Toxicity and Feeding Deterrent Effect of 2-Methylanthraquinone from the Wood Extractives of Tectona grandis on the Subterranean Termites Coptotermes formosanus and Reticulitermes speratus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Termites and T. grandis Heartwood Sampling
2.2. Extraction and Fractionation
2.3. Primary No-Choice Feeding Tests against R. speratus
2.4. No-Choice Feeding Tests against R. speratus and C. formosanus Using Fractions of Teak Extractives
2.5. Statistical Analysis
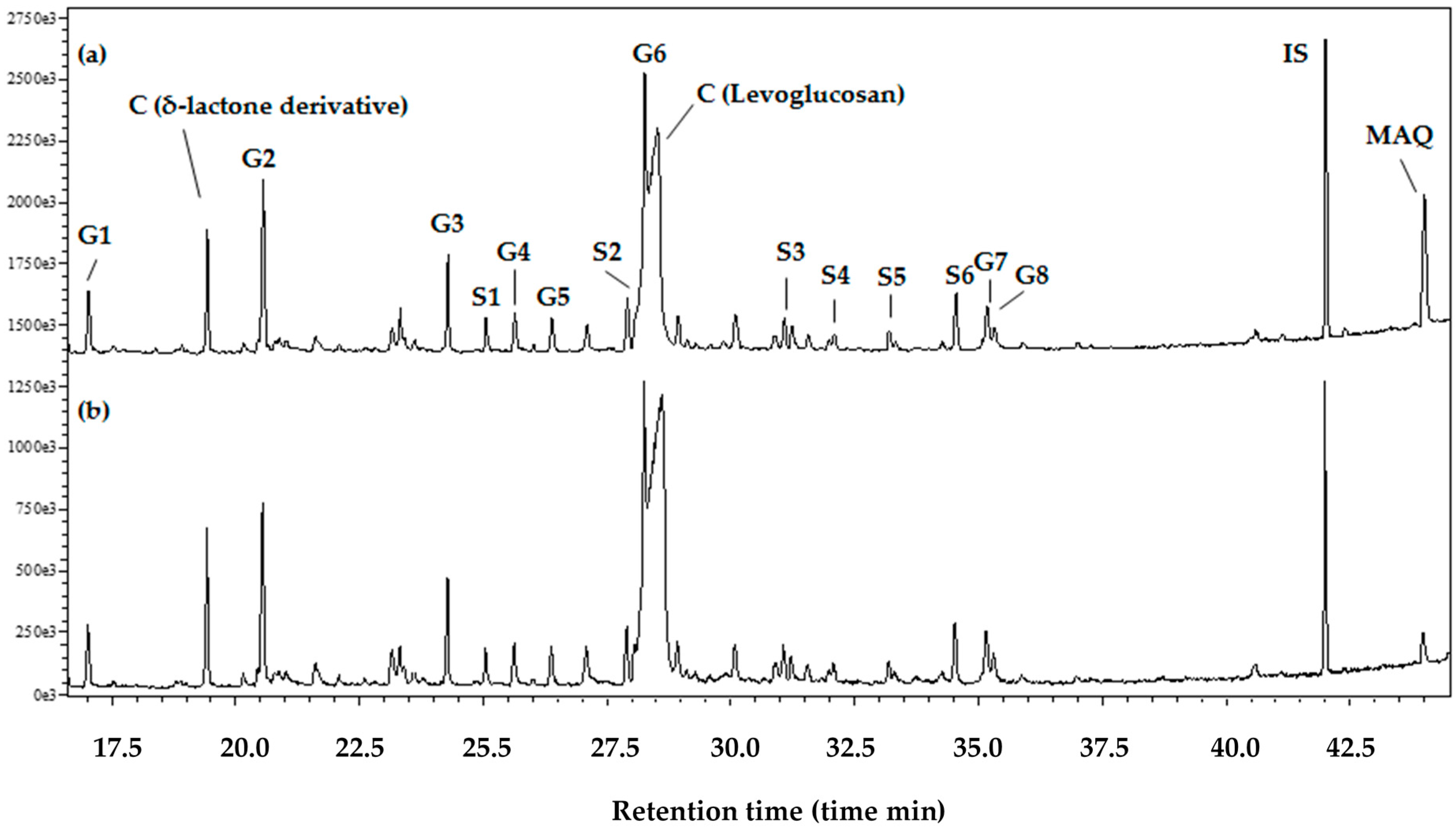
2.6. Gas Chromatography (GC) and Pyrolysis-Gas Chromatography–Mass Spectroscopy (Py-GC/MS)
3. Results
3.1. Extraction, Fractionation, and Determination of MAQ Content
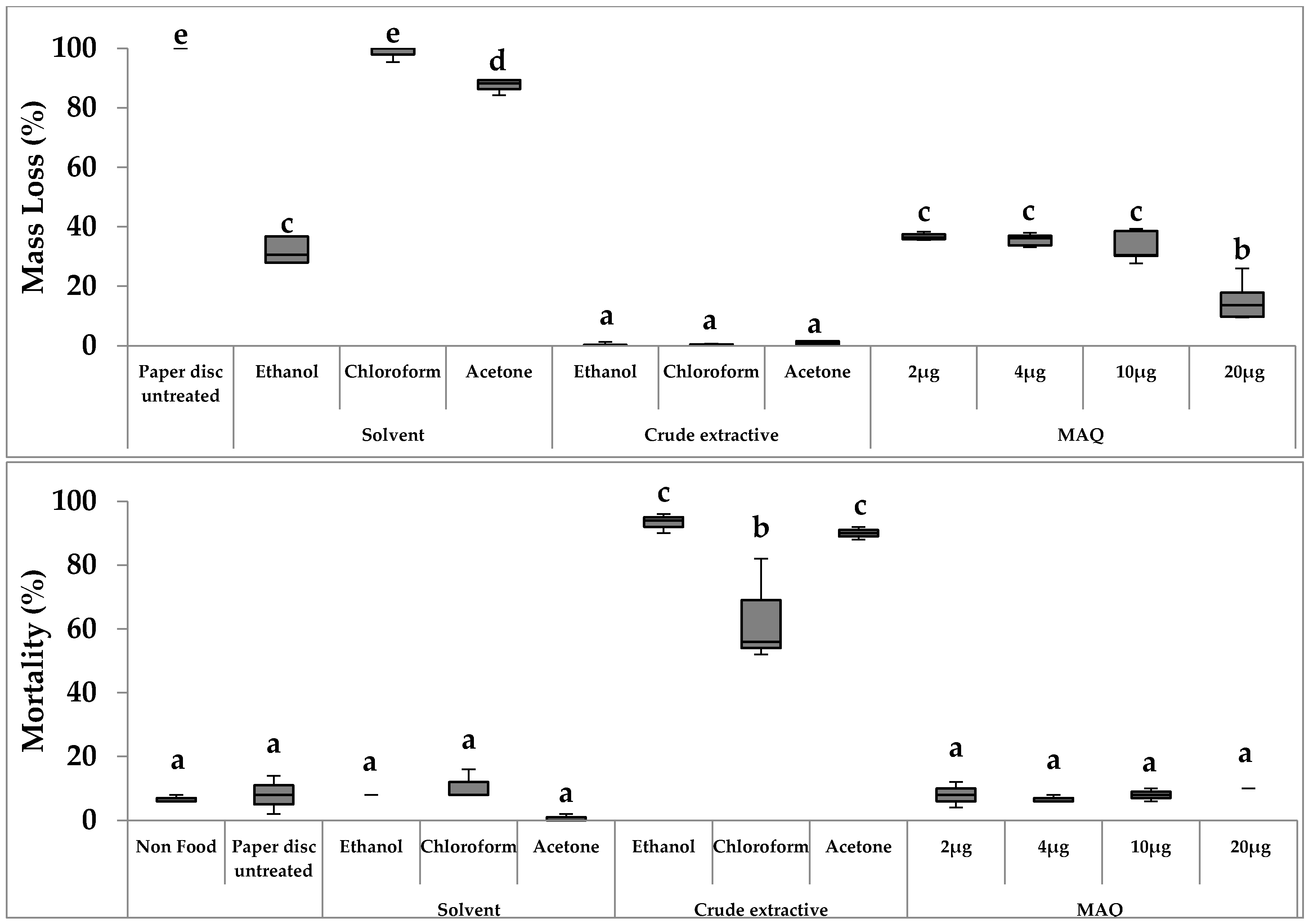
3.2. Primary No-Choice Feeding Test of Crude Extractives in R. speratus
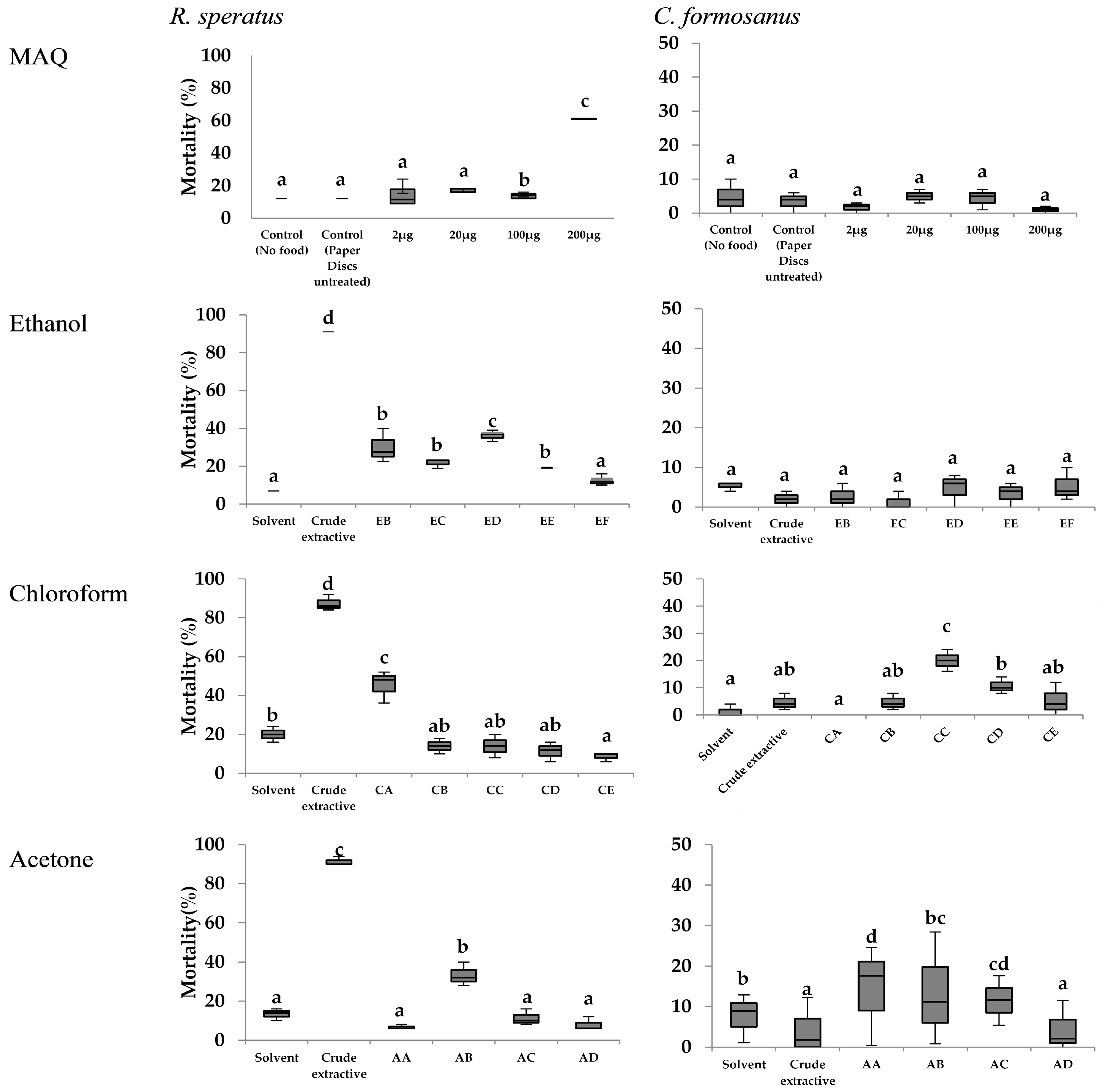
3.3. No-Choice Feeding Tests of Crude Extractives in R. speratus and C. formosanus
3.3.1. Mass Loss
3.3.2. Termite Mortality
4. Discussion
4.1. Determination of MAQ in Teak Wood and PLC Fractions
4.2. Primary No-Choice Feeding Tests of Crude Extractives against R. speratus
4.3. Toxicity and the Feeding Deterrent Effect of Wood Extractives (Including MAQ) against R. speratus and C. formosanus
4.3.1. Effect of Crude Extractives
4.3.2. Effect of the Fractions Derived from Crude Extractives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Da Costa, E.W.B.; Rudman, P.; Gay, F.J. Investigations on the durability of Tectona grandis. Emp. For. Rev. 1958, 37, 291–298. [Google Scholar]
- Rudman, P.; Da Costa, E.W.B.; Gay, F.J. Wood quality in plus trees of teak (Tectona grandis L.f.): An assessment of decay and termite resistance. Sylvae Genet. 1967, 16, 102–105. [Google Scholar]
- Lukmandaru, G.; Ogiyama, K. Bioactive compounds from ethyl acetate extract of teakwood (Tectona grandis L.f.). In Proceedings of the 6th International Wood Sciences Symposium LIPI-JSPS Core, Bali, Indonesia, 9–31 August 2005; pp. 346–350.
- Thulasidas, P.K.; Bhat, K.M. Chemical extractive compounds determining the brown-rot decay resistance of teak wood. Holz als Roh- und Werkstoff 2007, 65, 121–124. [Google Scholar] [CrossRef]
- Lukmandaru, G.; Takahashi, K. Radial distribution of quinones in plantation teak (Tectona grandis L.f.). Ann. For. Sci. 2009. [Google Scholar] [CrossRef]
- Haupt, M.; Leithoff, H.; Meier, D.; Puls, J.; Richter, H.G.; Faix, O. Heartwood extractives and natural durability of plantation-grown teakwood (Tectona grandis L.f.)—A case study. Holz als Roh- und Werkstoff 2003, 61, 473–474. [Google Scholar] [CrossRef]
- Wolcott, G.N. The permanence of termite repellent. J. Econ. Entom. 1947, 40, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Windeisen, E.; Klassen, A.; Wegener, G. On the chemical characterization of plantation teakwood from Panama. Holz als Roh- und Werkstoff 2003, 61, 416–418. [Google Scholar] [CrossRef]
- Ohmura, W.; Doi, S.; Aoyama, M.; Ohara, S. Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. J. Wood Sci. 2000, 46, 149–153. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Fortmann, I. Thermal degradation products of wood. Gas chromatography separation and mass spectrometric characterization of monomeric lignin derived products. Holz als Roh- und Werkstoff 1990, 48, 281–285. [Google Scholar]
- Lukmandaru, G.; Takahashi, K. Variation in the natural termite resistance of teak (Tectona grandis Linn fil) wood as a function of tree age. Ann. For. Sci. 2008. [Google Scholar] [CrossRef]
- Von Sandermann, W.; Simatupang, M.H. Zur chemie und biochemie des teakholzes (Tectona grandis L. fil). Holz als Roh- und Werkstoff 1966, 24, 190–204. (In German) [Google Scholar] [CrossRef]
- Niamke, F.B.; Amusant, N.; Charpentier, J.P.; Chaix, G.; Baissac, Y.; Boutahar, N.; Amissa-Adima, A.; KatiKoulibaly, S.; Jay-Allemand, C. Relationships between biochemical attributes (non-structural carbohydrates and phenolics) and natural durability against fungi in dry teak wood (Tectona grandis L.f.). Ann. For. Sci. 2011, 68, 201–211. [Google Scholar] [CrossRef]
- Lourenco, A.; Neiva, D.M.; Gominho, J.; Marques, A.V.; Pereira, H. Characterization of lignin in heartwood, sapwood and bark from Tectona grandis using Py-GC-MS/FID. Wood Sci. Technol. 2015, 49, 159–175. [Google Scholar] [CrossRef]
- Ohi, H. Rapid analysis of 2-methylanthraquinone in tropical hardwoods and its effect on polysulfide-AQ pulping. In Proceedings of the 11th International Symposium of Wood and Pulping Chemistry Vol II, Nice, France, 11–14 June 2001; pp. 63–66.
- Tsunoda, K. Improved management of termites to protect Japanese homes. In Proceedings of the Fifth International Conference on Urban Pests, Singapore, 11–13 July 2005; Lee, C.Y., Robinson, W.H., Eds.; pp. 33–37.
- Becker, V.G. On the examination and estimation of the natural resistance of wood to termites. Holz als Roh- und Werkstoff 1961, 19, 278–290. [Google Scholar] [CrossRef]
- Ngee, P.S.; Toshiro, A.; Yoshimura, T.; Jaal, Z.; Lee, C.Y. Wood preference of selected Malaysian subterranean termites (Isoptera: Rhinotermitidae, Termitidae). Sociobiology 2004, 43, 535–550. [Google Scholar]


| Extraction Solvent | Crude Extractives Recovered (%) 1 | MAQ Content in Crude Extractives (%) 1 | MAQ Content in Extracted Residue (%) 2 | Dosage in No-Choice Feeding 3 | |
|---|---|---|---|---|---|
| Crude Extractives (μg) | MAQ (μg) | ||||
| Ethanol | 6.5 ± 0.6 | 0.11 ± 0.01 | 0.05 ± 0.01 | 602 | 10 |
| Chloroform | 8.7 ± 0.2 | 0.10 ± 0.01 | 0.05 ± 0.02 | 606 | 6.8 |
| Acetone | 6.6 ± 0.2 | 0.15 ± 0.01 | 0.04 ± 0.01 | 666 | 15 |
| Fraction 1 | Rf | Extractives Weight (μg) | MAQ Weight (μg) | Dosage in No-Choice Feeding Test 2 | |
|---|---|---|---|---|---|
| Extractives (μg) | MAQ (μg) | ||||
| Ethanol crude extractives | 1296 | 22 | |||
| EB | 0.52 ± 0.16 | 5590 | 468 | 656 | 55 |
| EC | 0.27 ± 0.01 | 2470 | 64.4 | 600 | 16 |
| ED | 0.21 ± 0.05 | 2890 | 2.55 | 573 | 0.5 |
| EE | 0.07 ± 0.04 | 10,300 | 2.37 | 687 | 0.2 |
| EF | 0.00 ± 0.01 | 30,400 | 2.37 | 779 | 0.1 |
| Chloroform crude extractives | 1960 | 22 | |||
| CA | 0.55 ± 0.02 | 21,900 | 25.3 | 683 | 0.8 |
| CB | 0.41 ± 0.04 | 14,000 | 0.81 | 698 | 0 |
| CC | 0.34 ± 0.03 | 14,600 | 0.12 | 795 | 0 |
| CD | 0.06 ± 0.03 | 20,700 | 0.35 | 848 | 0 |
| CE | 0.00 ± 0.01 | 15,500 | 0.46 | 764 | 0 |
| Acetone crude extractives | 946 | 22 | |||
| AA | 0.69 ± 0.06 | 2540 | 3.06 | 585 | 0.7 |
| AB | 0.56 ± 0.04 | 3730 | 887 | 681 | 162 |
| AC | 0.24 ± 0.16 | 7450 | 68.6 | 828 | 7.6 |
| AD | 0.00 ± 0.01 | 10,120 | 26.5 | 707 | 1.9 |
| Fraction | Dosage | R. speratus | C. formosanus | |||
|---|---|---|---|---|---|---|
| Extractives (μg) | MAQ (μg) | Toxicity | Feeding Deterrent | Toxicity | Feeding Deterrent | |
| MAQ | 0 | 200 | ++ | ++ | - | ++ |
| MAQ | 0 | 100 | - | ++ | - | ++ |
| MAQ | 0 | 20 | - | + | - | - |
| Ethanol crude extractives | 1296 | 22 | ++ | ++ | - | ++ |
| EB | 656 | 55 | + | ++ | - | ++ |
| EC | 600 | 16 | + | ++ | - | ++ |
| ED | 573 | 0.5 | ++ | ++ | - | ++ |
| EE | 687 | 0.2 | - | ++ | - | ++ |
| EF | 779 | 0.1 | - | + | - | + |
| Chloroform crude extractives | 1960 | 22 | ++ | ++ | - | ++ |
| CA | 683 | 0.8 | ++ | ++ | - | + |
| CB | 698 | 0 | - | - | - | + |
| CC | 795 | 0 | - | + | - | - |
| CD | 848 | 0 | - | + | - | - |
| CE | 764 | 0 | - | - | - | - |
| Acetone crude extractives | 946 | 22 | ++ | ++ | - | + |
| AA | 585 | 0.7 | - | - | - | + |
| AB | 681 | 162 | + | ++ | - | ++ |
| AC | 828 | 7.6 | - | ++ | - | + |
| AD | 707 | 1.9 | - | - | - | ++ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismayati, M.; Nakagawa-Izumi, A.; Kamaluddin, N.N.; Ohi, H. Toxicity and Feeding Deterrent Effect of 2-Methylanthraquinone from the Wood Extractives of Tectona grandis on the Subterranean Termites Coptotermes formosanus and Reticulitermes speratus. Insects 2016, 7, 63. https://doi.org/10.3390/insects7040063
Ismayati M, Nakagawa-Izumi A, Kamaluddin NN, Ohi H. Toxicity and Feeding Deterrent Effect of 2-Methylanthraquinone from the Wood Extractives of Tectona grandis on the Subterranean Termites Coptotermes formosanus and Reticulitermes speratus. Insects. 2016; 7(4):63. https://doi.org/10.3390/insects7040063
Chicago/Turabian StyleIsmayati, Maya, Akiko Nakagawa-Izumi, Nadia Nuraniya Kamaluddin, and Hiroshi Ohi. 2016. "Toxicity and Feeding Deterrent Effect of 2-Methylanthraquinone from the Wood Extractives of Tectona grandis on the Subterranean Termites Coptotermes formosanus and Reticulitermes speratus" Insects 7, no. 4: 63. https://doi.org/10.3390/insects7040063






