprofruit Workshop, Centre Ctifl Lanxade, Prigonrieux, France, 8–10 June 2011; pp. 66–67.
- Stallinga, H.; Van de Zande, J.C.; Wenneker, M.; Michielsen, J.M.G.P. Nozzle classification for drift reduction in orchard spraying: effect of nozzle type in full leave stage orchards. In Proceedings of 11th Workshop, Sustainable Plant Protection Techniques in Fruit Growing, Bergerac, France, 8–10 June 2011; pp. 70–71.
- Loquet, B.; Destombes, J. Nozzles and spray application in orchards: efficiency and spray quality. In Proceedings of 11th Workshop, Sustainable Plant Protection Techniques in Fruit Growing, Bergerac, France, 8–10 June 2011; pp. 54–55.
- Bondesan, D.; Rizzi, C.; Angeli, G.; Ioriatti, C. Evaluation of spray drift in apple orchards of Trentino: Comparison of different solutions to reduce environmental contamination. IOBC-WPRS Bull. 2013, 91, 493–499. [Google Scholar]
- Koch, H.; Weisser, P. Sensor equipped orchard spraying – efficacy, savings and drift reduction. Asp. Appl. Biol. 2000, 57, 357–362. [Google Scholar]
- Balsari, P.; Marucco, P.; Tamagnone, M. A crop identification system (CIS) to optimise pesticide applications in orchards. J. Hortic. Sci. Biotech. 2009, 84, 113–116. [Google Scholar]
- Solanelles, F.; Escola, A.; Planas, S.; Rosell, J.R.; Camp, F.; Gracia, F. An Electronic Control System for Pesticide Application Proportional to the Canopy Width of Tree Crops. Biosyst. Eng. 2006, 95, 473–481. [Google Scholar] [CrossRef]
- Doruchowski, G.; Jaeken, P.; Hołownicki, R. Target detection as a tool of selective spray application on trees and weeds in orchards. In Precision Agriculture and Biological Quality; Bellingham, W.A., Meyer, G.E., DeShazer, J.A., Eds.; Publisher: Boston, MA, USA, 1998; pp. 290–301. [Google Scholar] [CrossRef]
- Walklate, P.J.; Cross, J.V.; Richardson, G.M.; Murray, R.A.; Baker, D.E. Comparison of Different Spray Volume Deposition Models Using LIDAR Measurements of Apple Orchards. Biosyst. Eng. 2002, 82, 253–267. [Google Scholar] [CrossRef]
- Cross, J.; Balsari, P.; Doruchowski, G.; Douzals, J.P.; Herbst, A.; Marucco, P.; Nuyttens, D.; Walklate, P. Orchard spray application in Europe—State of the art and research challenges. IOBC-WPRS Bull. 2013, 91, 465–475. [Google Scholar]
- Dorigoni, A.; Lezzer, P.; Micheli, F.; Dallabetta, N.; Pasqualini, J.; Guerra, A. Parete fruttifera stretta per mele redditizie e sostenibili. Inf. Agrar. 2009, 65, 54–58. [Google Scholar]
- Dorigoni, A.; Micheli, F. Possibilities for multi-leader trees. Eur. Fruit Mag. 2014, 2, 18–20. [Google Scholar]
- Jamar, L.; Mostade, O.; Huyghebaert, B.; Pigeon, O. Comparative performance of recycling tunnel and conventional sprayers using standard and drift-mitigating nozzles in dwarf apple orchards. Crop Prot. 2010, 29, 561–566. [Google Scholar] [CrossRef]
- Agnello, A.; Landers, A. Progress in the Development of an In-Canopy Fixed Spraying System for High-Density Apple Orchards. In Proceedings of the 88th Annual Orchard Pest and Disease Management Conference, Portland, OR, USA, 8–10 January 2014.
- Sévérac, G.; Romet, L. Des arbres bien enveloppés avec Alt’Carpo! L’Arboriculture Frutière 2007, 620–621, 24–28. [Google Scholar]
- Sauphanor, B.; Severac, G.; Maugin, S.; Toubon, J.-F.; Capowiez, Y. Exclusion netting may alter reproduction of the codling moth (Cydia pomonella) and prevent associated fruit damage to apple orchards. Entomol. Exp. Appl. 2012, 145, 134–142. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations Statistics Department. Available online: http://faostat3.fao.org/home/E (accessed on 10 June 2015).
- Pesticide Use Reduction Strategies in Europe. Six case studies. Available online: http://www.paneurope.info/Resources/Reports/Pesticide_Use_Reduction_Strategies_in_Europe.pdf (accessed on 10 June 2015).
- Wauchope, R.D.; Buttler, T.M.; Hornsby, A.G.; Augustijn-Beckers, P.W.M.; Burt, J.P. SCS/ARS/CES pesticides properties database for environmental decision making. Rev. Environ. Contam. Toxicol. 1992, 123, 1–157. [Google Scholar] [PubMed]
- Boller, E.F.; Avilla, J.; Gendrier, J.P.; Jörg, E.; Malavolta, C. Integrated Production in Europe. 20 years after the declaration of Ovrannaz. IOBC-WPRS Bull. 1998, 21, 41. [Google Scholar]
- IOBC/WPRS. Guidelines for Integrated Production of Pome Fruits in Europe. IOBC-WPRS Bull. 2002, 25, 1–8. [Google Scholar]
- Hough, W.S. Relative resistance to arsenical poisoning of two codling moth strains. J. Econ. Entomol. 1928, 21, 325–329. [Google Scholar] [CrossRef]
- Waldner, W. Ruckblick und Vorschau auf die Bekämpfung des Apfelwicklers. Obstbau-Weinbau 1993, 12, 355–357. [Google Scholar]
- Riedl, H.; Zelger, R. Erste Ergebnisse der Untersuchungen zur resistenz des Apfelwickler gegen uber Diflubenzuron. Obstbau Weinbau 1994, 4, 107–109. [Google Scholar]
- Sauphanor, B.; Benoit, M.; Bouvier, J.C.; Perron, G.; Fremond, J.C. Un cas de r´esistance du carpocapse des pommes au diflubenzuron dans le sud-est de la France. Phytoma 1994, 458, 46–49. [Google Scholar]
- Charmillot, P.J.; Pasquier, D.; Sauphanor, B.; Bouvier, J.C.; Olivier, R. Carpocapse des pommes: premier cas de r´esistance au diflubenzuron en Suisse. Rev Suisse Vitic Arboric Hortic 1999, 31, 129–132. [Google Scholar]
- Ioriatti, C.; Tasin, M.; Charmillot, P.J.; Reyes, M.; Sauphanor, B. Early detection of resistance to tebufenozide in field populations of Cydia pomonella L.: methods and mechanisms. J. Appl. Entomol. 2007, 131, 453–459. [Google Scholar] [CrossRef]
- Sauphanor, B.; Avilla, J.; Charmillot, P.J.; Ioriatti, C.; Michele, S.; Matias , C.; Waldner , W. Coping with insecticide resistance in fruit production: the example of codling moth in Europe. In Book of Abstracts of the VIth European Congress of Entomology; Brinnhofer, V., Soldan, T., Eds.; Publisher: City, County, 1998; pp. 619–620. [Google Scholar]
- Sauphanor, B.; Brosse, V.; Bouvier, J.C.; Speich, P.; Micoud, A.; Martinet, C. Monitoring resistance to diflubenzuron and deltamethrin in French codling moth populations (Cydia pomonella). Pest Manag Sci. 2000, 56, 74–82. [Google Scholar] [CrossRef]
- Charmillot, P.J.; Pasquier, D.; Grela, C.; Genini, M.; Olivier, R.; Ioriatti, C.; Butturini, A. Résistance du carpocapse Cydia pomonella aux insecticides. Revue Suisse Vitic. Arboric. Hortic. 2003, 35, 363–368. [Google Scholar]
- Rodriguez, M.A.; Dolors, B.; Avilla, J. Resistance of Spanish codling moth (Cydia pomonella) populations to insecticides and activity of detoxifying enzymatic systems. Entomol. Exp. Appl. 2011, 138, 184–192. [Google Scholar] [CrossRef]
- Voudouris, C.; Sauphanor, B.; Franck, P.; Reyes, M.; Mamuris, Z.; Tsitsipis, J.A.; Vontas, J.; Margaritopoulos, J.T. Insecticide resistance status of the coodling moth Cydia pomonella (Lepidoptera: Tortricidae) From Greece. Pectic Biochem. Physiol. 2011, 100, 229–238. [Google Scholar] [CrossRef]
- Gund, N.A.; Wagner, A.; Timm, A.E.; Schulze-Bopp, S.; Jehle, J.A.; Johannesen, J.; Reineke, A. Genetic analysis of Cydia pomonella (Lepidoptera: Tortricidae) populations with different levels of sensitivity towards the Cydia pomonella granulovirus (CpGV). Genetica 2012, 140, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.V. Susceptibility of the summer fruit tortrix moth, Adoxophyes orana (Lepidoptera: Tortricidae), to chlorpyrifos and strategies for insecticidal control in orchards. Ann. Appl. Biol. 1997, 131, 197–212. [Google Scholar] [CrossRef]
- Salamin, C.; Charmillot, P.J.; Pasquier, D. Nouveau cas de resistance aux insecticides de la tordeuse de la pelure capua (Adoxophyes orana). Revue Suisse Vitic. Arboric. Hortic 2007, 39, 179–183. [Google Scholar]
- Kehrli, P.; Pasquier, D.; Roux, P.A. Variabilite phenologique et sensibilite aux insecticides de capua (Adoxophyes orana) en Valais. Revue Suisse Vitic. Arboric. Hortic 2009, 41, 263–268. [Google Scholar]
- Cao, G.; Han, Z. Tebufenozide resistance selected in Plutella xylostella and its cross-resistance and fitness cost. Pest Manage. Sci. 2006, 62, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Ioriatti, C.; Charmillot, P.J.; Forno, F.; Mattedi, L.; Pasquier, D.; Rizzi, C. Control of Codling moth Cydia pomonella L. using insecticides: field efficacy in relation to the susceptibility of the insect. IOBC-WPRS Bull. 2005, 28, 259–264. [Google Scholar]
- Charmillot, P.J.; Blanc, G.; Pasquier, D. Premier cas de résistance en Suisse de la tordeuse de la pelure capua (Adoxophyes orana) aux insecticides. Revue suisse Vitic. Arboric. Hortic. 2006, 38, 87–93. [Google Scholar]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Analysis of Global Pesticide Resistance. In Arthropods; CABI Publishing, CAB International: Wallingford, UK, 2008; pp. 5–32. [Google Scholar]
- Newcomer, E.J.; Dean, F.P. Orchards mites resistant to Parathion in Washington. J. Econ. Entomol. 1952, 45, 1076–1078. [Google Scholar] [CrossRef]
- Kumral, N.A.; Susurluk, H.; Gençer, S.N.; Gűrkan, M.O. Resistance to chlorpyrifos and lambda-cyhalothrin along with detoxifying enzyme activities in field-collected female populations of European red mite. Phytoparasitica 2009, 37, 7–15. [Google Scholar] [CrossRef]
- Kramer, T.; Nauen, R. Monitoring of spirodiclofen susceptibility in field populations of European red mites, Panonychus ulmi (Koch) (Acari: Tetranychidae), and the cross-resistance pattern of a laboratory-selected strain. Pest Manag. Sci. 2011, 67, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Duso, C.; Castagnoli, M.; Simoni, M.; Angeli, G. The impact of eriophyds on crops: recent issues on Aculus schlechtendali, Calepitrimerus vitis and Aculops lycopersici. Exp. Appl. Acarol. 2010, 51, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.V.G.; Anderson, N.H. Notes on parathion-resistant strains of two phytophagous mites and a predacious mite in British Columbia. Can. Entomol. 1958, 90, 92–97. [Google Scholar] [CrossRef]
- Marcic, D. Acaricides in modern management of plant-feeding mites. J. Pest Sci. 2012, 85, 395–408. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Dermauw, W.; Grbic, M.; Tirry, L.; Feyereisen, R. Spider mite control and resistance management: Does a genome help? Pest Manag. Sci. 2012, 69, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Arthropod Pesticide Resistance Database. Available online: www.pesticideresistance.com/search.php (accessed on 10 June 2015).
- Beckerman, J.; Sundin, G.W.; Rosenberger, D.A. Do some IPM concepts contribute to the development of fungicide resistance? Lessons learned from the apple scab pathosystem in the United States. Pest Manag. Sci. 2015, 71, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Dux, H.; Sierotzki, H.; Gisi, U. Sensitivity of Venturia inaequalis populations to anilinopyrimidyne, DMI and QoI fungicides. In Proceedings of the 14th International Reinhardsbrunn Symposium “Modern fungicides and antifungal compounds”, Reinhardsbrunn, Germany, 25–29 April 2004; pp. 40–41.
- Köller, W.; Wilcox, W.F. Evidence for predisposition of fungicide-resistant isolates of Venturia inaequalis to a preferential selection for resistance to other fungicides. Phytopathology 2001, 91, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Köller, W.; Wilcox, W.F.; Parker, D.M. Sensitivity of Venturia inaequalis populations to anilinopyrimidine fungicides and their contribution to scab management in New York. Plant Dis. 2005, 89, 357–365. [Google Scholar] [CrossRef]
- Fiaccadori, R.; Portillo, I.; Roberti, R.; Brunelli, A. Isolation of antagonistic fungi towards Venturia inaequalis and preliminary applications in sanitation practice reducing ascospore inoculums. IOBC-WPRS Bull. 2013, 91, 59–66. [Google Scholar]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål)(Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Nielsen, A.L.; Hamilton, G.C. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J. Econ. Entomol 2009, 102, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the invasive brown marmorated stink bug, Halyomorpha halys, in mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche 2012. [Google Scholar] [CrossRef]
- Milonas, P.G.; Partsinevelos, G.K. First report of the brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) in Greece. EPPO Bull. 2014, 44, 183–186. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; Herbert, A.; Hoelmer, K.; Hooks, C.R.; Jones, A.; Krawczyk, G.; Kuhar, T.; Martinson, H.; Mitchell, W.; Nielsen, A.L.; Pfeiffer, D.G.; Raupp, M.J.; Rodriguez-Saona, C.; Shearer, P.; Shrewsbury, P.; Venugopal, P.D.; Whalen, J.; Wiman, N.G.; Leskey, T.C.; Tooker, J.F. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemipetra: Pentatomidae). J. Int. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Dalpiaz, A. Innovazione e organizzazione, le uniche risposte per uscire dalla crisi. Frutticoltura 2014, 11, 2–6. [Google Scholar]
- Waldner, W. Le catene alimentari e la riduzione numerica di residui di agro farmaci. Frutta e Vite 2009, 4, 182–186. [Google Scholar]
- Krawczyk, G.; Hull, L.A.; Bohnenblust, E. Utilization of mating disruption and codling moth granulosis virus (CMGV) in conventional commercial apple orchards in Pennsylvania, USA. IOBC/WPRS Bull. 2010, 54, 71–74. [Google Scholar]
- Agnolin, C.; Ioriatti, C.; Pontalti, M.; Venturelli, M.B. IFP experiences in Trentino, Italy. Acta Holticulturae 2000, 525, 45–49. [Google Scholar]
- Ioriatti, C.; Anfora, G.; Angeli, G.; Civolani, S.; Schmidt, S.; Pasqualini, E. Toxicity of emamectin benzoate to Cydia pomonella (L.) and Cydia molesta (Busck) (Lepidoptera: Tortricidae): laboratory and field tests. Pest Manag. Sci. 2009, 65, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Baldessari, M.; Giuliani, G.; Chiesa, S.; Larcher, R.; Ioriatti, C.; Angeli, G. Pesticide residue free fruits: The aim of Trentino apple production system. Commun. Agric. Appl. Biol. Sci. 2013, 78, 133–137. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damos, P.; Colomar, L.-A.E.; Ioriatti, C. Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept? Insects 2015, 6, 626-657. https://doi.org/10.3390/insects6030626
Damos P, Colomar L-AE, Ioriatti C. Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept? Insects. 2015; 6(3):626-657. https://doi.org/10.3390/insects6030626
Chicago/Turabian StyleDamos, Petros, Lucía-Adriana Escudero Colomar, and Claudio Ioriatti. 2015. "Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept?" Insects 6, no. 3: 626-657. https://doi.org/10.3390/insects6030626
APA StyleDamos, P., Colomar, L.-A. E., & Ioriatti, C. (2015). Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept? Insects, 6(3), 626-657. https://doi.org/10.3390/insects6030626