Assessment of Trichogramma japonicum and T. chilonis as Potential Biological Control Agents of Yellow Stem Borer in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Performance of the Trichogramma Species on Substitute Host
2.3. Cage Tests for Parasitism
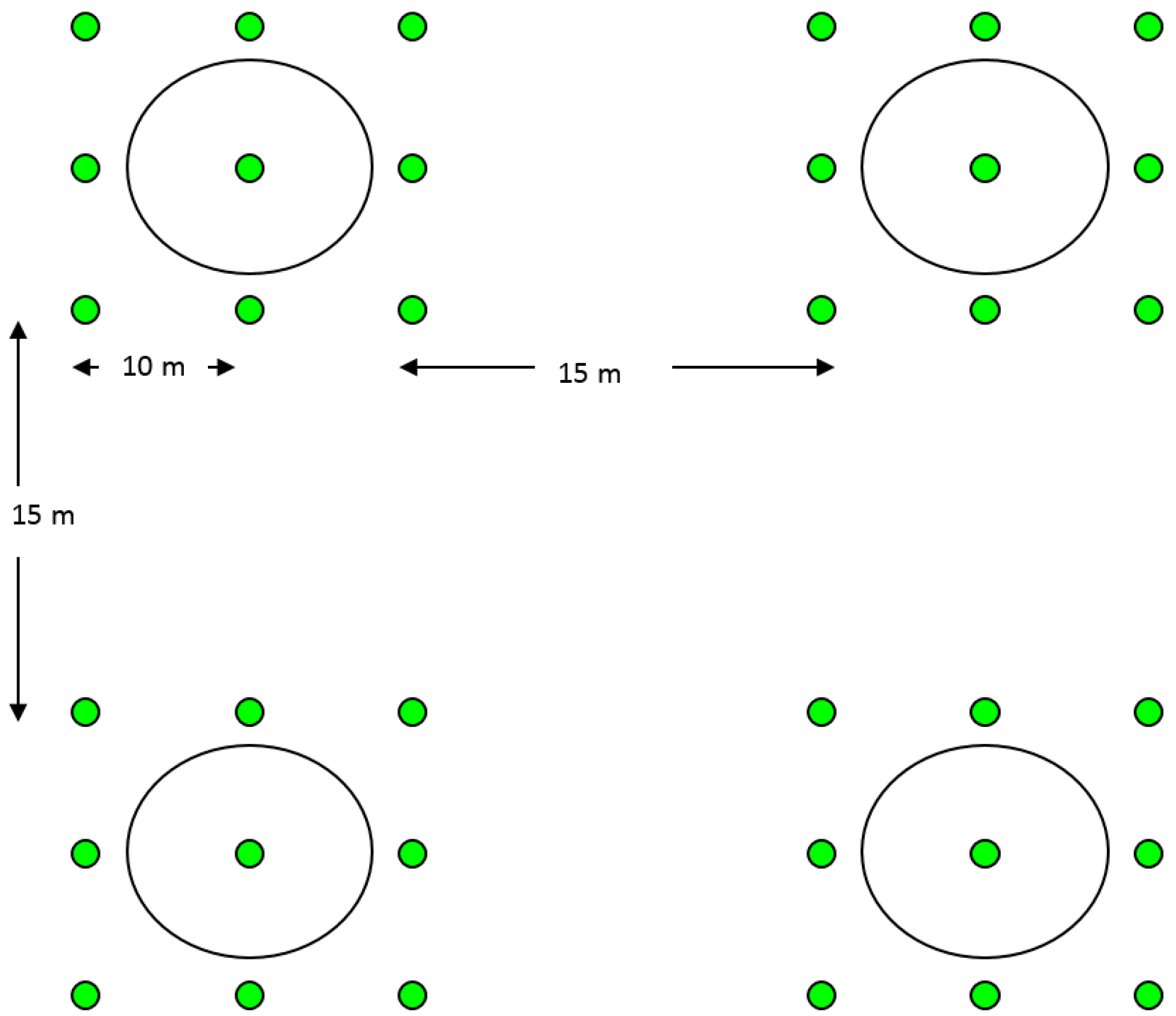
2.4. Field Tests for Parasitism
2.5. Morphological Observations
2.6. Data Analysis
3. Results
3.1. Performance of the Trichogramma Species on Substitute Host
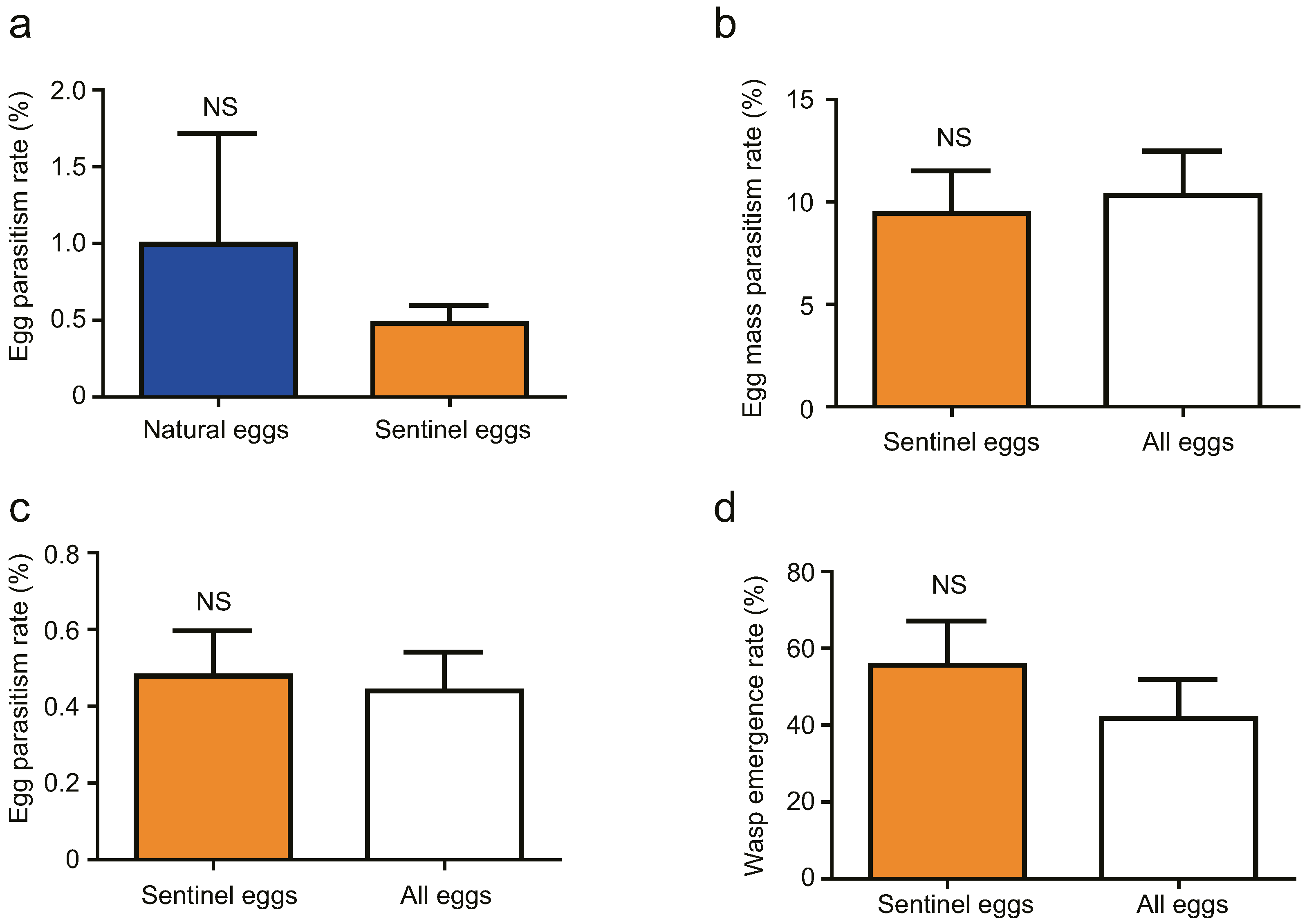
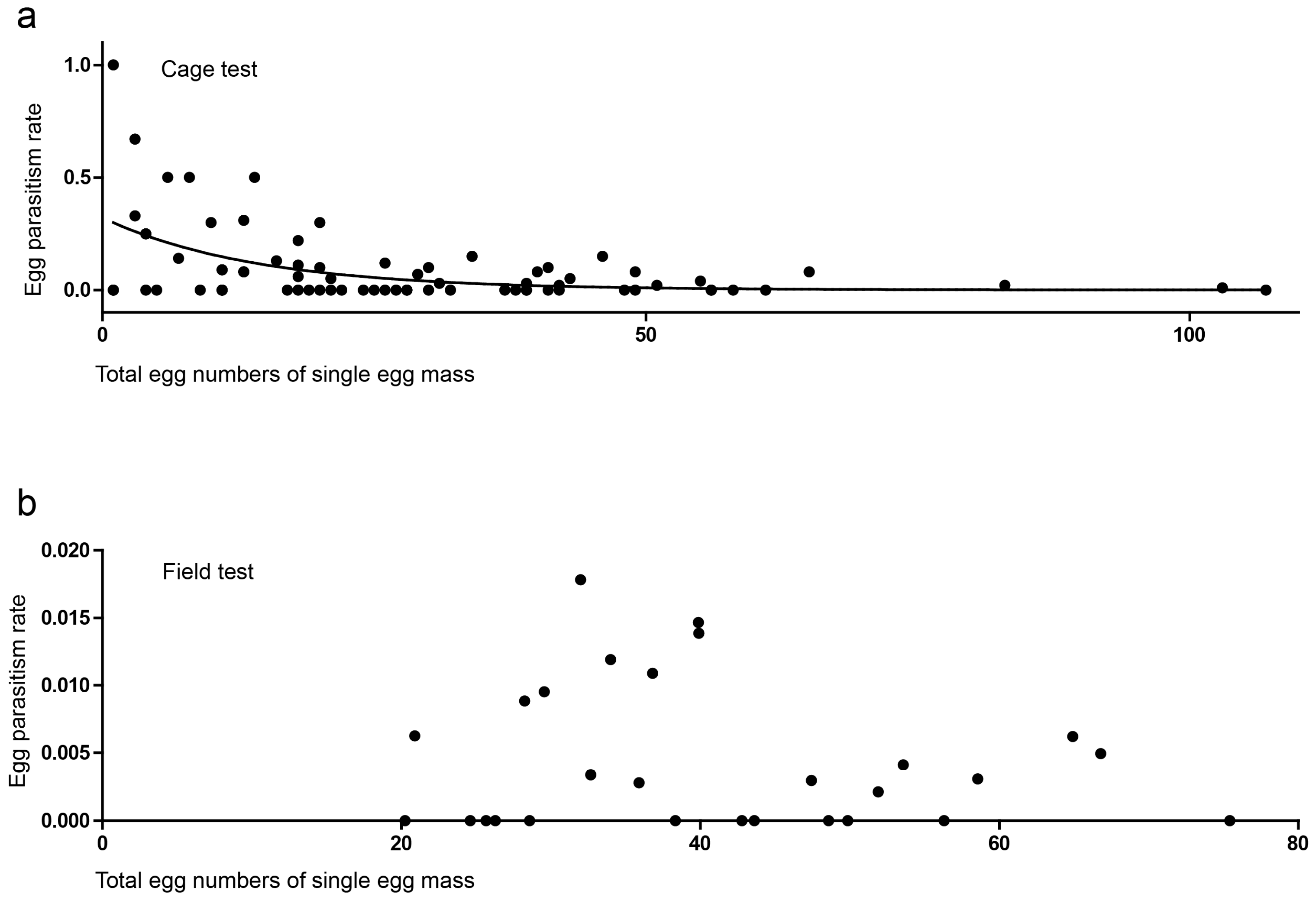
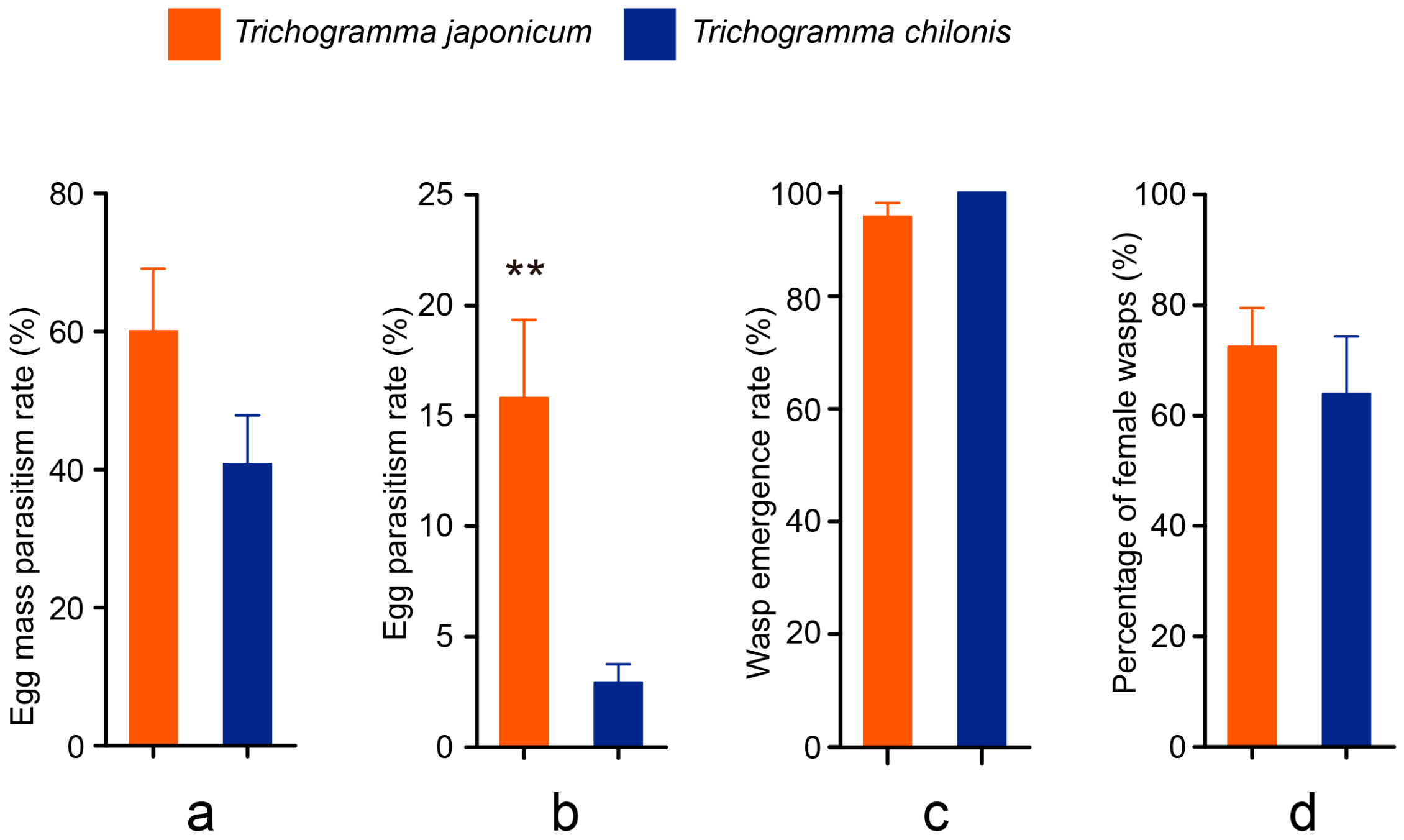
3.2. Cage Tests
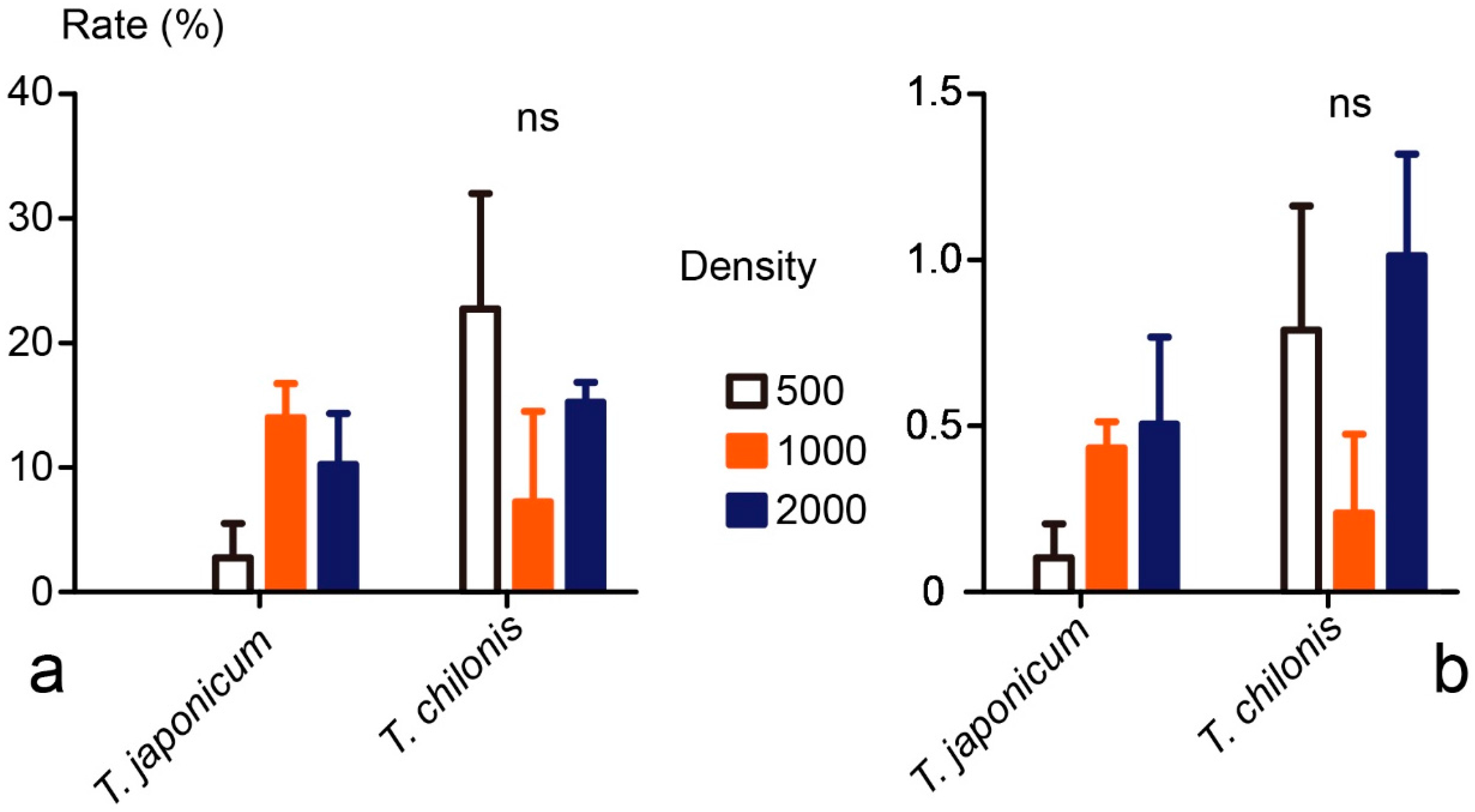
3.3. Field Tests
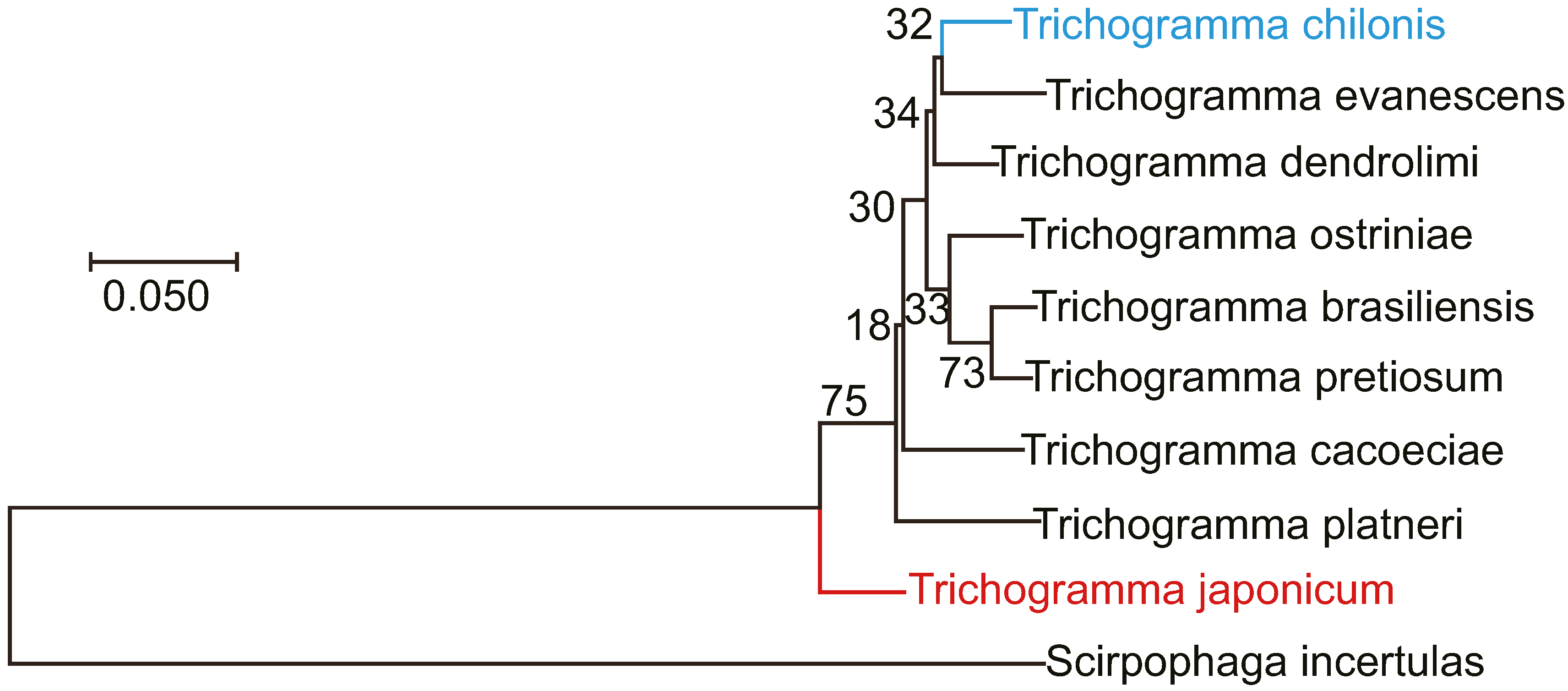
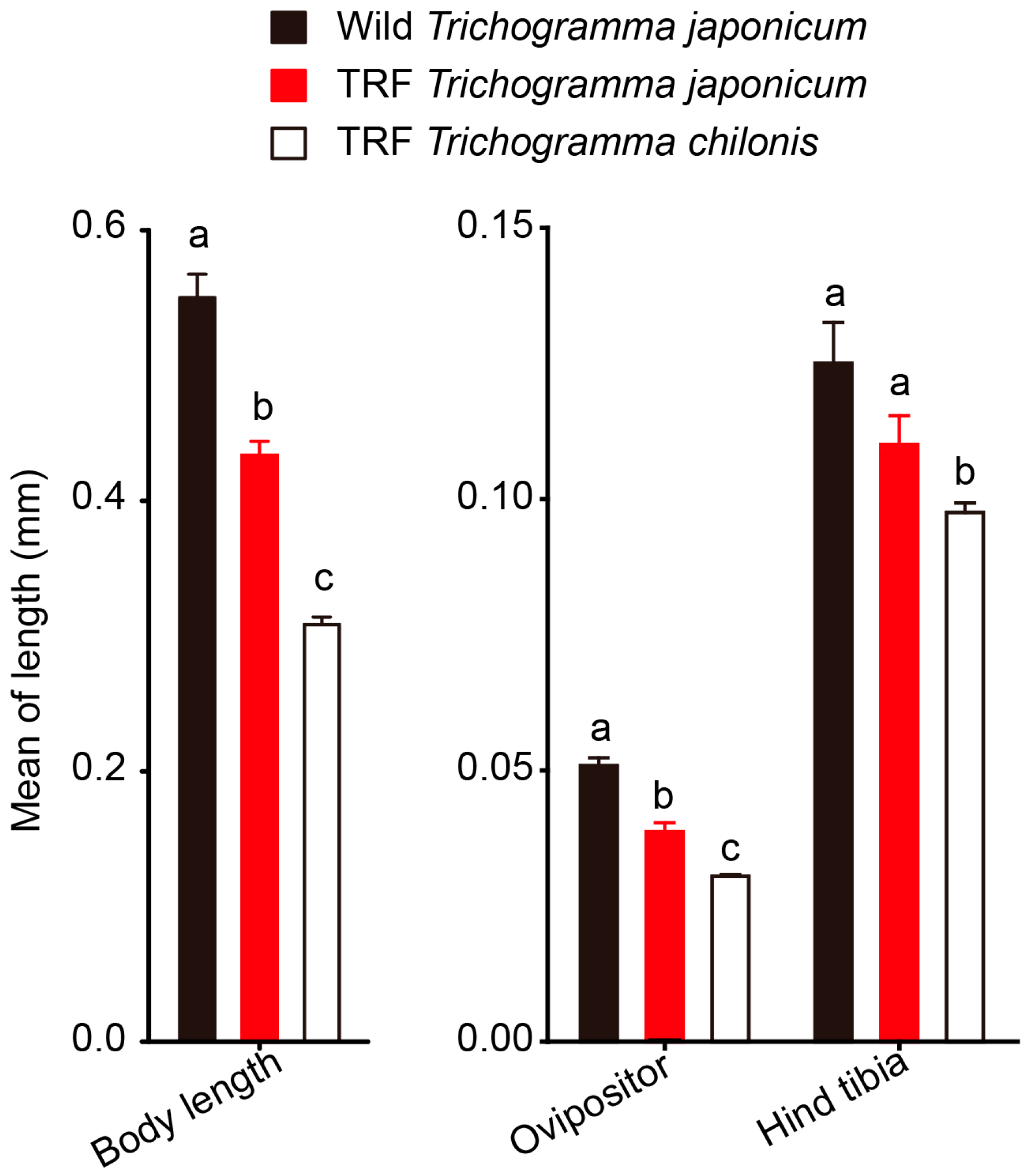
3.4. Morphological Comparison
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Species | Area (cm2) | Number of Cards Produced Per mL eggs | Total Egg Number per Card | Parasitized Egg Number Per Card | Emerged Wasp Number Per Card | Parasitism Rate (%) | Emergence Rate (%) | Female Rate (%) |
|---|---|---|---|---|---|---|---|---|
| T. japonicum | 1.8 | 23 | 1087 | 623.9 | 547 | 57.4 + 1.1 | 87.8 + 1.2 | 75.7 + 1.0 |
| T. chilonis | 1.8 | 23 | 1087 | 545.8 | 506.2 | 50.2 + 0.7 | 92.8 + 0.9 | 74.2 + 0.8 |
References
- Zeigler, R.; Barclay, A. The Relevance of Rice. Rice 2008, 1, 3–10. [Google Scholar] [CrossRef]
- Savary, S.; Mila, A.; Willocquet, L.; Esker, P.D.; Carisse, O.; McRoberts, N. Risk factors for crop health under global change and agricultural shifts: A framework of analyses using rice in tropical and subtropical Asia as a model. Phytopathology 2011, 101, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Boles, S.; Liu, J.; Zhuang, D.; Frolking, S.; Li, C.; Salas, W.; Boore, B. Mapping paddy rice agriculture in southern China using multi-temporal MODIS images. Remote Sens. Environ. 2005, 95, 480–492. [Google Scholar] [CrossRef]
- O’Shannassy, T.O. Greater Mekong Subregion (GMS): Context. Southeast Asian J. Trop Med. Public Health 2013, 44 (Suppl. 1), S1–S45. [Google Scholar]
- Johnston, R.; Hoanh, C.T.; Lacombe, G.; Noble, A.; Smakhtin, V.; Suhardiman, D.; Pheng, K.S.; Sze, C.P. Rethinking agriculture in the Greater Mekong Subregion: How to sustainably meet food needs, enhance ecosystem services and cope with climate change. Iwmi Res. Rep. 2010. [Google Scholar] [CrossRef]
- Ko, K.; Liu, Y.; Hou, M.; Babendreier, D.; Zhang, F.; Song, K. Evaluation for potential Trichogramma (Hymenoptera: Trichogrammatidae) strains for control of the striped stem borer (Lepidoptera: Crambidae) in the Greater Mekong Subregion. J. Econ. Entomol. 2014, 107, 955–963. [Google Scholar] [CrossRef]
- Kaur, R.; Brar, K.S.; Singh, J.; Shenhmar, M. Large-scale evaluation of bio-intensive management for leaf folder and stem borer on basmati rice. J. Biol. Control 2007, 21, 255–259. [Google Scholar]
- Otuka, A. Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Front. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Milazzo, J.; Adreit, H.; Fournier, E.; Tharreau, D. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol. 2014, 201, 1440–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Zhang, Z.; Wu, M.; Gao, C. Geographic susceptibility of Chilo suppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China. Pest Manag. Sci. 2014, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.G.; Zhang, G.R.; Zhang, W.Q.; Hu, Y.; Zhang, J. Reprint of: Biological control of rice insect pests in China. Biol. Control 2014, 68, 103–116. [Google Scholar] [CrossRef]
- Cheng, X.; Chang, C.; Dai, S.M. Responses of striped stem borer, Chilo suppressalis (Lepidoptera: Pyralidae), from Taiwan to a range of insecticides. Pest Manag. Sci. 2010, 66, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Jacob, S.; Purushothaman, S.M. Field evaluation of egg parasitoids, Trichogramma japonicum Ashmead and Trichogramma chilonis Ishii, against rice yellow stem borer and leaf folder. J. Biol Control. 2007, 21, 261–265. [Google Scholar]
- Jin, J.Y.; Li, Z.Q.; Zhang, Y.N.; Liu, N.Y.; Dong, S.L. Different roles suggested by sex-biased expression and pheromone binding affinity among three pheromone binding proteins in the pink rice borer, Sesamia inferens (Walker) (Lepidoptera: Noctuidae). J. Insect Physiol. 2014, 66, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Hua, X.; Turlings, T.C.; Cheng, J.; Chen, X.; Ye, G. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J. Chem. Ecol. 2006, 32, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, A.; Bandani, A.R.; Fazeli-Dinan, M.; Zibaee, I.; Sendi, J.J.; Maleki, F.A. A trypsin-like protease in rice green semi-looper, Naranga aenescens Moore (Lepidoptera: Noctuidae): Purification and characterization. Arch. Insect Biochem. Physiol. 2011, 78, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deka, S.; Barthakur, S. Overview on current status of biotechnological interventions on yellow stem borer Scirpophaga incertulas (Lepidoptera: Crambidae) resistance in rice. Biotechnol. Adv. 2010, 28, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Tian, J.H.; Chen, P. Population dynamics of Tryporyza incertulas (Walker) in Menghai, Yunnan from 2001–2011. J. Yunnan Univ. 2012, 34, 367–372. (In Chinese) [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.R.; Hendawy, A.S.; El-Habashy, M.M. Utilization of Trichogramma evanescens (Ashmead) for controlling rice stem borer, Chilo Agamemnon Bles. in rice fields in Egypt. Egypt J. Biol. Pest Control 2008, 18, 11–16. [Google Scholar]
- Kaur, R.; Brar, K.S. Evaluation of different doses of Trichogramma species for the management of leaf folder and stem borer on Basmati rice. J. Biol. Control 2008, 22, 131–135. [Google Scholar]
- Shi, P.; Zhong, L.; Sandhu, H.S.; Ge, F.; Xu, X.; Chen, W. Population decrease of Scirpophaga incertulas Walker (Lepidoptera Pyralidae) under climate warming. Ecol. Evol. 2012, 2, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hikjm, I.S. Seasonal parasitism by egg parasites of the yellow rice borer, Scirpophaga incertulas [Lepidoptera Pyralidae]. Entomophaga 1988, 33, 115–124. [Google Scholar] [CrossRef]
- Chakraborty, K. Relative composition of egg parasitoid species of yellow stem borer, Scirpophaga incertulas Wlk. in paddy field at Uttar Dinajpur, West Bengal, India. Curr. Biot. 2012, 6, 42–52. [Google Scholar]
- Samara, R.Y.; Monje, J.C.; Zebitz, C.P.W. Comparison of different European strains of Trichogramma aurosum (Hymenoptera: Trichogrammatidae) using fertility life tables. Biocontrol Sci. Technol. 2008, 18, 75–86. [Google Scholar] [CrossRef]
- Guo, H.F.; Fang, J.C.; Xie, Y.F.; Du, Z.W. Egg parasitism of rice stem borers in regions with different rice stem borer occurring patterns. Chin. J. Biol. Control 2002, 18, 13–16. (In Chinese) [Google Scholar]
- Riba, T.; Sarma, A.K. Efficacy of Trichogrmma japonicum Ashmead against yellow stem borer, Scirpophaga incertulas Walk on rice in Nagaland. J. Appl. Zool. Res. 2006, 17, 196–200. [Google Scholar]
- Smith, S.M.; Hubbes, M. Strains of the egg parasitoid Trichogramma minutum Riley. 1. Biochemical and biological characterization. J. Appl. Entomol. Z. Angew. Entomol. 1986, 101, 223–239. [Google Scholar] [CrossRef]
- Babendreier, D.; Kuske, S.; Bigler, F. Parasitism of non-target butterflies by Trichogramma brassicae (Hymenoptera: Trichogrammatidae) under semifield and field conditions. Biol. Control 2003, 26, 139–145. [Google Scholar] [CrossRef]
- He, J.H. A method for investigation parasitism rate for yellow stem borer eggs. Plant Prot. 1966, 1, 38–40. (In Chinese) [Google Scholar]
- ICM Team. Field survey on natural parasitism rate for yellow stem borer eggs in Wuhu City. J. Anhui Norm. Univ. (Nat. Sci.) 1974, 1, 50–54. (In Chinese) [Google Scholar]
- Luo, L.Z.; Shepard, B.M. Predation and parasitism of yellow stem borer Scirpophaga incertulas (Walker) eggs influenced by rice plant density and growth stages. Acta Ecol. Sin. 1994, 37, 298–304. (In Chinese) [Google Scholar]
- Sugiura, S.; Yamazaki, K. Caterpillar hair as a physical barrier against invertebrate predators. Behav. Ecol. 2014, 25, 975–983. [Google Scholar] [CrossRef]
- Castellanos, I.; Barbosa, P.; Zuria, I.; Tammaru, T.; Christman, M.C. Contact with caterpillar hairs triggers predator-specific defensive responses. Behav. Ecol. 2011, 22, 1020–1025. [Google Scholar] [CrossRef]
- Gentry, G.L.; Dyer, L.A. On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 2002, 83, 3108–3119. [Google Scholar] [CrossRef]
- Stireman, J.O.; Singer, M.S. Determinants of parasitoid-host associations: Insights from a natural tachinid-Lepidopteran community. Ecology 2003, 84, 296–310. [Google Scholar] [CrossRef]
- Song, K.; Tang, R.; Babendreier, D.; Zhang, F.; Hou, M.L. Institute of Plant Protection, Beijing, China. Unpublished data. 2017. [Google Scholar]
- Nicol, C.M.Y.; Mackauer, M. The scaling of body size and mass in a host-parasitoid association: Influence of host species and stage. Entomol. Exp. Appl. 1999, 90, 83–92. [Google Scholar] [CrossRef]
- King, B.H. Host-size-dependent sex ratios among parasitoid wasps: Does host growth matter? Oecologia 1989, 78, 420–426. [Google Scholar] [CrossRef]
- Kazmer, D.J.; Luck, R.F. Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology 1995, 76, 412–425. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Babendreier, D.; Zhang, F.; Kang, M.; Song, K.; Hou, M.-L. Assessment of Trichogramma japonicum and T. chilonis as Potential Biological Control Agents of Yellow Stem Borer in Rice. Insects 2017, 8, 19. https://doi.org/10.3390/insects8010019
Tang R, Babendreier D, Zhang F, Kang M, Song K, Hou M-L. Assessment of Trichogramma japonicum and T. chilonis as Potential Biological Control Agents of Yellow Stem Borer in Rice. Insects. 2017; 8(1):19. https://doi.org/10.3390/insects8010019
Chicago/Turabian StyleTang, Rui, Dirk Babendreier, Feng Zhang, Min Kang, Kai Song, and Mao-Lin Hou. 2017. "Assessment of Trichogramma japonicum and T. chilonis as Potential Biological Control Agents of Yellow Stem Borer in Rice" Insects 8, no. 1: 19. https://doi.org/10.3390/insects8010019






