Flight Synchrony among the Major Moth Pests of Cranberries in the Upper Midwest, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trapping Moths
2.2. Degree-Day Calculations
2.3. Modeling of Flight Dynamics
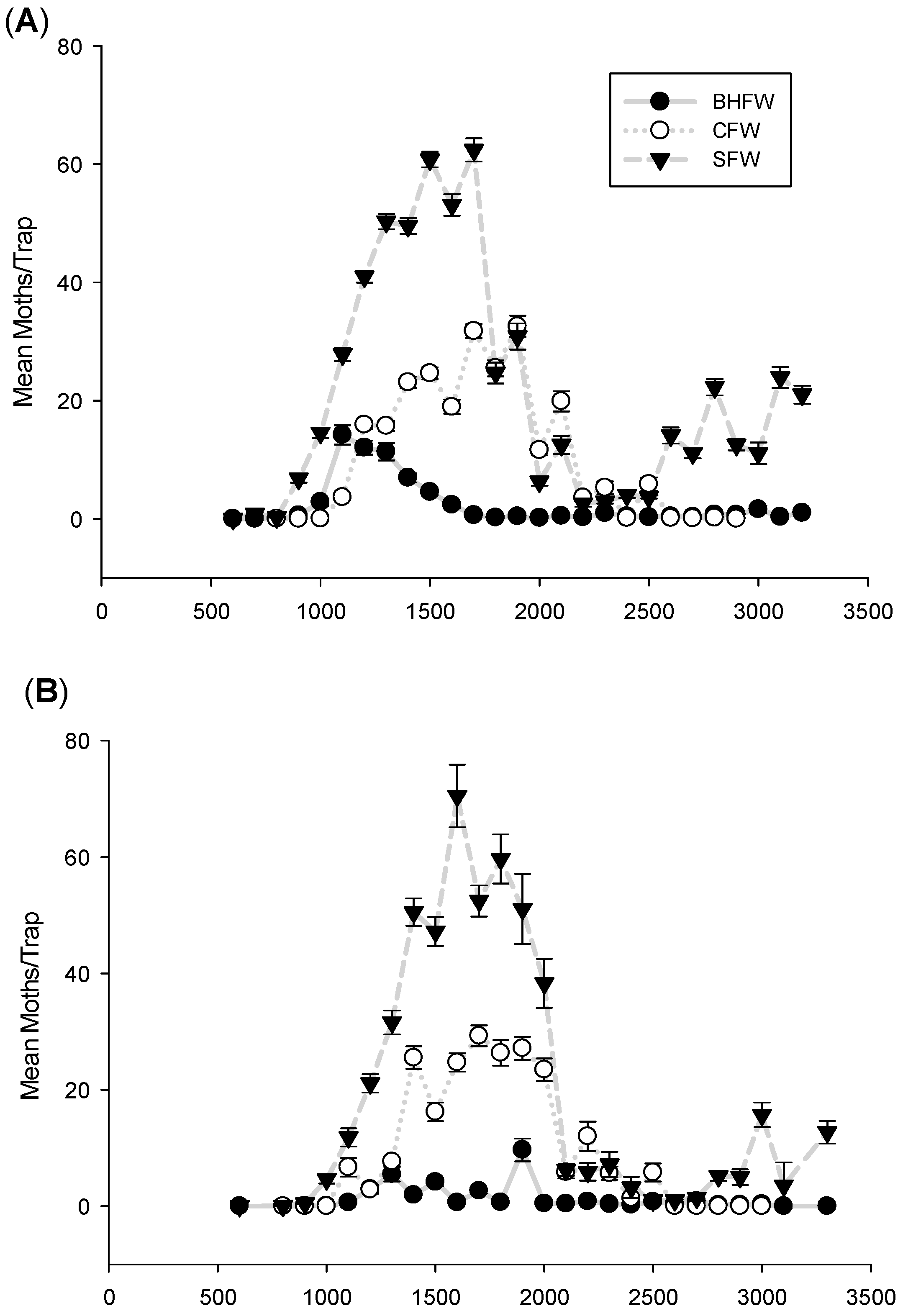
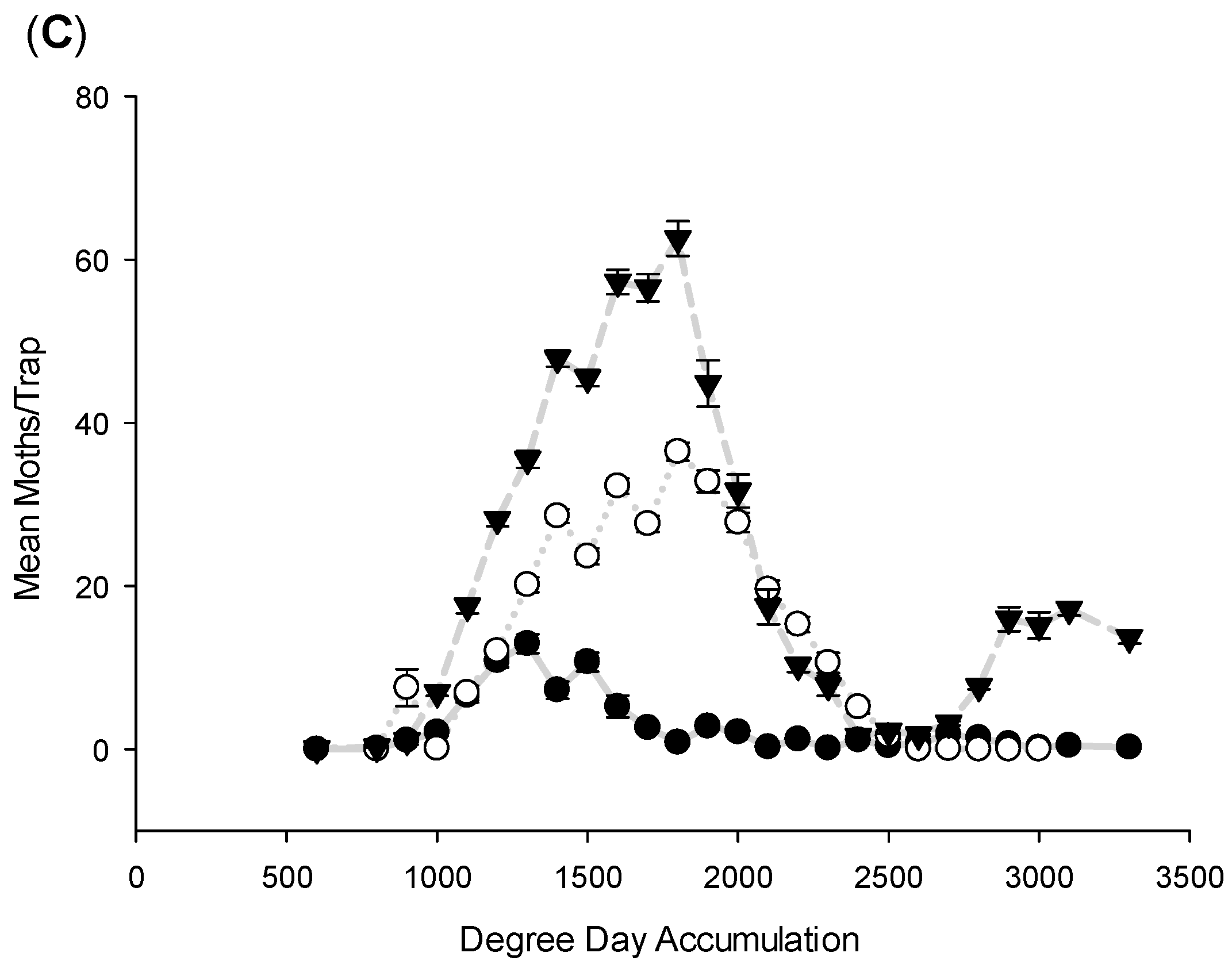
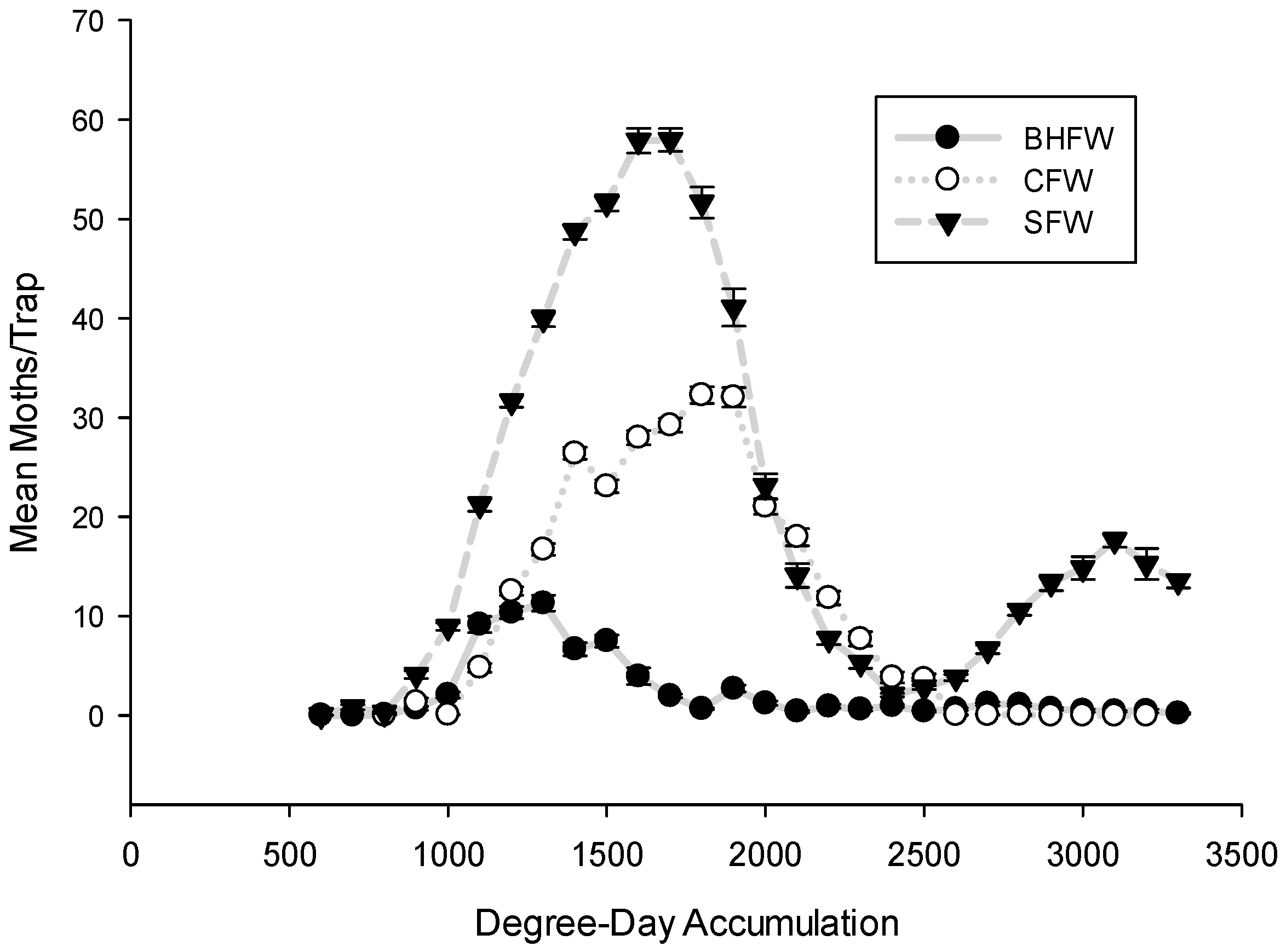
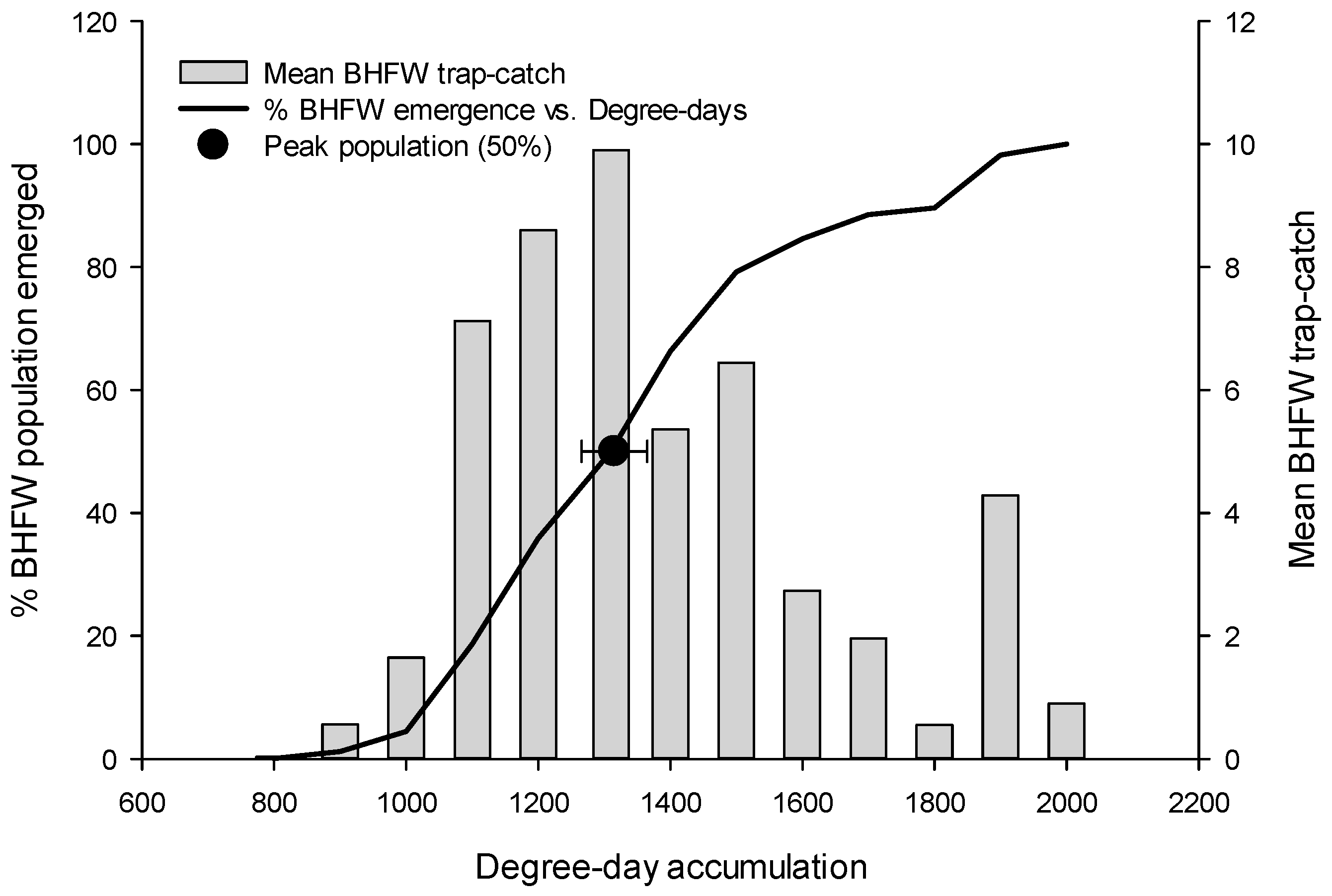
3. Results and Discussion
3.1. Flight Initiation, by Species
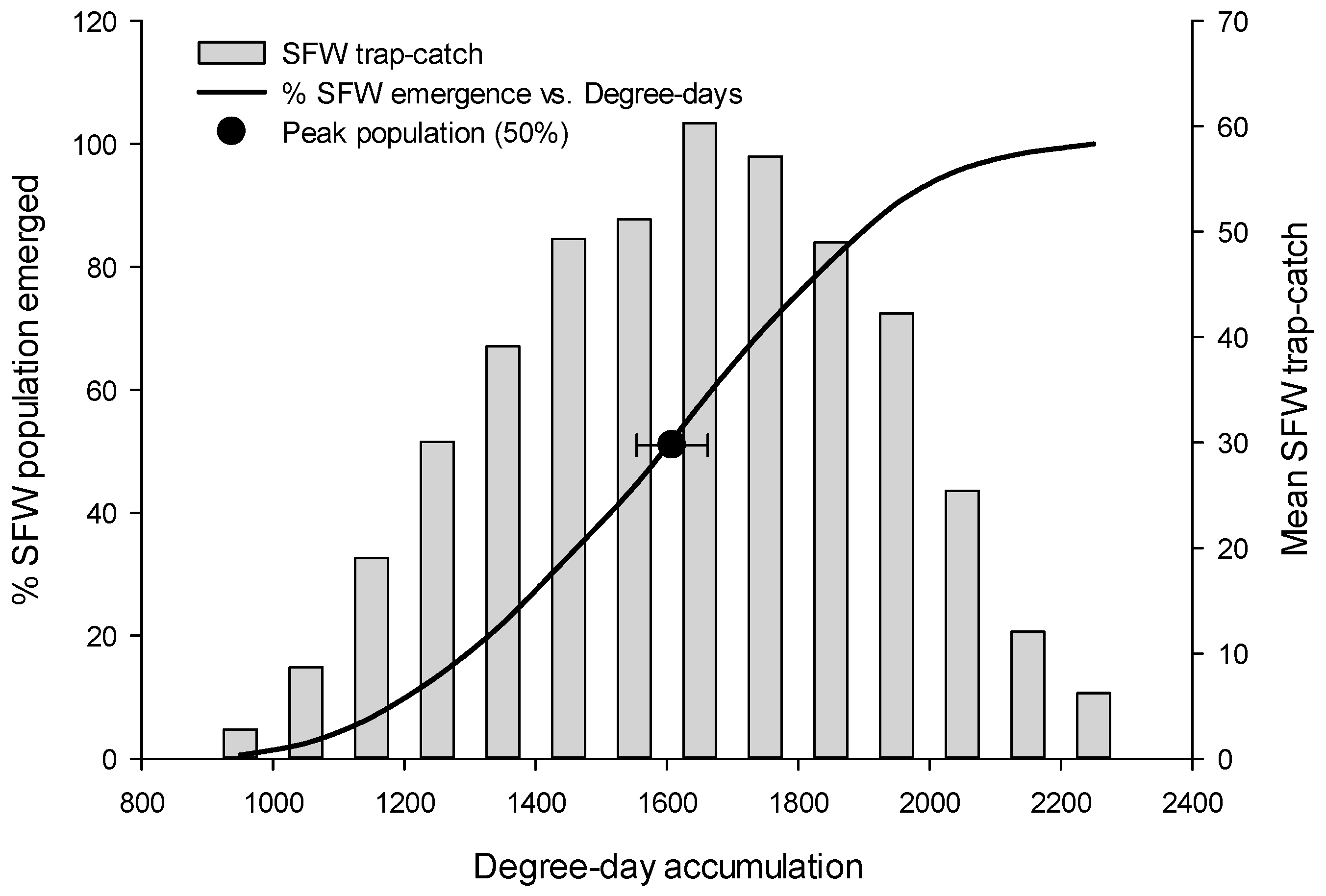
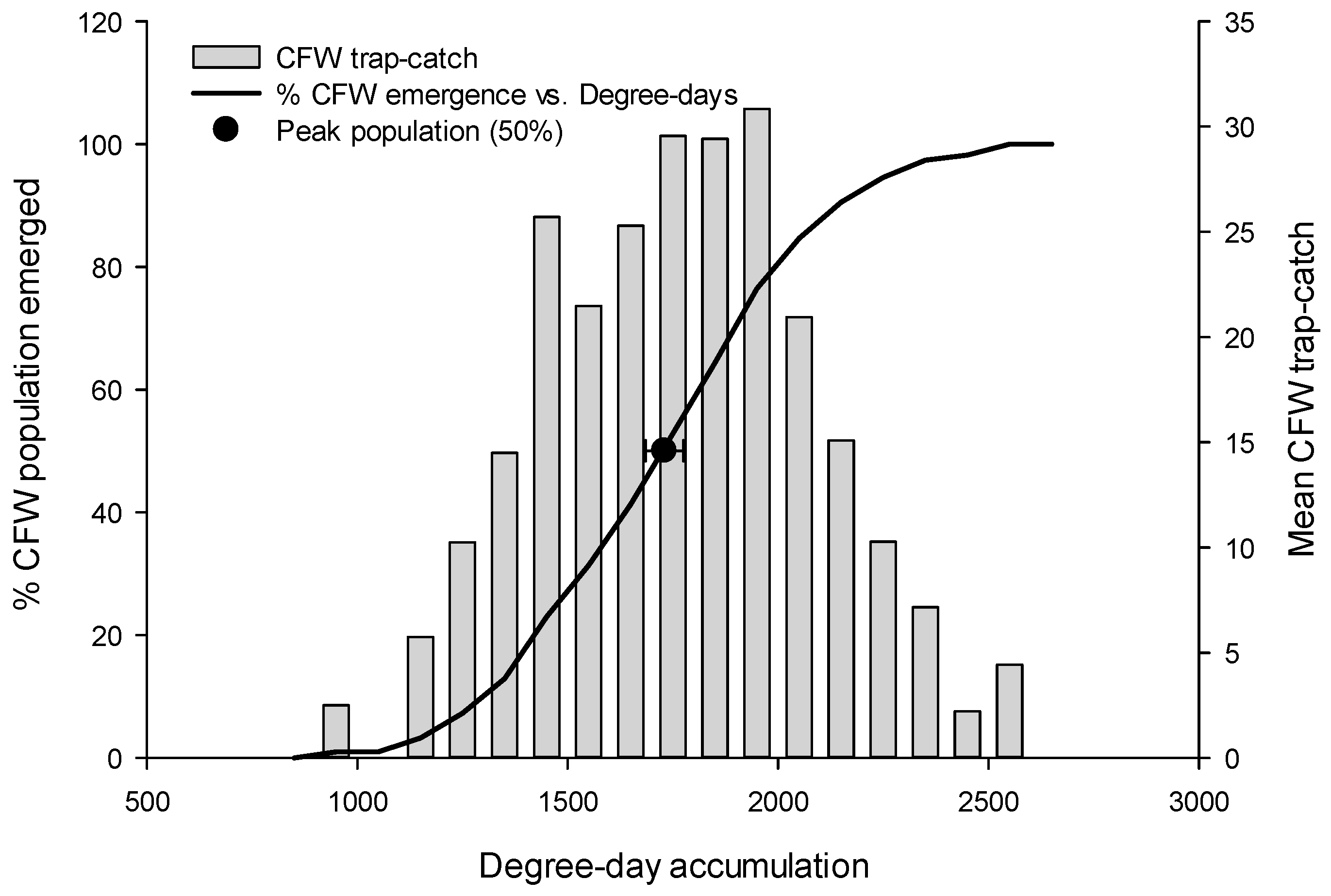
3.2. Peak Flight, by Species
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stern, V.; Smith, R.; Van Den Bosch, R.; Hagen, K. The integration of chemical and biological control of the spotted alfalfa aphid: The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Prokopy, R.; Kogan, M. Integrated Pest Management. In Encyclopedia of INSECTS; Resh, V.H., Carde, R.T., Eds.; Elsevier Inc.: Burlinton, VT, USA, 2009; pp. 523–528. [Google Scholar]
- Hagen, A.F. The biology and control of the western bean cutworm in dent corn in Nebraska. J. Econ. Entomol. 1962, 55, 628–631. [Google Scholar] [CrossRef]
- Moon, R.D.; Wilson, L.T. Sampling for detection, estimation and IPM decision making. In Integrated Pest Management: Concepts, Tactics, Strategies and Case Studies; Radcliffe, E.B., Hutchison, W.D., Cancelado, R.E., Eds.; Cambridge University Press: New York, NY, USA, 2009; pp. 75–89. [Google Scholar]
- Legg, D.E. The relevance of modelling in successful implementation of IPM. In Integrated Pest Management: Potential, Constraints, and Challenges; Koul, O., Dhaliwal, G.S., Cuperus, G.W., Eds.; CABI Publishing: Cambridge, MA, USA, 2004; pp. 39–54. [Google Scholar]
- Damos, P.T.; Savopoulou-Soultani, M. Development and statistical evaluation of models in forecasting moth phenology of major lepidopterous peach pest complex for integrated pest management programs. Crop Prot. 2010, 29, 1190–1199. [Google Scholar] [CrossRef]
- Pruess, K.P. Day-degree methods for pest management. Environ. Entomol. 1983, 12, 613–619. [Google Scholar] [CrossRef]
- Nietschke, B.S.; Magarey, R.D.; Borchert, D.M.; Calvin, D.D.; Jones, E. A developmental database to support insect phenology models. Crop Prot. 2007, 26, 1444–1448. [Google Scholar] [CrossRef]
- Miller, J.; West, K.J.; Hanson, P.E. Temperature requirements for development of Autographa californica (Lepidoptera: Noctuidae). Environ. Entomol. 1984, 13, 593–594. [Google Scholar] [CrossRef]
- Deutsch, A.E.; Rodriguez-Saona, C.R.; Zalapa, J.E.; Steffan, S.A. Temperature-mediated development thresholds of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. Environ. Entomol. 2015, 44, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Mahr, D.L. Development of cranberry girdler, Chrysoteuchia topiaria (Lepidoptera: Pyralidae) in relation to temperature. Gt. Lakes Entomol. 1986, 19, 85–90. [Google Scholar]
- Cockfield, S.D.; Butkewhich, S.L.; Samoil, K.S.; Mahr, D.L. Forecasting flight activity of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. J. Econ. Entomol. 1994, 87, 193–196. [Google Scholar] [CrossRef]
- Cockfield, S.D.; Fitzpatrick, S.M.; Patten, K.; Henderson, D.; Dittl, T.; Poole, A.; Shanks, C.; Mahr, D.L. Modeling of blackheaded fireworm (Lepidoptera: Tortricidae) oviposition and pheromone-trap catches. J. Econ. Entomol. 1994, 87, 787–792. [Google Scholar] [CrossRef]
- Deutsch, A.E.; Rodriguez-saona, C.R.; Kyryczenko-roth, V.; Sojka, J.; Zalapa, J.E.; Steffan, S.A. Degree-day benchmarks for Sparganothis sulfureana (Lepidoptera: Tortricidae) development in cranberries. J. Econ. Entomol. 2014, 107, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Chasen, E.M.; Steffan, S.A. Temperature-mediated growth thresholds of Acrobasis vaccinii (Lepidoptera: Pyralidae). Environ. Entomol. 2016, 45, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Eck, P. The American Cranberry; Rutgers University Press: New Brunswick, NJ, USA, 1990. [Google Scholar]
- Peltier, G.L. A History of the Cranberry Industry in Wisconsin; Harlo Press: Detroit, MI, USA, 1970. [Google Scholar]
- Guédot, C.; Lippert, M.; McManus. Cranberry School Grower Survey Results. Available online: https://fruit.wisc.edu/wp-content/uploads/sites/36/2016/10/2014-Cranberry-School-Proceedings-red.pdf (accessed on 13 November 2016).
- USDA-NASS United States Department of Agriculture—National Agricultural Statistics Service 2013 Wisconsin Agricultural Statistics. Available online: https://www.nass.usda.gov/Statistics_by_State/Wisconsin/Publications/Annual_Statistical_Bulletin/bulletin2013_web.pdf (accessed on 9 May 2016).
- Mahr, D.L. Cranberry Fruitworm. Available online: https://fruit.wisc.edu/wp-content/uploads/sites/36/2011/05/Cranberry-Fruitworm.pdf (accessed on 28 September 2015).
- Demoranville, C.J.; Sandler, H.A.; Shumaker, D.E.; Averill, A.L.; Caruso, F.L.; Sylvia, M.M.; Pober, D.M. Fall flooding for management of cranberry fruitworm (Acrobasis vaccinii) and dewberry (Rubus hispidus) in Massachusetts cranberry production. Crop Prot. 2005, 24, 999–1006. [Google Scholar] [CrossRef]
- Mahr, D.L. Biological control in a high-value native crop: Status, opportunities, and constraints in cranberry. In Biological Control of Native or Indigenous Pests: Challenges, Constraints, and Potential; Entomological Society of America: Lanham, MD, USA, 1999. [Google Scholar]
- Mahr, D.L. Sparganothis Fruitworm. Available online: https://fruit.wisc.edu/wp-content/uploads/sites/36/2011/05/Sparganothis-Fruitworm.pdf (accessed on 7 November 2016).
- Teixeira, L.A.F.; Averill, A.L.; Teixeira, L.S. Evaluation of flooding for cultural control of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberry bogs. Environ. Entomol. 2006, 35, 670–675. [Google Scholar] [CrossRef]
- Marucci, E.P.; Moulter, J.H. Biological contgrol of sparganothis fruitworm on cranberries in New Jersey. Cranberries 1992, 56, 11–15. [Google Scholar]
- Mahr, D.L. Blackheaded Fireworm. Available online: https://fruit.wisc.edu/wp-content/uploads/sites/36/2011/05/Blackheaded-Fireworm.pdf (accessed on 7 November 2016).
- Cockfield, S.D.; Mahr, D.L.; Blackheaded Fireworm, T.H.E. Flooding cranberry beds to control blackheaded fireworm (Lepidoptera: Tortricidae). J. Econ. Entomol. 1992, 85, 2383–2388. [Google Scholar] [CrossRef]
- DeMoranville, C.J. Cranberry Nutrients, Phenology, and N-P-K Fertilization; University of Massachusetts: Amherst, MA, USA, 1992. [Google Scholar]
| Moth Species | South | Central | East | All Regions |
|---|---|---|---|---|
| A. vaccinii | 1714.9 | 1776.3 | 1801.7 | 1760.9 ± 44.0 |
| S. sulfureana | 1571.7 | 1622.9 | 1638.3 | 1608.9 ± 59.8 |
| R. naevana | 1335.7 | 1402.3 | 1350.2 | 1357.8 ± 26.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffan, S.A.; Singleton, M.E.; Sojka, J.; Chasen, E.M.; Deutsch, A.E.; Zalapa, J.E.; Guédot, C. Flight Synchrony among the Major Moth Pests of Cranberries in the Upper Midwest, USA. Insects 2017, 8, 26. https://doi.org/10.3390/insects8010026
Steffan SA, Singleton ME, Sojka J, Chasen EM, Deutsch AE, Zalapa JE, Guédot C. Flight Synchrony among the Major Moth Pests of Cranberries in the Upper Midwest, USA. Insects. 2017; 8(1):26. https://doi.org/10.3390/insects8010026
Chicago/Turabian StyleSteffan, Shawn A., Merritt E. Singleton, Jayne Sojka, Elissa M. Chasen, Annie E. Deutsch, Juan E. Zalapa, and Christelle Guédot. 2017. "Flight Synchrony among the Major Moth Pests of Cranberries in the Upper Midwest, USA" Insects 8, no. 1: 26. https://doi.org/10.3390/insects8010026






