Antagonistic Interactions between the African Weaver Ant Oecophylla longinoda and the Parasitoid Anagyrus pseudococci Potentially Limits Suppression of the Invasive Mealybug Rastrococcus iceryoides
Abstract
:1. Introduction
2. Experimental Section
2.1. Mealybug and Parasitoid Wasp Colonies
2.2. Ant Colonies
2.3. Effect of O. longinoda on Parasitism of R. iceryoides by A. pseudococci and Parasitoid Sex Ratio
2.4. Host Handling Time and Oviposition Success of A. pseudococci in the Presence and Absence of O. longinoda
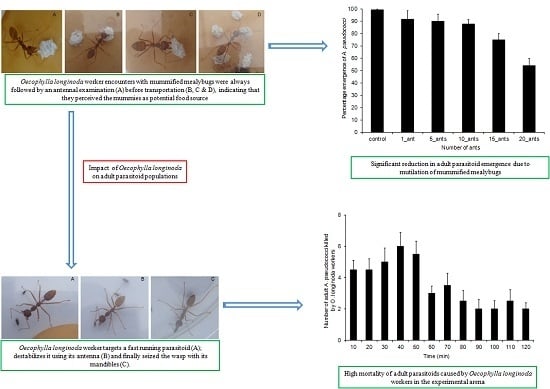
2.5. Effect of O. longinoda Abundance on Parasitoid Emergence
2.6. Behavioural Experiment of Parasitoid Wasp
2.7. Statistical Analysis
3. Results
3.1. Effect of O. longinoda on Parasitism of R. iceryoides by A. pseudococci, Parasitoid Emergence and Parasitoid Sex Ratio
| Parameters | Treatment | t-Test | |||
|---|---|---|---|---|---|
| Ant-Tended | Ant-Excluded | t | df | P | |
| Parasitized Nymphs (%) | 51.4 ± 4.13 b | 86.6 ± 1.31 a | 8.45 | 18 | <0.0001 |
| Adult parasitoid eclosion (%) | 85.42 ± 2.72 b | 94.54 ± 0.55 a | 3.34 | 18 | 0.0069 |
| Sex ratio (%) | 62.2 ± 3.28 b | 71.67 ± 1.71 a | 2.54 | 18 | 0.0204 |
3.2. Host-Handling Time and Oviposition Success of A. pseudococci in the Presence and Absence of O. longinoda
| Parameters | Treatment | t-Test | |||
|---|---|---|---|---|---|
| Ant-Tended | Ant-Excluded | t | df | P | |
| Searching time (seconds) | 440.9 ± 28.42 a | 334.5 ± 25.83 b | 2.77 | 38 | 0.0086 |
| Host handling time (seconds) | 71.07 ± 1.68 b | 79.53 ± 2.56 a | 2.72 | 28 | 0.0112 |
| No. of ovipositor penetration/h | 5.87 ± 0.72 b | 11.27 ± 1.29 a | 7.18 | 28 | 0.0093 |
| No. of successful oviposition/h | 4.13 ± 0.70 b | 9.87 ± 1.25 a | 4.02 | 28 | 0.0006 |
3.3. Effect of O. longinoda Abundance on Parasitoid Emergence



3.4. Mortality of A. pseudococci by O. longinoda and Parasitoid Behaviour Displayed to Evade Encounters



4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, D.J. The mealybug genus Rastrococcus Ferris (Hemiptera: Pseudococcidae). J. Syst. Entomol. 1989, 14, 433–486. [Google Scholar] [CrossRef]
- Luhanga, W.W.; Gwinner, J. Mango mealybug (Rastrococcus iceryoides) on Mangifera indica in Malawi. FAO Plant Prot. Bull. 1993, 41, 125–126. [Google Scholar]
- CABI. Crop Protection Compendium. In Global Module, 2nd ed.; CAB International Publishing: Wallingford, UK, 2000. [Google Scholar]
- Tanga, M.C. Bio-Ecology of the Mango Mealybug, Rastrococcus iceryoides Green (Hemiptera: Pseudococcidae) and Its Associated Natural Enemies in Kenya and Tanzania. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2012. [Google Scholar]
- Moore, D. Biological control of Rastrococcus invadens. Biocontrol News Inf. 2004, 25, 17–27. [Google Scholar]
- Agounké, D.; Agricola, U.; Bokonon-Ganta, A. Rastrococcus invadens Williams (Hemiptera, Pseudococcidae), a serious exotic pest of fruit trees and other plants in West Africa. Bull. Entomol. Res. 1988, 78, 695–702. [Google Scholar] [CrossRef]
- Ben-Dov, Y. A Systematic Catalogue of the Mealybugs of the World (Insecta: Homoptera: Coccoidea: Pseudococcidae and Putoidae) with Data on Geographical Distribution, Host Plants, Biology and Economic Importance; Intercept Limited: Andover, UK, 1995; pp. 435–436. [Google Scholar]
- Bokonon-Ganta, A.H.; de Groote, H.; Neuenschwander, P. Socio-economic impact of biological control of mango mealybug in Benin. Agric. Ecosyst. Environ. 2002, 93, 367–378. [Google Scholar] [CrossRef]
- Schabel, H.G. Forest Entomology in East Africa: Forest Insects of Tanzania; Springer: Berlin, Germany, 2006; p. 328. [Google Scholar]
- Rawat, R.R.; Jakhmola, S.S. Bionomics of the mango-coccid (Rastrococcus iceryoides Green; Homoptera, Coccidae). Indian J. Agric. Sci. 1970, 40, 140–144. [Google Scholar]
- CPC. Crop Protection Compendium Database; CAB International: Wallingford, UK, 2002. [Google Scholar]
- Kairo, M.T.K.; Pollard, G.V.; Peterkin, D.D.; Lopez, V.F. Biological control of the hibiscus mealybug, Maconellicoccus hirsutus Green (Hemiptera: Pseudococcidae) in the Caribbean. Integr. Pest Manag. Rev. 2000, 5, 241–254. [Google Scholar] [CrossRef]
- Meyerdirk, D.E.; Warkentin, R.; Attavian, B.; Gersabeck, E.; Francis, A.; Adams, M.; Francis, G. Biological Control of the Pink Hibiscus Mealybug Project Manual; United States Department of Agriculture: Washington, DC, USA, 1998.
- McKenzie, H.L. Mealybugs of California with Taxonomy, Biology and control of North American Species (Homoptera: Coccoidea: Pseudococcidae); University of California Press: Berkeley, LA, USA, 1967; p. 534. [Google Scholar]
- Sagarra, L.A.; Peterkin, D.D. Invasion of the Carribean by the hibiscus mealybug, Maconellicoccus hirsutus Green (Homoptera: Pseudococcidae). Phytoprotection 1999, 80, 103–113. [Google Scholar] [CrossRef]
- Tanga, M.C.; Samira, A.M.; Govender, P.; Ekesi, S. Effect of host plant on bionomics and life history parameters of Anagyrus pseudococci (Hymenoptera: Encyrtidae), a parasitoid of the mango mealybug Rastrococcus iceryoides (Homoptera: Pseudococcidae). Biol. Control 2012, 65, 43–52. [Google Scholar] [CrossRef]
- Van Wijngaarden, P.M.; van Kessel, M.; van Huis, A. Oecophylla longinoda (Hymenoptera: Formicidae) as a biological control agent for cocoa capsids (Hemiptera: Miridae). Proc. Neth Entomol. Soc. Meet. 2007, 18, 21–30. [Google Scholar]
- Van Mele, P. A historical review of research on the weaver ant Oecophylla in biological control. Agric. For. Entomol. 2008, 10, 13–22. [Google Scholar] [CrossRef]
- Carroll, C.R.; Janzen, D.H. Ecology of foraging by ants. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 231–257. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990; p. 732. [Google Scholar]
- Jiggins, C.; Majerus, M.E.N.; Gough, U. Ant defence of colonies of Aphis fabae. Br. J. Entomol. Nat. Hist. 1993, 6, 129–137. [Google Scholar]
- Buckley, R.C. Interactions involving plants, Homoptera, and ants. Ann. Ecol. Syst. 1987, 18, 111–135. [Google Scholar] [CrossRef]
- Wimp, G.M.; Whitham, T.G. Biodiversity consequences of predation and host plant hybridization on an aphid-ant mutualism. Ecology 2001, 82, 440–452. [Google Scholar]
- Martinez-Ferrer, M.T.; Grafton-Cardwell, E.E.; Shorey, H.H. Disruption of parasitism of the California red scale (Homoptera: Diaspididae) by three ant species (Hymenoptera: Formicidae). Biol. Control 2003, 26, 279–286. [Google Scholar] [CrossRef]
- Chacón, J.M.; Landis, D.A.; Heimpel, G.E. Potential for biotic interference of a classical biological control agent of the soybean aphid. Biol. Control 2008, 46, 216–225. [Google Scholar] [CrossRef]
- Goeden, R.D.; Louda, S.M. Biotic interference with insects imported for weed-control. Ann. Rev. Entomol. 1976, 21, 325–342. [Google Scholar] [CrossRef]
- Van Mele, P.; Vayssières, J.F.; van Tellingen, E.; Vrolijks, J. Effects of an African weaver ant, Oecophylla longinoda, in controlling mango fruit flies (Diptera: Tephritidae) in Benin. J. Econ. Entomol. 2007, 100, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Van Mele, P.; Cuc, N.T.T.; van Huis, A. Direct and indirect influences of the weaver ant Oecophylla smaragdina on citrus farmers’ pest perceptions and management practices in Mekong Delta, Vietnam. Int. J. Pest Manag. 2002, 48, 225–232. [Google Scholar] [CrossRef]
- Ayenor, G.K.; Röling, N.G.; Padi, B.; van Huis, A.; Obeng-Ofori, D.; Atengdem, P.B. Converging farmers’ and scientists’ perspectives on researchable constraints on organic cocoa production in Ghana: Results of a diagnostic study. NJAS Wagening. J. Life Sci. 2004, 52, 261–284. [Google Scholar] [CrossRef]
- Offenberg, J.; Havanon, S.; Aksornkoae, S.; Macintosh, D.; Nielsen, M.G. Observations on the ecology of weaver ants (Oecophylla smaragdina Fabricius) in a Thai mangrove ecosystem and their effect on herbivory of Rhizophora mucronata Lam. Biotropica 2004, 36, 344–351. [Google Scholar] [CrossRef]
- Peng, R.K.; Christian, K. The weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), an effective biological control agent of the red-banded thrips, Selenothrips rubrocinctus (Thysanoptera: Thripidae) in mango crops in the Northern Territory of Australia. Int. J. Pest Manag. 2004, 50, 107–114. [Google Scholar] [CrossRef]
- Peng, R.K.; Christian, K.; Reilly, D. Weaver ants, Oecophylla smaragdina (Hymenoptera: Formicidae), as bio-control agents on African mahogany trees, Khaya senegalensis (Sapindales: Meliaceae), in the Northern Territory of Australia. Int. J. Pest Manag. 2010, 56, 363–370. [Google Scholar] [CrossRef]
- Sinzogan, A.A.C.; van Mele, P.; Vayssières, J.F. Implications of on-farm research for local knowledge related to fruit flies and the weaver ant Oecophylla longinoda in mango production. Int. J. Pest Manag. 2008, 54, 241–246. [Google Scholar] [CrossRef]
- Way, M.J.; Khoo, K.C. Role of ants in pest management. Ann. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Kaplan, I.; Eubanks, M.D. Disruption of cotton aphid (Homoptera: Aphididae)-natural enemy dynamics by red imported fire ants (Hymenoptera: Formicidae). Environ. Entomol. 2002, 31, 1173–1183. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.R.; Fallon, J.; Cooper, M.L. Impacts of Argentine ants on mealybugs and their natural enemies in California’s coastal vineyards. Ecol. Entomol. 2007, 32, 583–596. [Google Scholar] [CrossRef]
- Mgocheki, N.; Addison, P. Interference of ants (Hymenoptera: Formicidae) with biological control of the vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biol. Control 2009, 49, 180–185. [Google Scholar] [CrossRef]
- Appiah, E.F.; Ekesi, S.; Afreh-Nuamah, K.; Obeng-Ofori, D.; Mohamed, S.A. African weaver ant-produced semiochemicals impact on foraging behavior and parasitism by the Opiine parasitoid, Fopius arisanus on Bactrocera invadens (Diptera: Tephritidae). Biol. Control 2014, 79, 49–57. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The multiple recruitment systems of the African weaver ant Oecophylla longinoda (Latreille) (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 1978, 3, 19–60. [Google Scholar] [CrossRef]
- Whitehead, V.B. A Study of the Predators and Parasites of Planococcus citri (Risso) (Homoptera: Pseudococcidae) on Vines in the Western Cape Province, South Africa. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 1957. [Google Scholar]
- Van Lenteren, J.C. Oviposition Behavior of Aphytis. In Advances in the Study of Aphytis; Rosen, D., Ed.; Intercept: Andover, MA, USA, 1994; pp. 13–40. [Google Scholar]
- Barzman, M.S.; Daane, K.M. Host-handling behaviours in parasitoids of the black scale: A case for ant-mediated evolution. J. Anim. Ecol. 2001, 70, 237–247. [Google Scholar]
- Way, M.J. Studies on the association of the ant Oecophylla longinoda (Latr.) (Formicidae) with the scale insect Saissetia zanzibarensis Williams (Coccidae). Bull. Entomol. Res. 1954, 45, 113–134. [Google Scholar] [CrossRef]
- Islam, K.S.; Copland, M.J.W. Host preference and progeny sex ratio in a solitary koinobiont mealybug endoparasitoids, Anagyrus pseudococci (Girault) in response to its host stage. Biocontrol. Sci. Technol. 1997, 7, 449–456. [Google Scholar] [CrossRef]
- Lacey, E.P. What is an adaptive environmentally induced parental effect? In Maternal Effects as Adaptations; Mousseau, T.A., Fox, C.W., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 54–66. [Google Scholar]
- Van Kessel, M.; van Wijngaarden, M. The Behaviour of Oecophylla longinoda. How to Manipulate or Use Oecophylla Ants in Cocoa AgroEcosystems in Order to Suppress Cocoa Pests Such as Capsids? Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Rosenheim, J.A.; Rosen, D. Foraging and oviposition decisions in the parasitoid Aphytis lingnanensis: Distinguishing the influences of egg load and experience. J. Anim. Ecol. 1991, 60, 873–893. [Google Scholar] [CrossRef]
- Rousse, P.; Chiroleu, F.; Veslot, J.; Quilici, S. The host and microhabitat olfactory location by Fopius arisanus suggests a broad potential host range. Physiol. Entomol. 2007, 32, 313–321. [Google Scholar] [CrossRef]
- R Development Core Team. The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 21 July 2015).
- Malakar-Kuenen, R.; Daane, K.M.; Godfrey, K.E.; Ball, J.C.; Bentley, W.J.; Yokota, G.Y.; Martin, L.A.; Godfrey, K.; Ball, J. Population dynamics of the vine mealybug and its natural enemies in the Coachella and San Joaquin Valleys. Univ. Calif. Plant Prot. Q. 2001, 11, 1–3. [Google Scholar]
- Trjapitzin, S.V.; Trjapitzin, V.A. Parasitoids of the mealybugs on cultivated grapes in Argentina, with description of a new species of the genus Aenasius Walker (Hymentoptera: Encrytidae). Entomol. Obozr. 2002, 76, 174–179. [Google Scholar]
- Walton, V.M. Development of an Integrated Pest Management System for Vine Mealybug, Planococcus citrus (Signoret), in Vineyards in the Western Cape Province, South Africa. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2003. [Google Scholar]
- Walton, V.M.; Daane, K.M.; Bentley, W.J.; Millar, J.G.; Larsen, T.E.; Malakar-Kuenen, R. Pheromone-based mating disruption of Planococcus ficus (Hemiptera: Pseudococcidae) in California vineyards. J. Econ. Entomol. 2006, 99, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Triapitsyn, S.V.; Gonzalez, D.; Vickerman, D.B.; Noyes, J.S.; White, E.B. Morphological, biological and molecular comparisons among the different geographical populations of Anagyrus pseudococci (Hymenoptera: Encyrtidae), parasitoids of Planococcus spp. (Hemiptera: Pseudococcidae), with notes on Anagyrus dactylopii. Biol. Control 2007, 41, 14–24. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Dhouibi, M.H. Survey of mealybugs (Hemiptera: Pseudococcidae) and their natural enemies in Tunisian vineyards. Afr. Entomol. 2009, 17, 154–160. [Google Scholar] [CrossRef]
- Boulton, R.A.; Collins, L.A.; Shuker, D.M. Beyond sex allocation: The role of mating systems in sexual selection in parasitoid wasps. Biol. Rev. 2014, 90, 599–627. [Google Scholar] [CrossRef] [PubMed]
- Wylie, H.G. Interference among females of Nasonia vitripennis (Hymenoptera: Pteromalidae) and its effect on sex ratio of their progeny. Can. Entomol. 1976, 108, 655–661. [Google Scholar] [CrossRef]
- Jackson, D.J. Observation on the biology of Caraphractus cinctus (Walter) (Hymenoptera: Mymaridae), a parasitoid of the eggs of Dysticidae (Coleoptera). III. The adult life and sex ratio. Trans. R. Entomol. Soc. Lond. 1966, 118, 23–49. [Google Scholar] [CrossRef]
- Hcidari, M.; Jahan, M. A study of ovipositional behaviour of Anagyrus pseudococci a parasitoid of mealybugs. J. Agric. Sci. Technol. 2000, 2, 49–53. [Google Scholar]
- Flanders, S.E. The role of the ant in the biological control of homopterous insects. Can. Entomol. 1951, 83, 93–98. [Google Scholar] [CrossRef]
- Tollerup, K.; Rust, M.K.; Klotz, J.H. Formica perpilosa, an emerging pest in vineyards. J. Agric. Urban Entomol. 2007, 24, 147–158. [Google Scholar] [CrossRef]
- Mansour, R.; Gaetana, M.; Alessandra, L.P.; Kaouthar, G.L.; Agatino, R. A survey of scale insects (Hemiptera: Coccoidea) and tending ants in Tunisian vineyards. J. Plant Prot. Res. 2012, 51, 197–203. [Google Scholar] [CrossRef]
- Vinson, S.B.; Scarborough, T.A. Interaction between Solenopsis invicta (Hymenoptera: Formicidae), Rhopalosiphum maidis (Homoptera: Aphididae) and the parasitoid Lysiphlebus testaceipes Cresson (Hymenoptera: Aphidiidae). Ann. Entomol. Soc. Am. 1991, 84, 158–164. [Google Scholar] [CrossRef]
- Michaud, J.P. Sources of mortality in colonies of brown citrus aphid Toxoptera citricida. BioControl 1999, 44, 347–367. [Google Scholar] [CrossRef]
- Hill, S.L.; Hoy, M.A. Interactions between the red imported fire ant Solenopsis invicta and the parasitoid Lipolexis scutellaris potentially affecting a classical biological control agent of the aphid Toxoptera citricida. Biol. Control 2003, 27, 11–19. [Google Scholar] [CrossRef]
- Shiojiri, K.; Takabayashi, J. Parasitoid preference for host-infested plants in affected by the risk of intraguild predation. J. Insect Behav. 2005, 18, 567–576. [Google Scholar] [CrossRef]
- Nault, L.R.; Montogomery, M.E.; Bowers, W.S. Ant-aphid association: Role of aphid alarm pheromone. Science 1976, 192, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.M.; Ward, S.A.; Dixon, A.F.G. Limitation of superparasitism by Aphidius rhopalosiphi: A consequence of aphid defensive behavior. Ecol. Entomol. 1984, 9, 149–155. [Google Scholar] [CrossRef]
- Gerling, D.; Roitberg, B.D.; Mackauer, M. Instar-specific defense of the pea aphid, Acyrthosiphon pisum: Influence on oviposition success of the parasite Aphelinus asychis. J. Insect Behav. 1990, 3, 501–514. [Google Scholar] [CrossRef]
- Buckley, R.; Gullan, P. More aggressive ant species (Hymenoptera: Formicidae) provide better protection for soft scale and mealybugs (Homoptera: Pseudococcidae). Biotropica 1991, 23, 282–286. [Google Scholar] [CrossRef]
- Jahn, G.C.; Beardsley, J.W.; González-Hernández, H. A review of the association of ants with mealybug wilt disease of pineapple. Proc. Hawaii Entomol. Soc. 2003, 36, 9–28. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanga, C.M.; Ekesi, S.; Govender, P.; Nderitu, P.W.; Mohamed, S.A. Antagonistic Interactions between the African Weaver Ant Oecophylla longinoda and the Parasitoid Anagyrus pseudococci Potentially Limits Suppression of the Invasive Mealybug Rastrococcus iceryoides. Insects 2016, 7, 1. https://doi.org/10.3390/insects7010001
Tanga CM, Ekesi S, Govender P, Nderitu PW, Mohamed SA. Antagonistic Interactions between the African Weaver Ant Oecophylla longinoda and the Parasitoid Anagyrus pseudococci Potentially Limits Suppression of the Invasive Mealybug Rastrococcus iceryoides. Insects. 2016; 7(1):1. https://doi.org/10.3390/insects7010001
Chicago/Turabian StyleTanga, Chrysantus M., Sunday Ekesi, Prem Govender, Peterson W. Nderitu, and Samira A. Mohamed. 2016. "Antagonistic Interactions between the African Weaver Ant Oecophylla longinoda and the Parasitoid Anagyrus pseudococci Potentially Limits Suppression of the Invasive Mealybug Rastrococcus iceryoides" Insects 7, no. 1: 1. https://doi.org/10.3390/insects7010001
APA StyleTanga, C. M., Ekesi, S., Govender, P., Nderitu, P. W., & Mohamed, S. A. (2016). Antagonistic Interactions between the African Weaver Ant Oecophylla longinoda and the Parasitoid Anagyrus pseudococci Potentially Limits Suppression of the Invasive Mealybug Rastrococcus iceryoides. Insects, 7(1), 1. https://doi.org/10.3390/insects7010001