Collectively Facilitated Behavior of the Neonate Caterpillars of Cactoblastis cactorum (Lepidoptera: Pyralidae)
Abstract
:1. Introduction
2. Experimental Section
2.1. Insects
2.2. Rate of Eclosion
2.3. Eclosion During the Photophase and Scotophase of Light Cycle
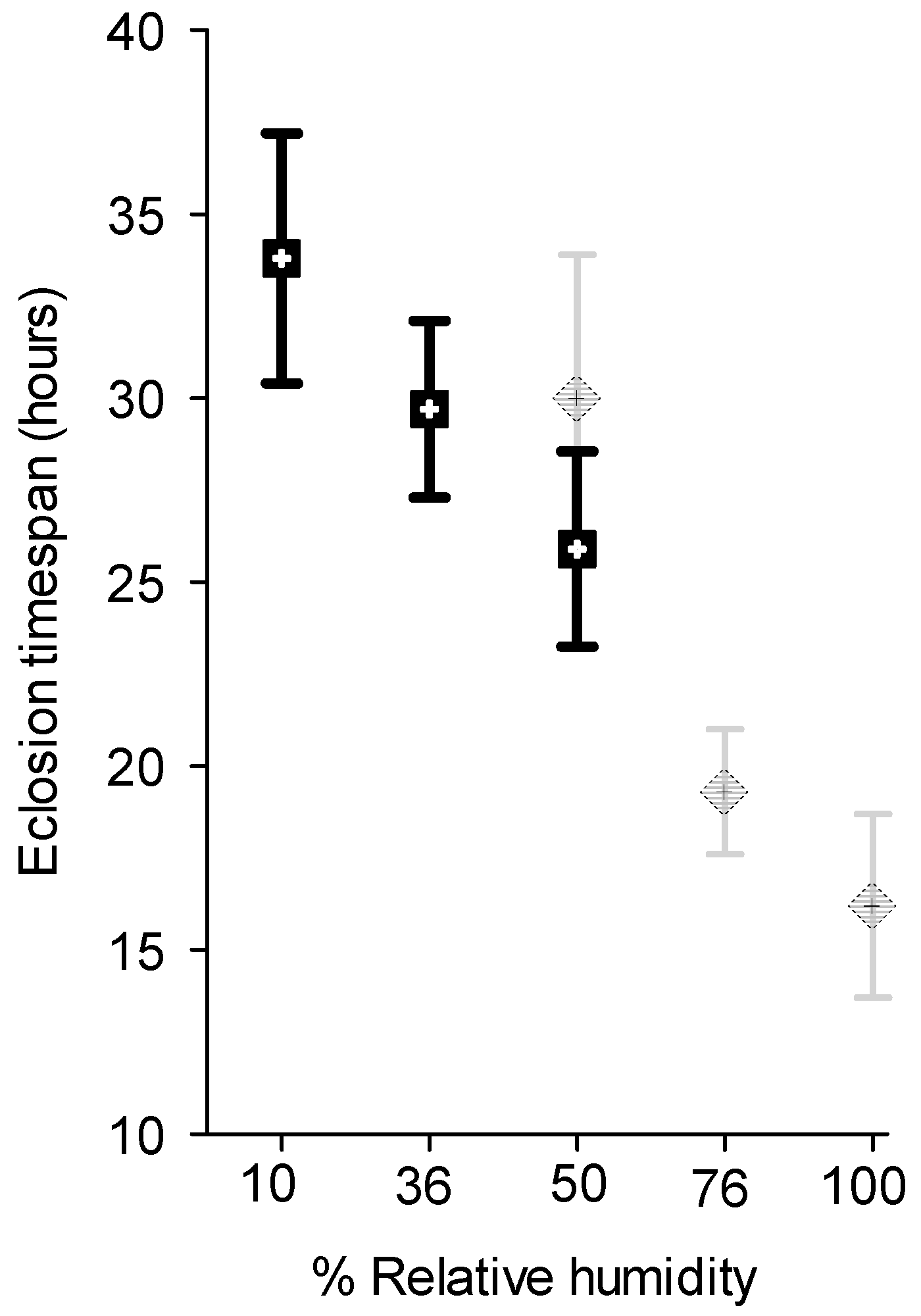
2.4. Effect of RH on Egg Hatch
2.5. Aggregation and the Initiation of Excavation
2.6. Ability of Individual and Small Groups of Caterpillars to Penetrate a Cladode
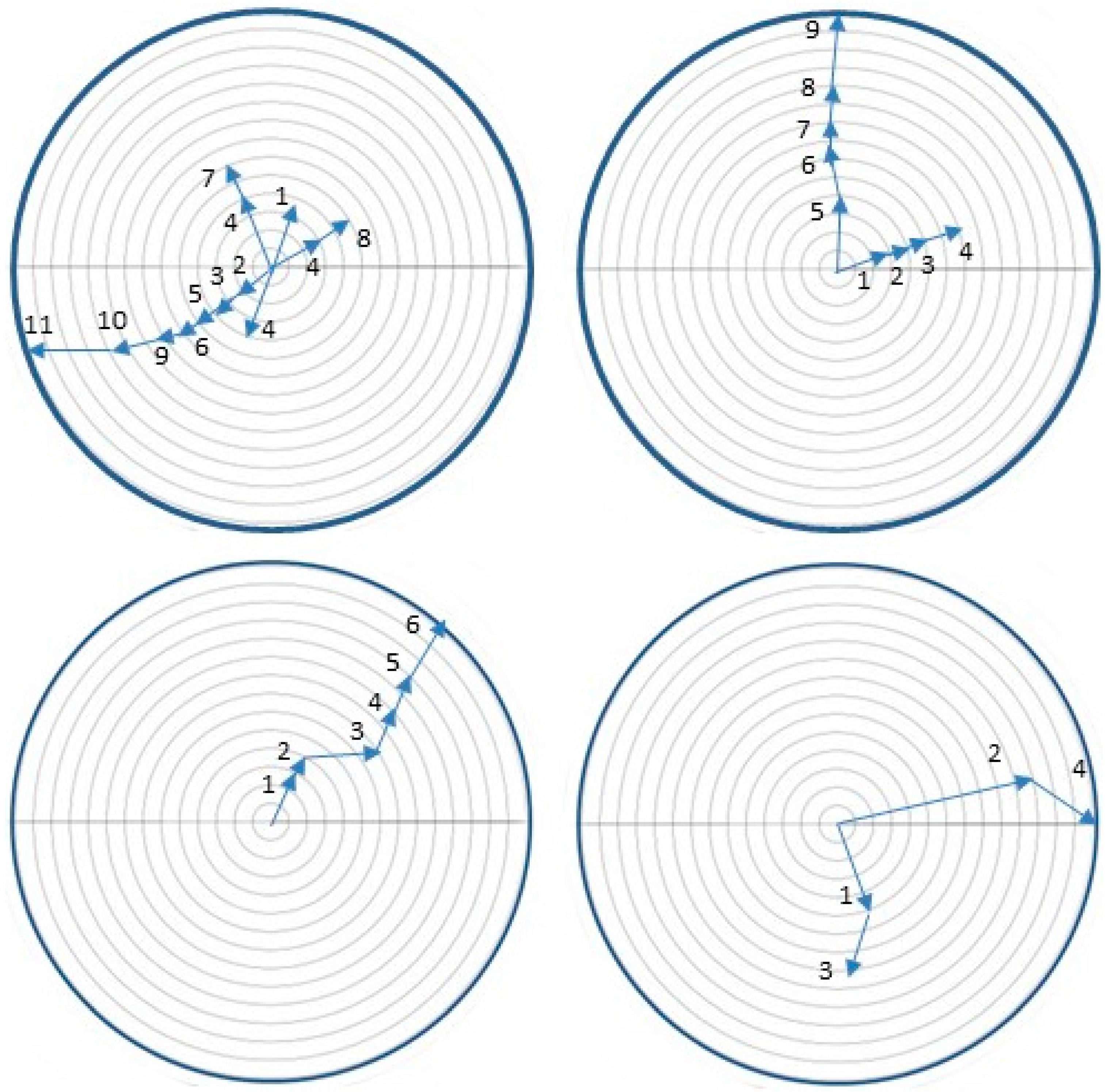
2.7. Orientation of Neonates to Host Volatiles
2.8. Group Excavation Behavior
2.9. Role of Mandibular Gland Secretion in Pre-Excavation Aggregation
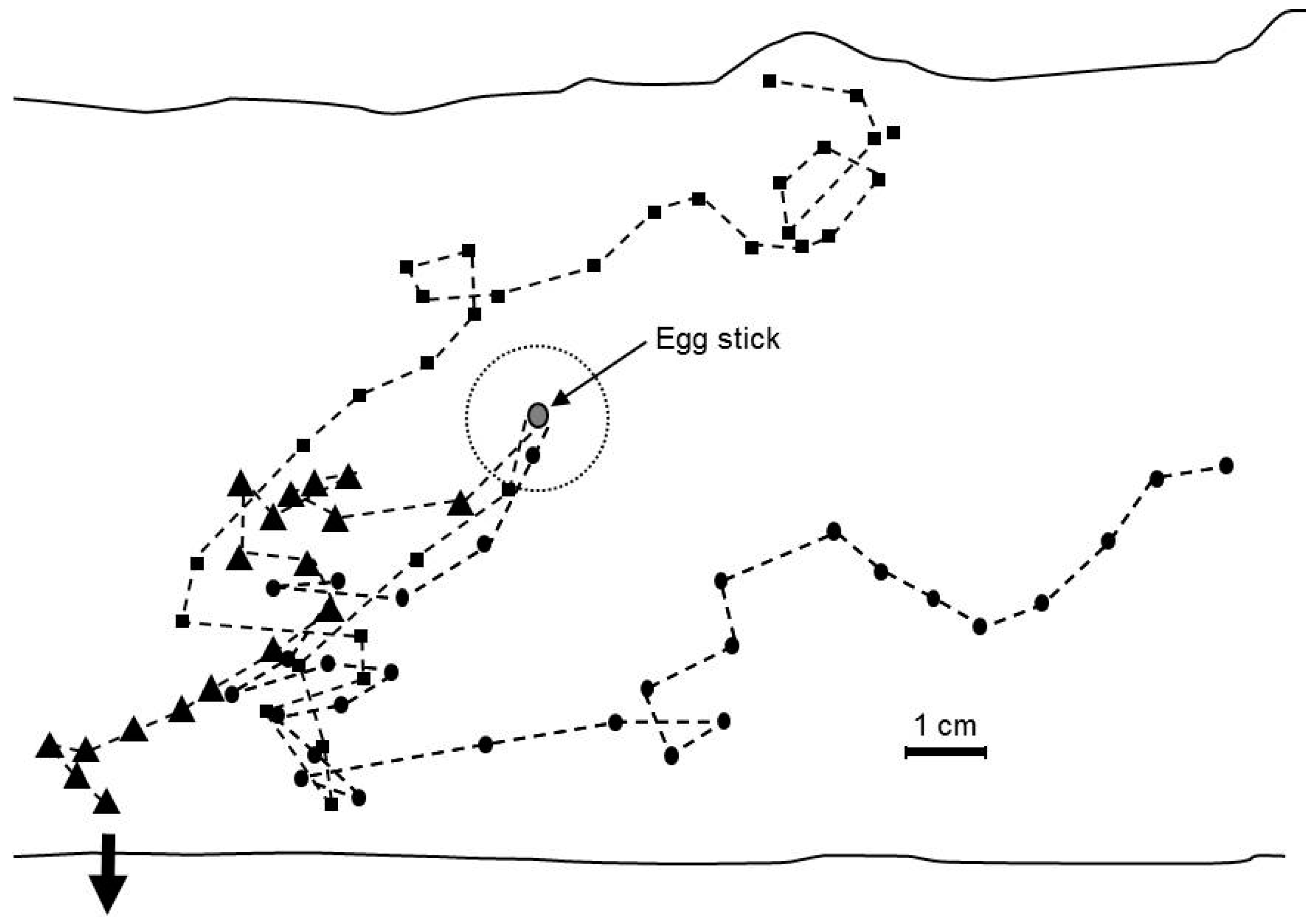
2.10. Trail Marking When the Cladode Cannot Be Excavated at the Eclosion Site
2.11. Data Analysis
3. Results
3.1. Rate of Eclosion
3.2. Eclosion During Photo- and Scoto- Phases of a Light Cycle
3.3. Effect of Relative Humidity on Egg Hatch
3.4. Aggregation and the Initiation of Excavation
3.5. Ability of Individual and Small Groups of Caterpillars to Penetrate a Cladode
3.6. Orientation of Neonates to Host Volatiles
3.7. Group Excavation Behavior
3.8. Role of Mandibular Gland Secretion in Pre-Excavation Aggregation
3.9. Trail Marking When the Cladode Cannot be Excavated Near the Eclosion Site
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dodd, A.P. The Biological Campaign against Prickly Pear; Commonwealth Prickly Pear Board: Brisbane, Australia, 1940. [Google Scholar]
- Pettey, F.W. The biological control of prickly pears in South Africa Science Bulletin. Dept. Agric. Union S. Afr. 1947, 271, 1–163. [Google Scholar]
- Zimmermann, H.G.; Moran, V.C.; Hoffmann, J.H. The renowned cactus moth, Cactoblastis cactorum: Its natural history and threat to native Opuntia floras in Mexico and the United States of America. Divers. Distrib. 2000, 6, 259–269. [Google Scholar] [CrossRef]
- Legaspi, J.C.; Baez, I.; Legaspi, B.C., Jr. Phenology and egg production of the cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae): Comparison of field census data and life stage development in the field. J. Entomol. Sci. 2009, 44, 341–352. [Google Scholar]
- Robertson, H.G. The Ecology of Cactoblastis cactorum (Berg) (Lepidoptera: Phycitidae) in Relation to Its Effectiveness as a Biological Control Agent of Prickly Pear and Jointed Cactus in South Africa. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 1985. [Google Scholar]
- Hoffmann, J.H.; Zimmermann, H.G. Ovipositional and Feeding Habits in Cactophagous Pyralids: Prediction for Biological Control of Cactus Weeds in South Africa. In Proceedings of the VII International Symposium on Biological Control of Weeds, Rome, Italy, 6–11 March 1988; Delfosse, E.S., Ed.; Ministero deH’Agricoltura e delle Foreste: Rome, Italy; CSIRO: Melbourne, Australia, 1989; pp. 395–399. [Google Scholar]
- Varone, L.; Manteca Acosta, M.; Logarzo, G.A.; Briano, J.A.; Hight, S.D.; Carpenter, J.E. Laboratory performance of Cactoblastis cactorum (Lepidoptera: Pyralidae) on South and North American Opuntia species occurring in Argentina. Fla. Entomol. 2012, 95, 1163–1173. [Google Scholar] [CrossRef]
- Fitzgerald, T.D.; Wolfin, M.; Rossi, F.; Carpenter, J.E.; Pescador-Rubio, A. Trail marking by larvae of the cactus moth, Cactoblastis cactorum. J. Insect Sci. 2014, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.D.; Kelly, M.; Potter, T.; Carpenter, J.E.; Rossi, F. Trail following response of larval Cactoblastis cactorum to 2-Acyl-1,3 cyclohexanediones. J. Chem. Ecol. 2015, 41, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. The Insect Societies; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Peterson, A. Entomological Techniques: How to Work with Insects; Edwards Brothers Inc.: Ann Arbor, MI, USA, 1934. [Google Scholar]
- Mclean, S.C.; Bloem, K.A.; Bloem, S.; Hight, S.D.; Carpenter, J.E. Effect of temperatures and length of exposure time on percent egg hatch of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2006, 89, 340–347. [Google Scholar] [CrossRef]
- Robertson, H.G.; Hoffmann, J.H. Mortality and life-tables of Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae) compared on two host-plant species. Bull. Entomol. Res. 1989, 79, 7–18. [Google Scholar] [CrossRef]
- Varone, L.; Logarzo, G.; Martinez, J.J.; Navarro, F.; Carpenter, J.E.; Hight, S.D. Field host range of Apanteles opuntiarum (Hymenoptera: Braconidae) in Argentina, a potential biocontrol agent of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2015, 98, 803–806. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.; Bisignano, G.; Faulds, C.; Waldron, K. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef] [PubMed]







| Colony | Number of Caterpillars Eclosed when Excavation Initiated | Time Elapsed between First Eclosion and Initiation of Excavation (HR) | Time between Initiation of Excavation Until One Body Length Penetration (HR) |
|---|---|---|---|
| 1 | 13 | 9.4 | 1.4 |
| 2 | 38 | 15.4 | 1.4 |
| 3 | 41 | 11.0 | 1.0 |
| 4 | 18 | 15.7 | 1.0 |
| 5 | 26 | 23.9 | 0.7 |
| 6 | 16 | 3.9 | 1.6 |
| 7 | 23 | 23.6 | 0.8 |
| Mean ± SE | 25.0 ± 4.1 | 14.7 ± 2.8 | 1.1 ± 0.13 |
| Replicate | Instar | Number of Caterpillars | Duration of Test (min) | Number of Choices for Treatment | ||
|---|---|---|---|---|---|---|
| Blank | Water | Cactus | ||||
| 1 | Fed 1st | 31 | 28 | 0 | 0 | 31 |
| 2 | Fed 1st | 29 | 88 | 0 | 1 | 28 |
| 3 | Fed 1st | 13 | 37 | 0 | 1 | 12 |
| 4 | 2nd–3rd | 15 | 52 | 3 | 0 | 10 |
| 5 | 2nd | 20 | 79 | 0 | 2 | 15 |
| 6 | Unfed 1st | 19 | 52 | 0 | 0 | 17 |
| 7 | Unfed 1st | 17 | 33 | 0 | 0 | 14 |
| 8 | Unfed 1st | 11 | 33 | 1 | 2 | 6 |
| 9 | Fed 1st | 20 | 24 | 3 | 0 | 18 |
| Mean ± SE | 0.79 ± 0.44 | 0.67 ± 0.29 | 16.78 ± 2.70 | |||
| Replicate | Mean Percent of Time | Episodes | ||
|---|---|---|---|---|
| Excavating | Other | Excavating | Other | |
| 1 | 36.1 | 63.9 | 12 | 23 |
| 2 | 74.3 | 25.7 | 8 | 7 |
| 3 | 65.0 | 35.0 | 18 | 21 |
| 4 | 80.0 | 20.0 | 11 | 10 |
| 5 | 40.8 | 59.2 | 38 | 40 |
| 6 | 21.1 | 78.9 | 66 | 112 |
| 7 | 45.8 | 54.2 | 33 | 43 |
| Mean ± SE | 51.9 ± 8.2 | 48.1 ± 8.2 | 26.6 ± 7.9 | 36.6 ± 13.6 |
| Group | Mean Percent of Time * | ||
|---|---|---|---|
| Excavating | Attempting to Excavate | Other | |
| 1 | 31.8 | 19.4 | 48.9 |
| 2 | 29.0 | 17.7 | 53.3 |
| 3 | 20.4 | 5.2 | 74.3 |
| 4 | 35.9 | 4.1 | 60.0 |
| 5 | 37.0 | 9.3 | 53.7 |
| 6 | 38.9 | 8.6 | 52.5 |
| 7 | 25.3 | 7.8 | 67.0 |
| 8 | 30 | 9.5 | 60.3 |
| Mean ± SE | 31.0 ± 2.2 | 10.2 ± 1.9 | 58.7 ± 3.0 |
| Caterpillar | Mean ± SE Interval between Successive Bouts of Defecation |
|---|---|
| 1 | 8.5 ± 0.49 |
| 2 | 10.4 ± 0.42 |
| 3 | 11.7 ± 0.48 |
| 4 | 10.2 ± 0.48 |
| 5 | 8.8 ± 0.36 |
| Mean ± SE * | 9.48 ± 0.23 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzgerald, T.D.; Wolfin, M.; Young, R.; Meyer, K.; Fabozzi, E. Collectively Facilitated Behavior of the Neonate Caterpillars of Cactoblastis cactorum (Lepidoptera: Pyralidae). Insects 2016, 7, 59. https://doi.org/10.3390/insects7040059
Fitzgerald TD, Wolfin M, Young R, Meyer K, Fabozzi E. Collectively Facilitated Behavior of the Neonate Caterpillars of Cactoblastis cactorum (Lepidoptera: Pyralidae). Insects. 2016; 7(4):59. https://doi.org/10.3390/insects7040059
Chicago/Turabian StyleFitzgerald, Terrence D., Michael Wolfin, Ryan Young, Katelyn Meyer, and Elizabeth Fabozzi. 2016. "Collectively Facilitated Behavior of the Neonate Caterpillars of Cactoblastis cactorum (Lepidoptera: Pyralidae)" Insects 7, no. 4: 59. https://doi.org/10.3390/insects7040059





