Spectral Signatures of Immature Lucilia sericata (Meigen) (Diptera: Calliphoridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
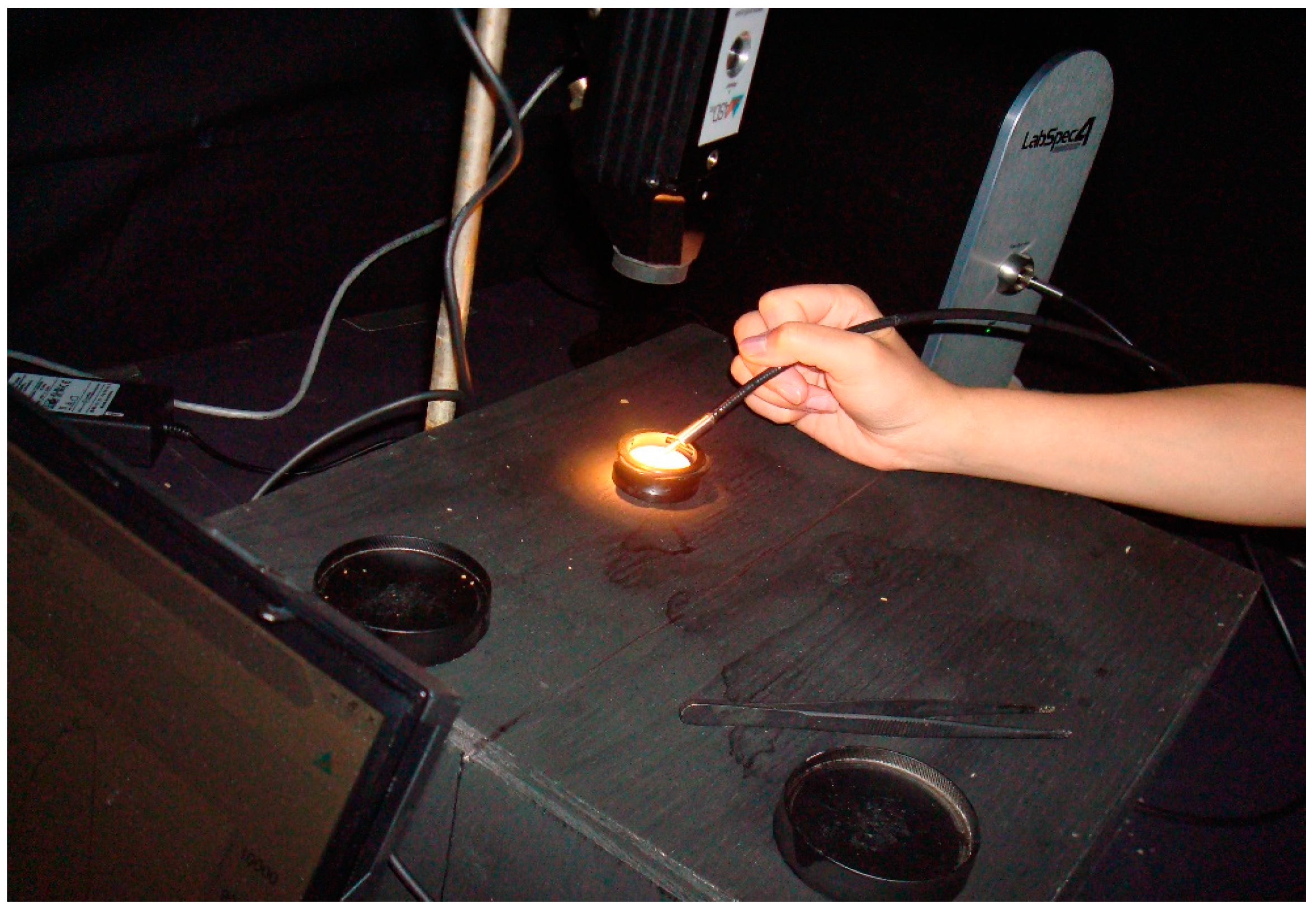
2.2. Experimental Protocol
2.3. Pre-Processing
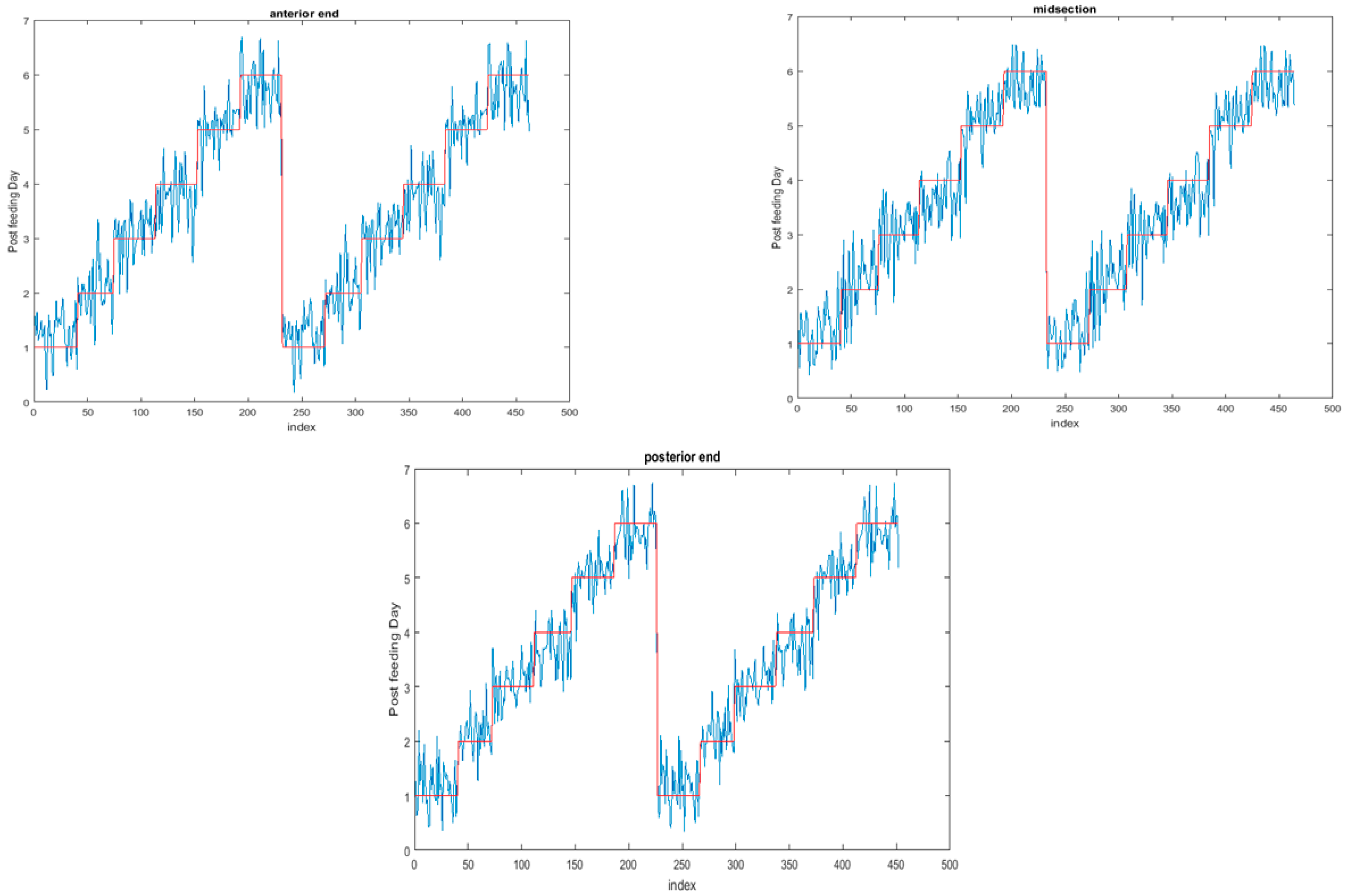
2.4. Functional Linear Model to Estimate Day of Development
2.4.1. Smoothing Individual Reflectance Measurements
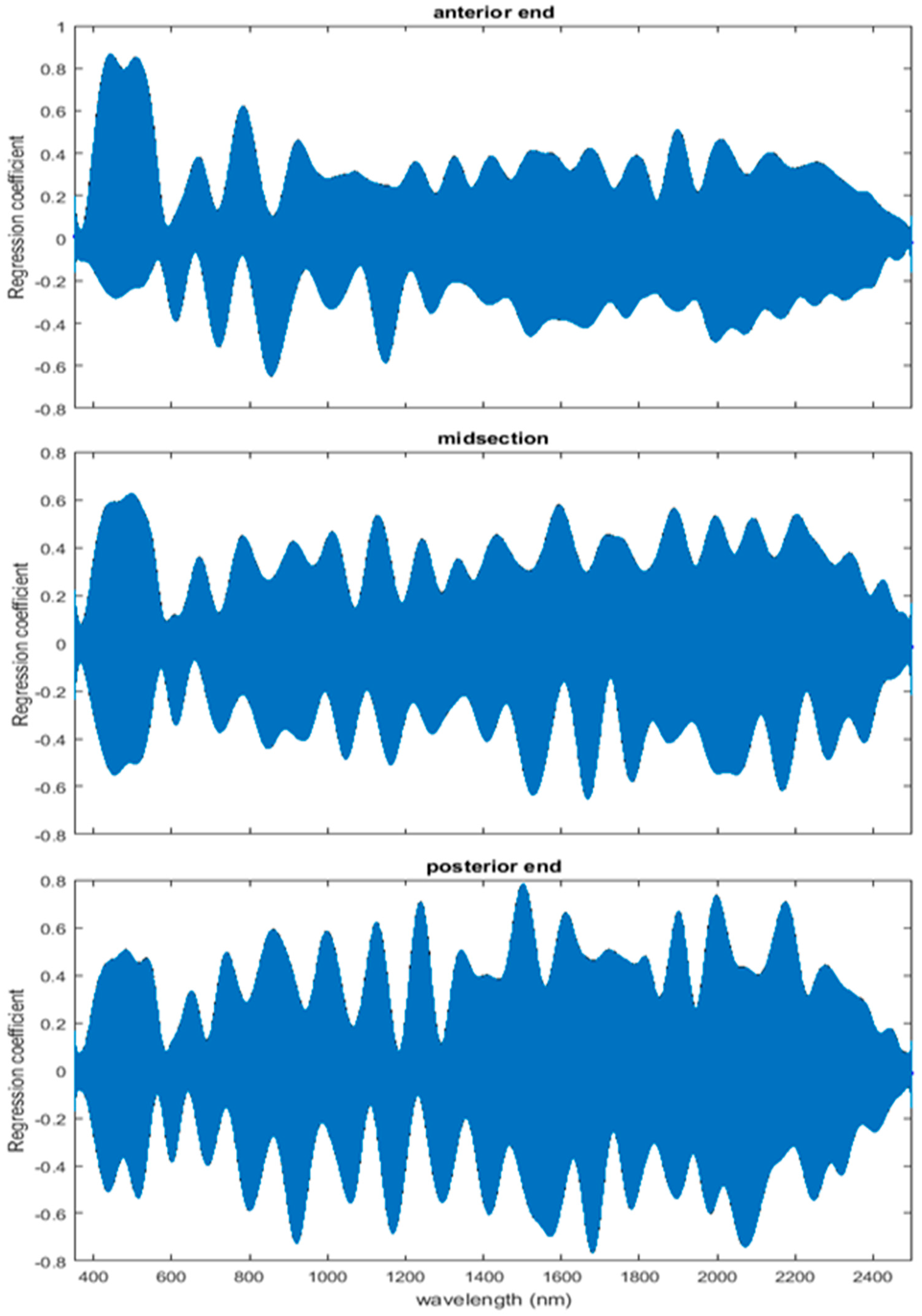
2.4.2. Functional Linear Model with Scalar Response
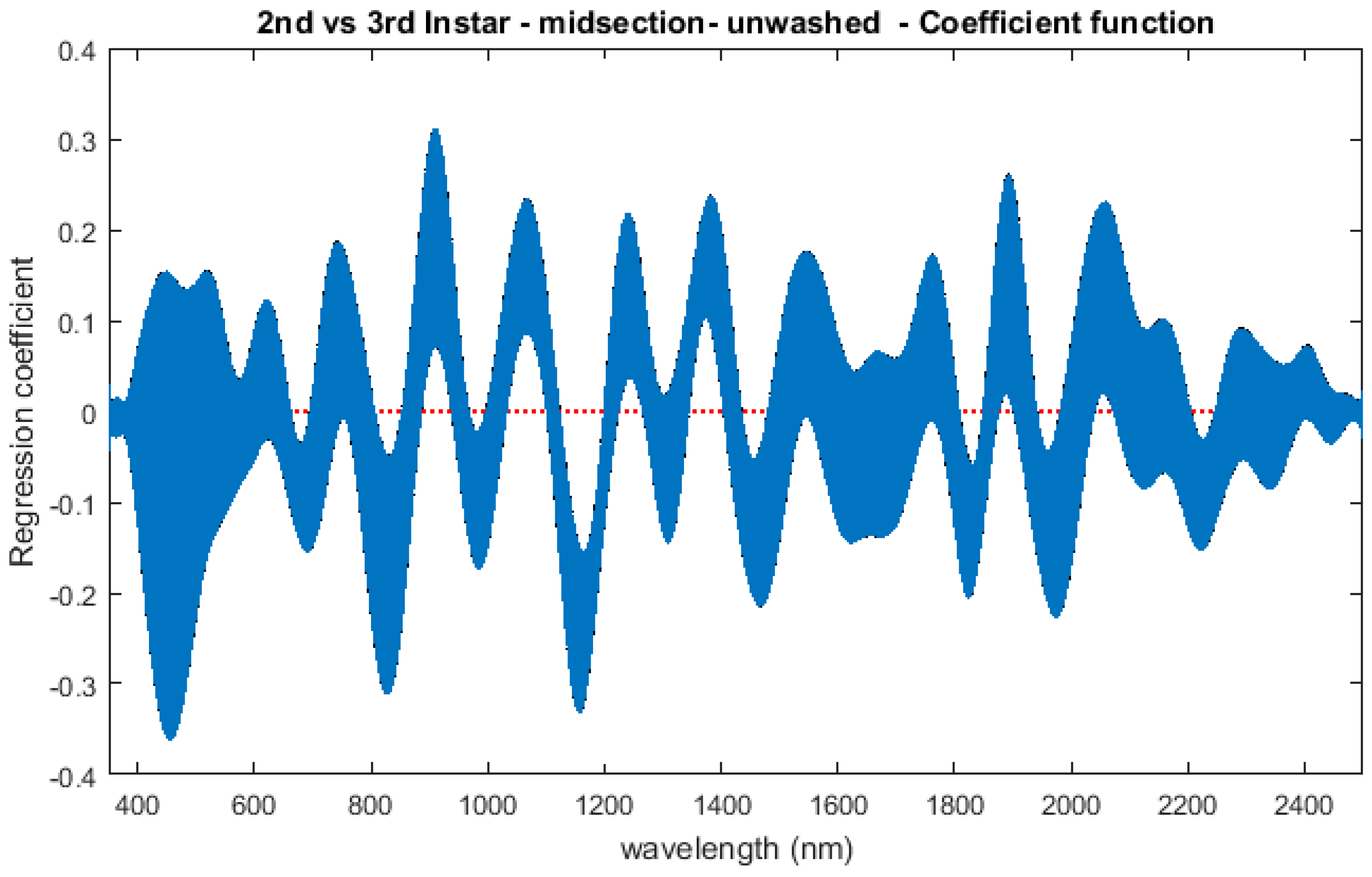
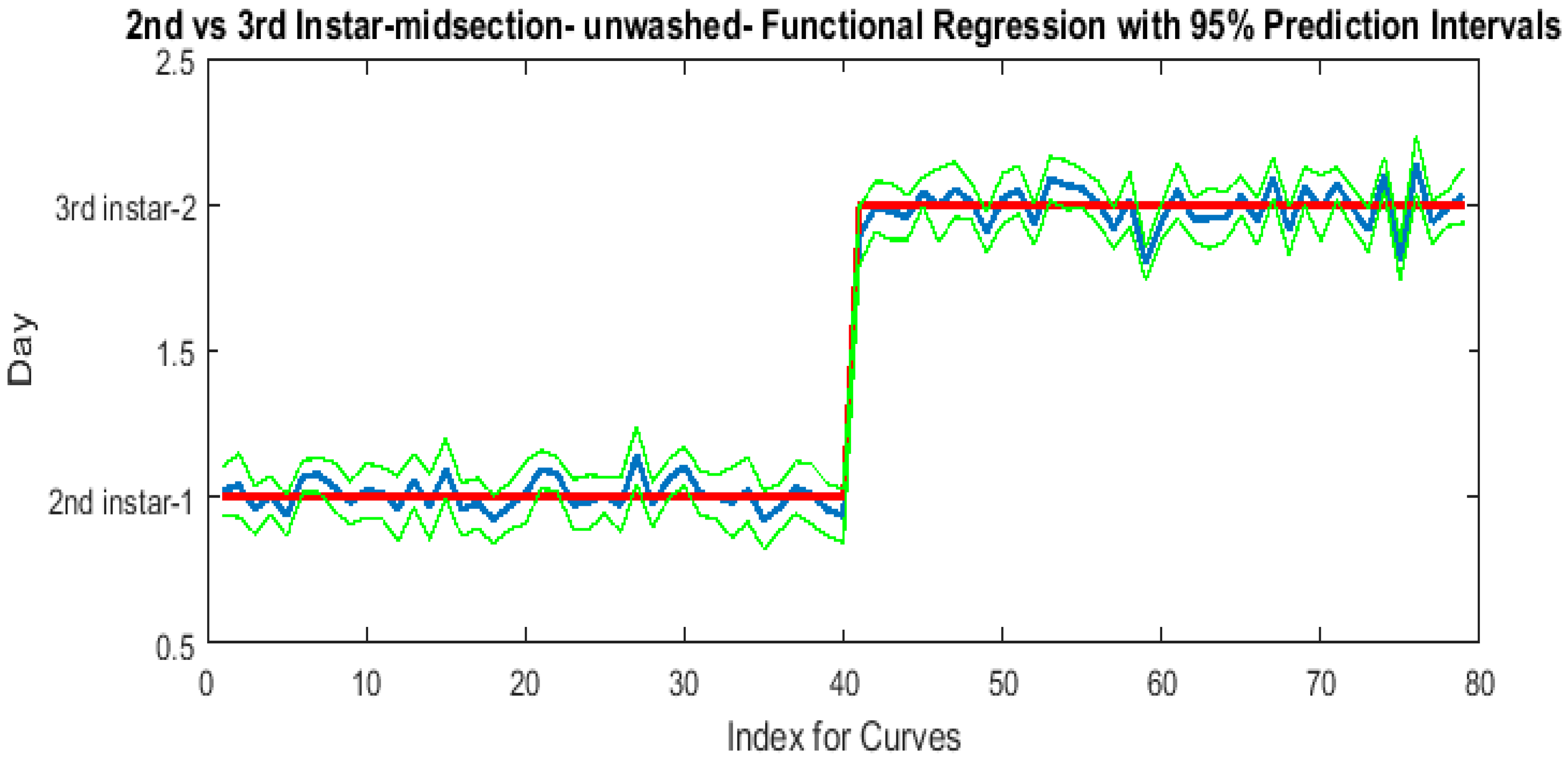
2.4.3. Model Fitting to Compare Washed to Unwashed Insects
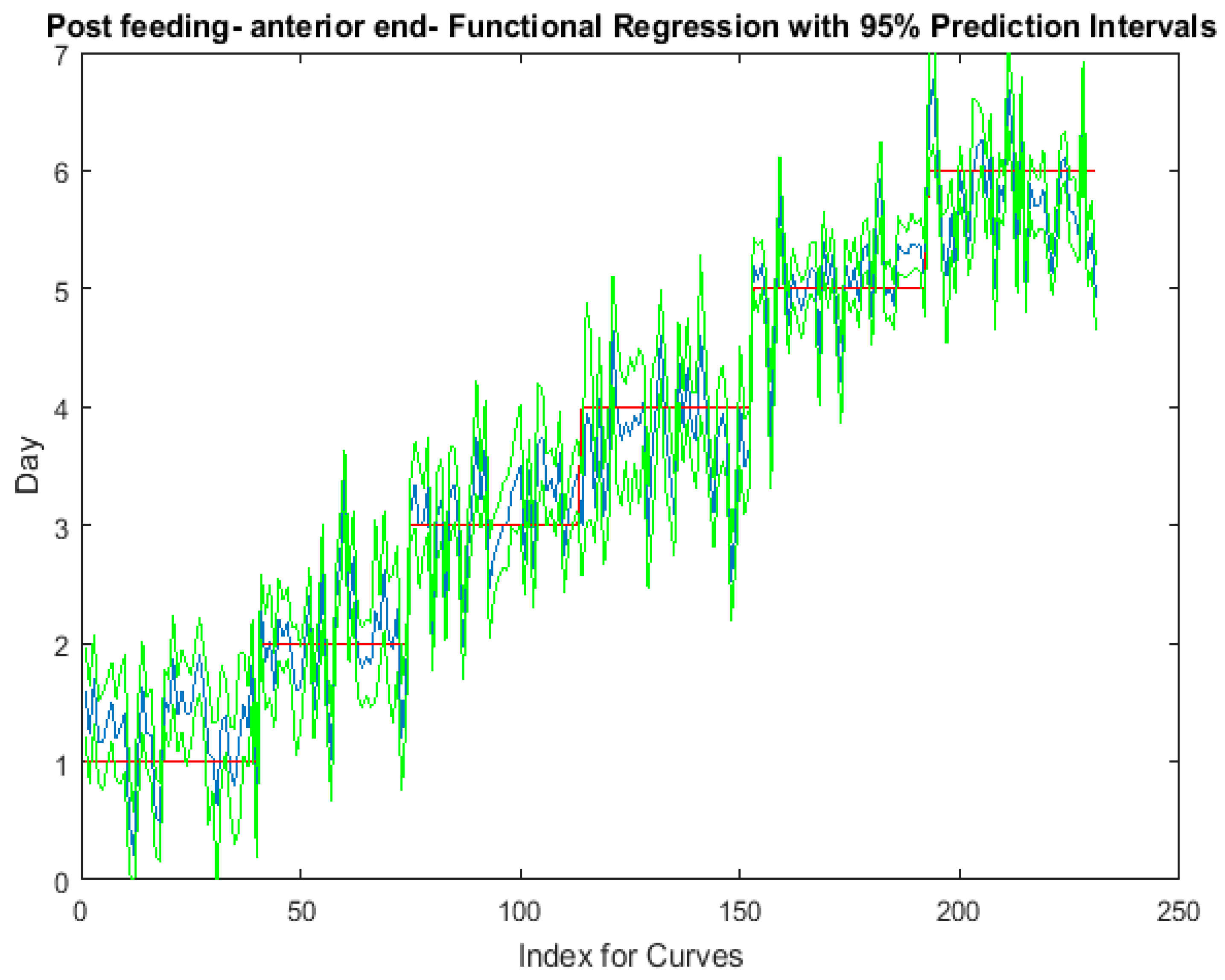
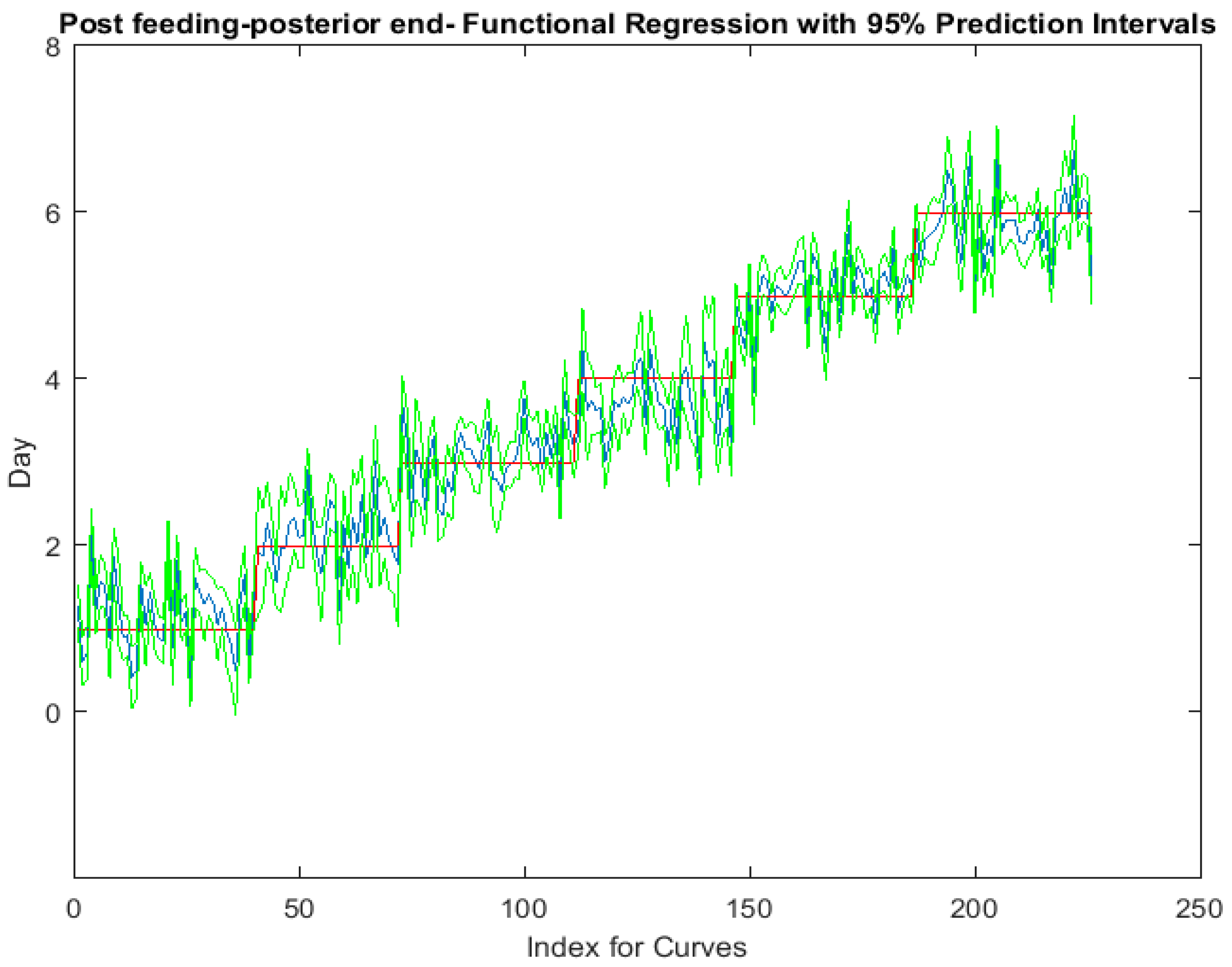
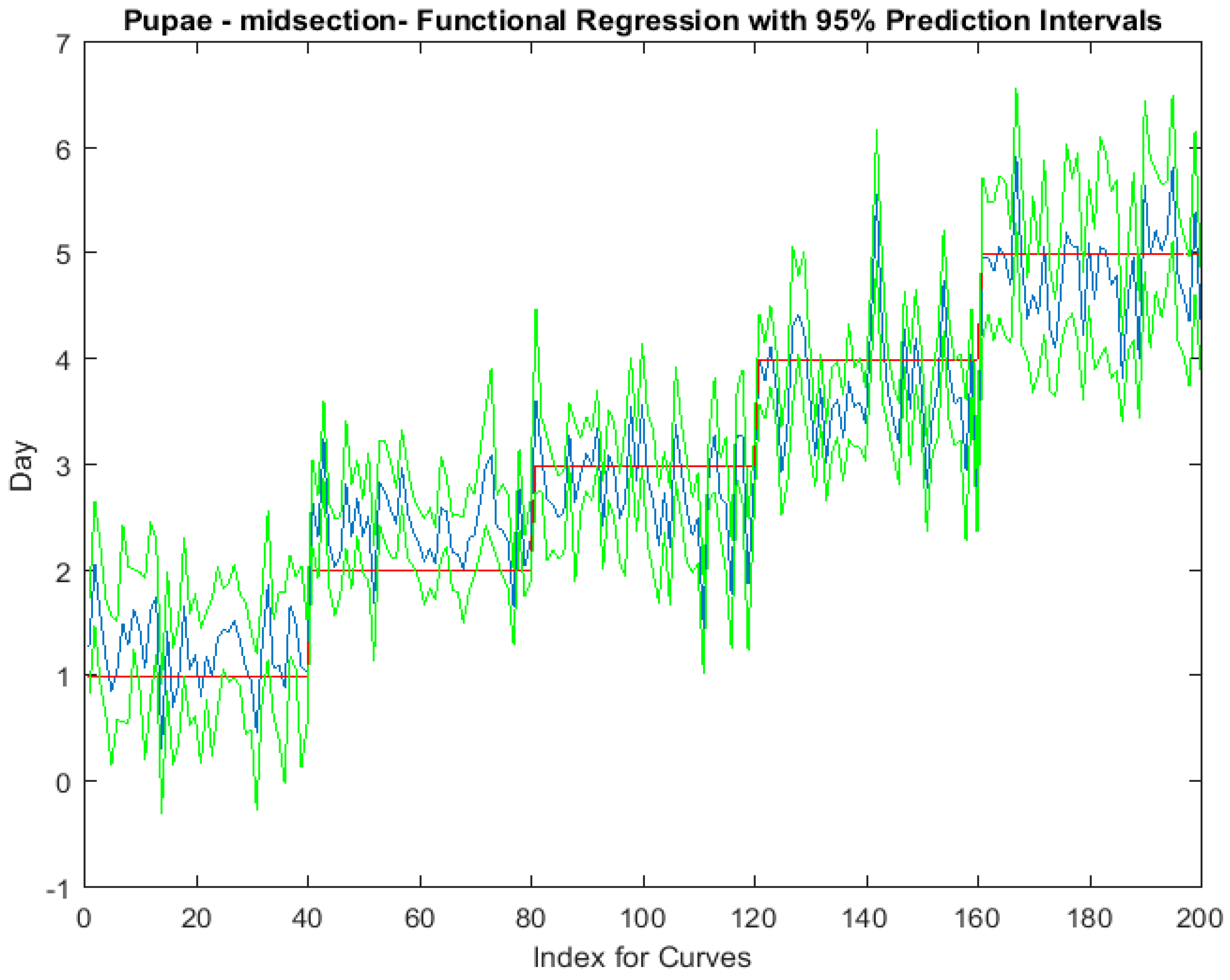
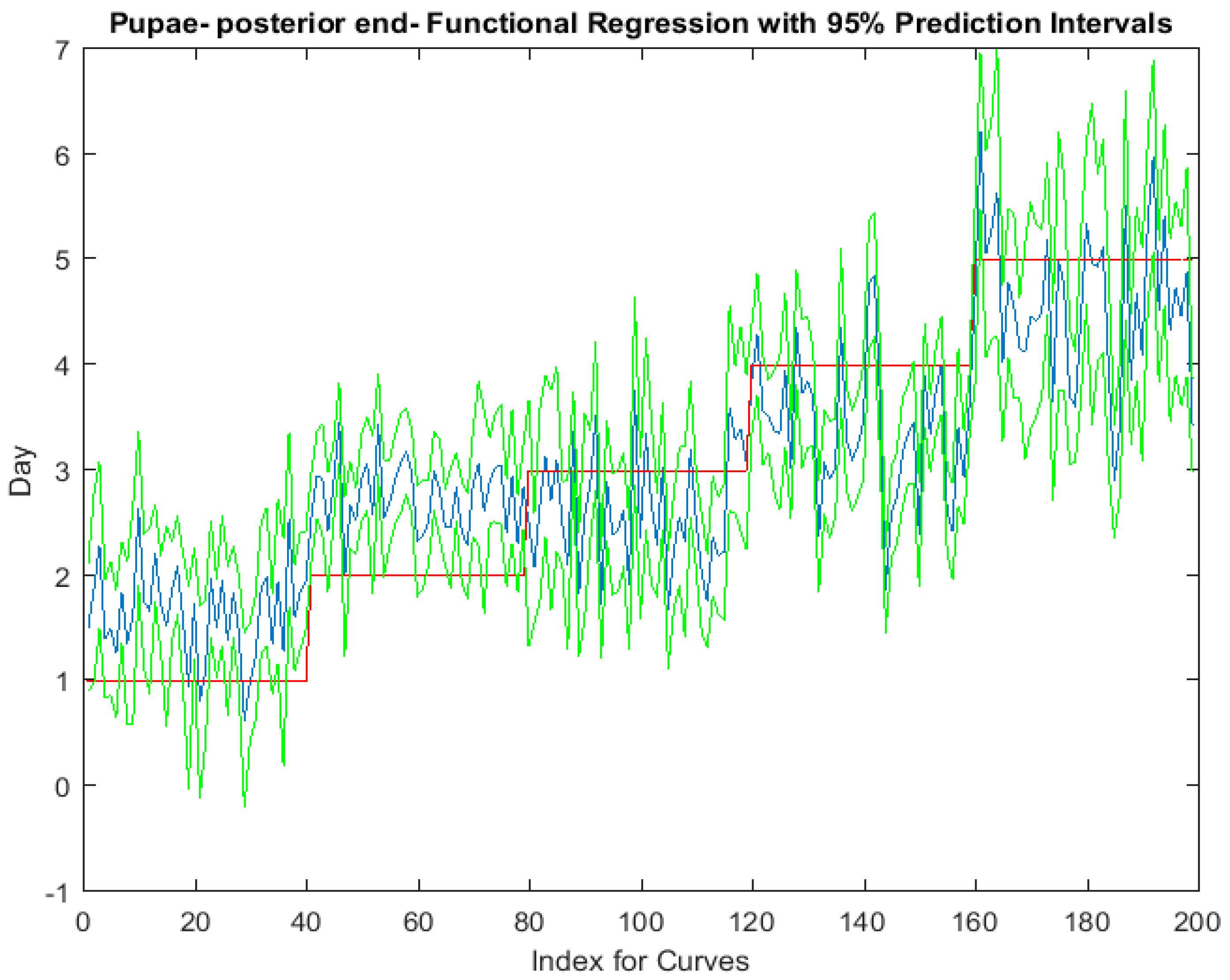
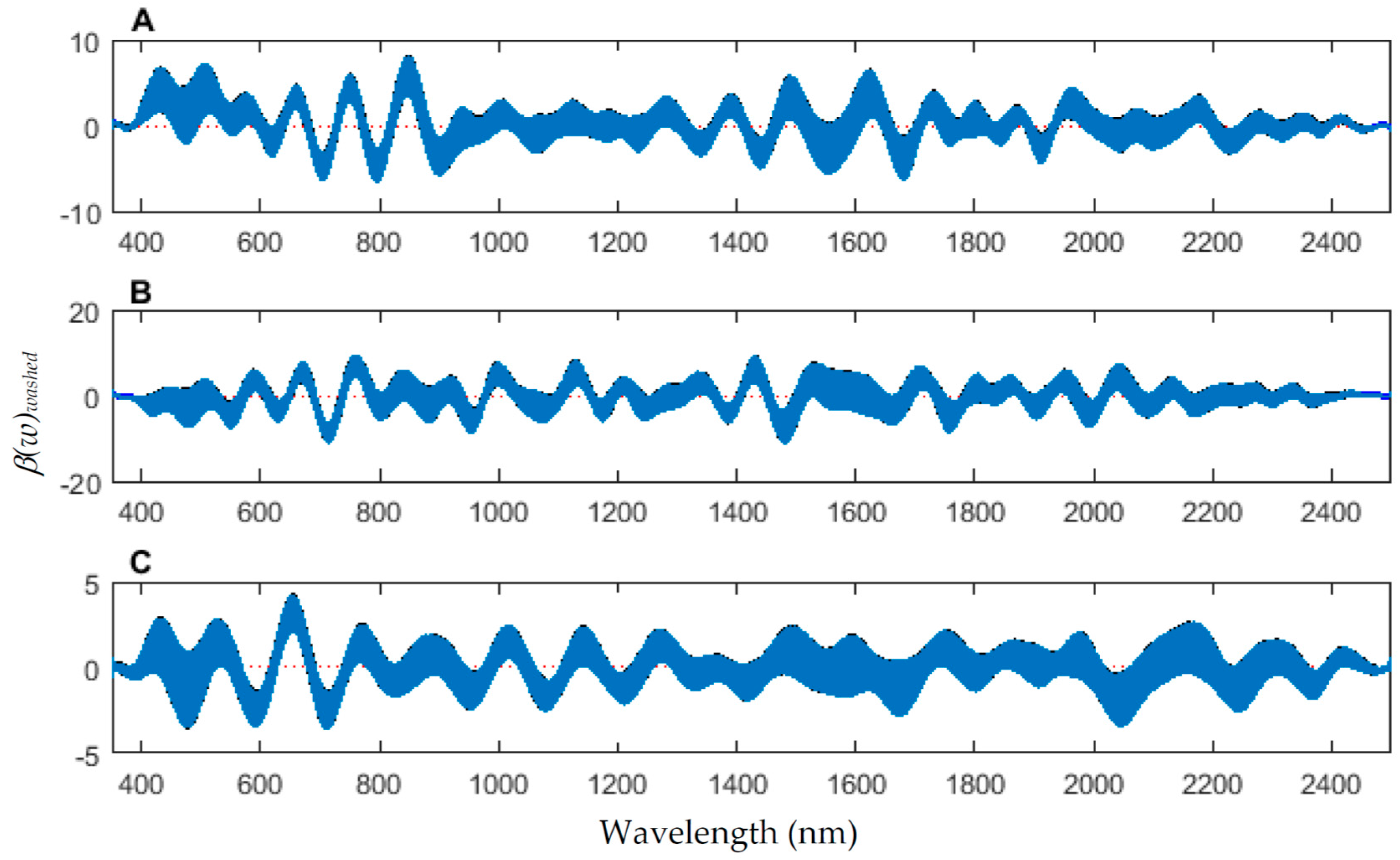
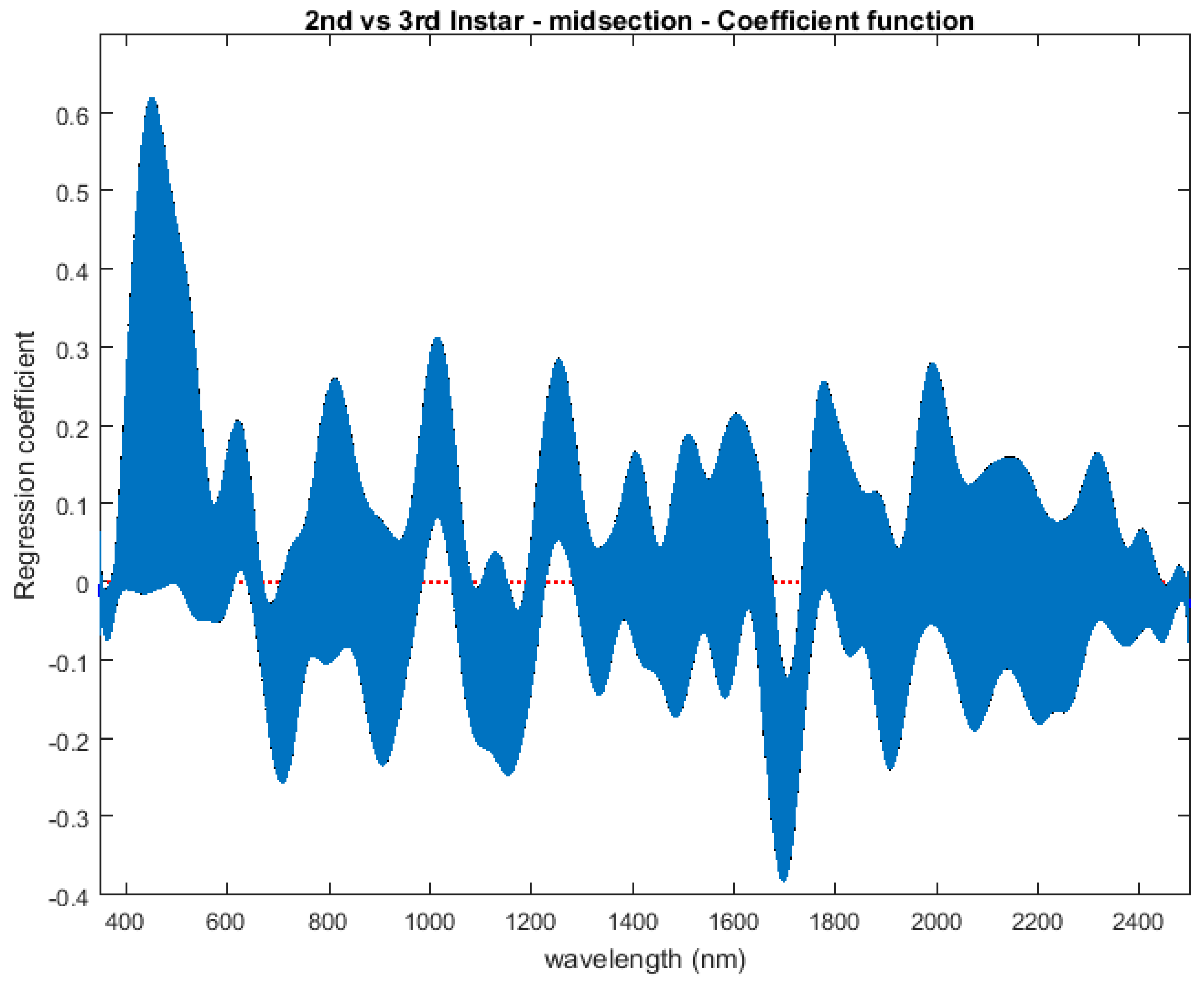
3. Results
3.1. Spectral Analysis
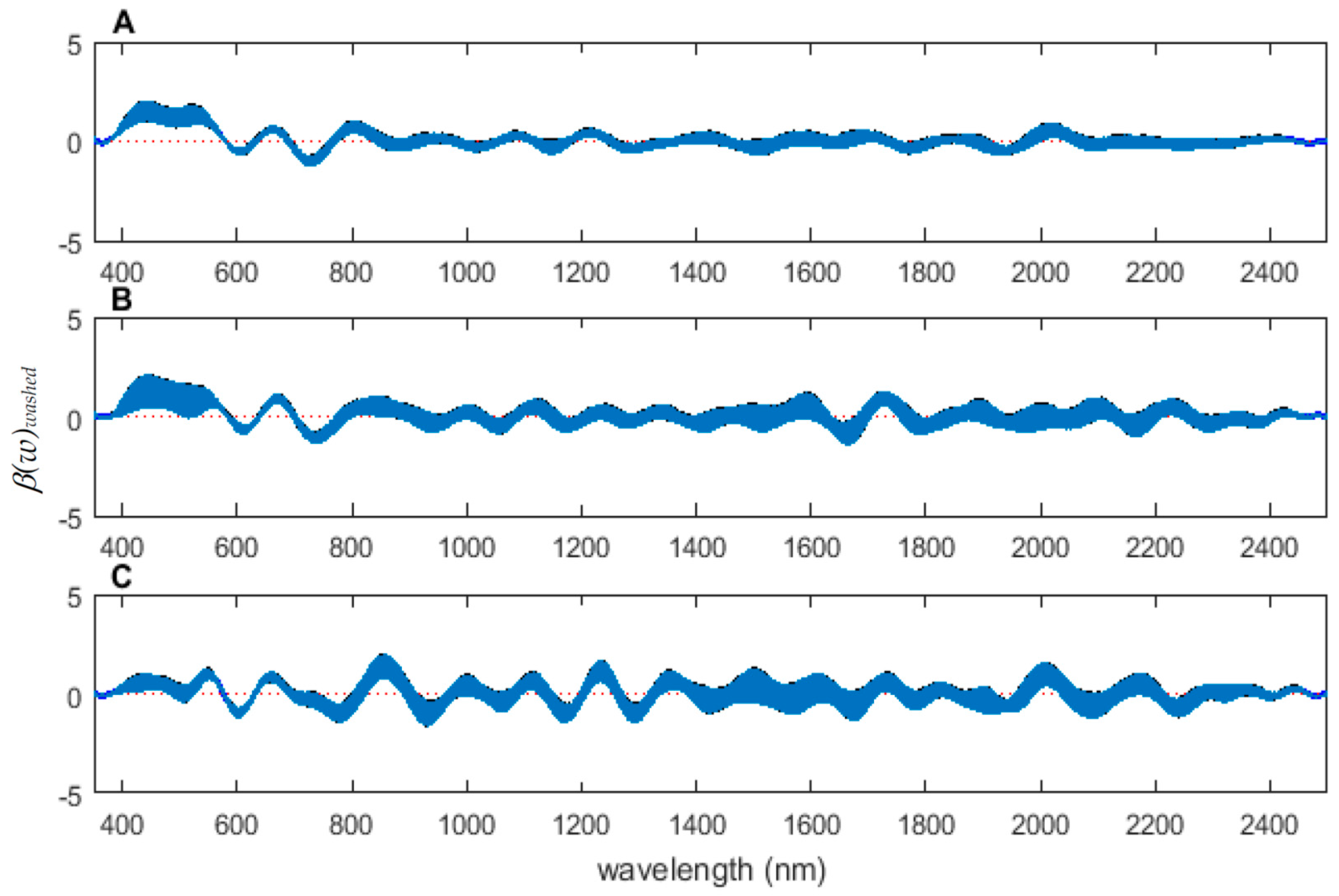
3.2. Washed Versus Unwashed
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nansen, C.; Ribeiro, L.P.; Dadour, I.; Roberts, J.D. Detection of temporal changes in insect body reflectance in response to killing agents. PLoS ONE 2015, 10, e0124866. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Jacques, S.L. Optical reflectance and transmittance of tissues: Principles and applications. IEEE J. Quantum Electron. 1990, 26, 2186–2199. [Google Scholar] [CrossRef]
- Carroll, M.W.; Glaser, J.A.; Hellmich, R.L.; Hunt, T.E.; Sappington, T.W.; Calvin, D.; Copenhaver, K.; Fridgen, J. Use of spectral vegetation indices derived from airborne hyperspectral imagery for detection of european corn borer infestation in Rowa corn plots. J. Econ. Entomol. 2008, 101, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.G.; Ingle, S.J.; Davis, M.R.; Mangum, C.; Higgins, A.; Boling, J.C. Some uses of infrared aerial color photography in entomology. In Proceedings of the Third Biennial Workshop on Aerial Color in the Plant Sciences, Gainesville, FL, USA, 2–4 March 1971; pp. 99–113. [Google Scholar]
- Lawrence, R.; Labus, M. Early detection of Douglas-fir beetle infestation with subcanopy resolution hyperspectral imagery. West. J. Appl. For. 2003, 18, 1–5. [Google Scholar]
- Mirik, M.; Ansley, R.J.; Steddom, K.; Rush, C.M.; Michels, G.J.; Workneh, F.; Cui, S.; Elliott, N.C. High spectral and spatial resolution hyperspectral imagery for quantifying russian wheat aphid infestation in wheat using the constrained energy minimization classifier. J. Appl. Remote Sens. 2014. [Google Scholar] [CrossRef]
- Mirik, M.; Michels, G.J., Jr.; Kassymzhanova-Mirik, S.; Elliot, N.C.; Bowling, R. Hyperspectral spectrometry as a means to differentiate uninfested and infested winter wheat by greenbug (Hemiptera: Aphididae). J. Econ. Entomol. 2006, 99, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Mirik, M.; Michels, G.J., Jr.; Kassymzhanova-Mirik, S.; Elliot, N.C.; Catana, V.; Jones, D.B.; Bowling, R. Using digital image analysis and spectral reflectance data to quantify damage by greenbug (Hemiptera: Aphididae) in winter wheat. Comput. Electron. Agric. 2006, 51, 86–98. [Google Scholar] [CrossRef]
- Moran, M.S.; Inoue, Y.; Barnes, E.M. Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sens. Environ. 1997, 61, 319–346. [Google Scholar] [CrossRef]
- Riley, J.R. Remote sensing in entomology. Annu. Rev. Entomol. 1989, 34, 247–271. [Google Scholar] [CrossRef]
- Singh, C.B.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Detection of insect-damaged wheat kernels using near-infrared hyperspectral imaging. J. Stored Prod. Res. 2009, 45, 151–158. [Google Scholar] [CrossRef]
- Solberg, S.; Eklundh, L.; Gjertsen, A.K.; Johansson, T.; Joyce, S.; Lange, H.; Naesset, E.; Olsson, H.; Pang, Y.; Solberg, A. Testing remote sensing techniques for monitoring large scale insect defoliation. Remote Sens. Environ. 2007, 99, 295–303. [Google Scholar]
- Williams, D.W.; Bartels, D.W.; Sawyer, A.J.; Mastro, V. Application of hyperspecral imaging to survey for emerald ash borer. Proceedings of XV U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and other invasive species Annapolis Maryland, Annapolis, MD, USA, 13–16 January 2004. [Google Scholar]
- Xing, J.; Guyer, D.; Ariana, D.; Lu, R. Determining optimal wavebands using genetic algorithm for detection of internal insect infestation in tart cherry. Sens. Instrum. Food Qual. 2008, 2, 161–167. [Google Scholar] [CrossRef]
- Beck, L.R.; Lobitz, B.M.; Wood, B.L. Remote sensing and human health: New sensors and new opportunities. Emerg. Infect. Dis. 2000, 6, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.E.; Diuk-Wasser, M.A.; Guan, Y.; Caskey, S.; Fish, D. Comparison of three satellite sensors at three spatial scales to predict larval mosquito presence in connecticut wetlands. Remote Sens. Environ. 2008, 112, 2301–2308. [Google Scholar] [CrossRef]
- Nansen, C.; Coelho, A., Jr.; Viera, J.M.; Parra, J.R.P. Reflectance-based identification of parasitized host eggs and adult Trichogramma specimens. J. Exp. Biol. 2014, 217, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Foran, D.R. Gene expression during blow fly development: Improving the precision of age estimates in forensic entomology. J. Forensic Sci. 2011, 56, S112–S122. [Google Scholar] [CrossRef] [PubMed]
- Catts, E.P. Problems in estimating the postmortem interval in death investigations. J. Agric. Entomol. 1992, 9, 245–255. [Google Scholar]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Leg. Med. 2016, 131, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Foran, D.R. Generalized additive models and Lucilia sericata growth: Assessing confidence intervals and error rates in forensic entomology. J. Forensic Sci. 2008, 53, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J. Forensic Sci. 2000, 45, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Reibe, S.; Doetinchem, P.V.; Madea, B. A new simulation-based model for calculating post-mortem intervals using developmental data for Lucilia sericata (Dipt.: Calliphoridae). Parisitol. Res. 2010, 107, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Day, D.M.; Wallman, J.F. Width as an alternative measurement to length for post-mortem interval estimations using calliphora augur (Diptera: Calliphoridae) larvae. Forensic Sci. Int. 2006, 159, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.D.; Lamotte, L.R. Chapter 9: Estimating the Postmortem Interval. In The Utility of Arthropods in Legal Investigations, 2nd ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Butcher, J.B.; Moore, H.E.; Day, C.R.; Adam, C.D.; Drijfhout, F.P. Artificial neural network analysis of hydrocarbon profiles for the ageing of Lucilia sericata for post mortem interval estimation. Forensic Sci. Int. 2013, 232, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.-P.; Verheggen, F.J.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E. Analysis of Cuticular Hydrocarbons in Forensically Important Blowflies Using Mass Spectrometry and Its Application in Post Mortem Interval Estimations. Doctoral Dissertation, Keele University, Newcastle, UK, 2013. [Google Scholar]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Potential use of hydrocarbons for aging Lucilia sericata blowfly larvae to establish the postmortem interval. J. Forensic Sci. 2013, 58, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Identifying 1st instar larvae for three forensically important blowfly species using “fingerprint” cuticular hydrocarbon analysis. Forensic Sci. Int. 2014, 240, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Pechal, J.L.; Moore, H.E.; Drijfhout, F.; Benbow, M.E. Hydrocarbon profiles throughout adult Calliphoridae aging: A promising tool for forensic entomology. Forensic Sci. Int. 2014, 245, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, G.-Y.; Xu, Y.; Hu, C.; Zhu, G.-H. Age-dependent changes in cuticular hydrocarbons of larvae in Aldrichina grahami (Aldrich) (Diptera: Calliphoridae). Forensic Sci. Int. 2014, 242, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Xu, X.H.; Yu, X.J.; Zhang, Y.; Wang, J.F. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci. Int. 2007, 169, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Jennings, K.C.; Foran, D.R. Aging blow fly eggs using gene expression: A feasibility study. J. Forensic Sci. 2007, 52, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Thorne, A.; Harvey, M. Calliphora vicina (Diptera: Calliphoridae) pupae: A timeline of external morphological development and a new age and PMI estimation tool. Int. J. Leg. Med. 2015, 129, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Harvey, M.L. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J. Forensic Sci. 2013, 58, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Defilippo, F.; Bonilauri, P.; Dottori, M. Effect of temperature on six different developmental landmarks within the pupal stage of the forensically important blowfly Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae). J. Forensic Sci.s 2013, 58, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S.; Simonsen, T.J.; Abel, R.L.; Hall, M.J.R.; Schwyn, D.A.; Wicklein, M. Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3d micro-computed tomography. Forensic Sci. Int. 2012, 220, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Picard, C.J.; Spiegelman, C.; Foran, D.R. Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J. Med. Entomol. 2011, 48, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.A. The Development of Protophormia terraenovae (Robineau-Desvoidy) (Diptera:Calliphoridae) at Constant and Fluctuating Temperatures. Master of Arts, Simon Fraser University, Burnaby, BC, Canada, 2006. [Google Scholar]
- Warren, J.A.; Anderson, G.S. Effect of fluctuating temperatures on the development of a forensically important blow fly, Protophormia terraenovae (Diptera: Calliphoridae). Environ. Entomol. 2013, 42, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.L.; Hands, J.R.; Fullwood, L.M.; Smith, J.A.; Baker, M.J. Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic Sci. Int. 2015, 249, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, T.L. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America north of Mexico. Proc. Entomol. Soc. Wash. 2006, 108, 689–725. [Google Scholar]
- Byrd, J.H.; Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA. Personal communication, 2016.
- Smith, K.G.V. A Manual of Forensic Entomology; British Museum (Natural History): London, UK, 1986; pp. 1–203. [Google Scholar]
- Kharbouche, H.; Augsburger, M.; Cherix, D.; Sporkert, F.; Giroud, D.; Wyss, C.; Champod, C.; Mangin, P. Codeine accumulation and elimination in larvae, pupae, and imago of the blowfly Lucilia sericata and effects on its development. Int. J. Leg. Med. 2008, 122, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.W.; Fuke, C.; Court, F.; Pounder, D.J. Drug accumulation and elimination in Calliphora vicina larvae. Forensic Sci. Int. 1995, 71, 191–197. [Google Scholar] [CrossRef]
- Gosselin, M.; Wille, S.M.R.; del Mar Ramirez Fernandez, M.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bourel, B.; Tournel, G.; Hedouin, V.; Deveaux, M.; Goff, M.L.; Gosset, D. Morphine extraction in necrophagous insects remains for determining ante-mortem opiate intoxication. Forensic Sci. Int. 2001, 120, 127–131. [Google Scholar] [CrossRef]
- Fleming, R.W.; Torralba, A.; Adelson, E.H. Specular reflections and the perception of shape. J. Vis. 2004, 4, 798–820. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.O.; Silverman, B.W. Functional Data Analysis; Springer: New York, NY, USA, 2005. [Google Scholar]
- Makki, R.; Cinnamon, E.; Gould, A.P. The development and functions of oenocytes. Annu. Rev. Entomol. 2014, 59, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zurek, L.; Dykstra, M.J.; Schal, C. Hydrocarbon synthesis by enzymatically dissociated oenocytes of the abdominal integument of the German cockroach, Blattella germanica. Naturwissenschaften 2003, 90, 121–126. [Google Scholar] [PubMed]
- Blomquist, G.J. Structure and analysis of insect hydrocarbons. In Insect Hydrocarbons Biology, Biochemistry and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 19–34. [Google Scholar]
- Dennell, R. A study of an insect cuticle: The Larval Cuticle of Sarcophaga Falculata Pand. (Diptera). Proc. R. Soc. Lond. Ser. B Biol. Sci. 1946, 348–373. [Google Scholar] [CrossRef]
- Dennell, R. A study of an insect cuticle: The formation of the puparium of Sarcophaga falculata pand. (Diptera). Proc. R. Soc. Lond. Ser. B Biol. Sci. 1947, 134, 79–110. [Google Scholar] [CrossRef]
- Zdarek, J.; Fraenkel, G. The mechanism of puparium formation in flies. J. Exp. Zool. 1972, 179, 315–324. [Google Scholar] [CrossRef]
- Foley, W.J.; McIlwee, A.; Lawler, I.; Aragones, L.; Woolnough, A.P.; Berding, N. Ecological applications of near infrared reflectance spectroscopy—A tool for rapid, cost-effective prediction of the composition of plant and animal tissues and aspects of animal performance. Oecologia 1998, 116, 293–305. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, J.-A.; Ratnasekera, T.D.P.; Campbell, D.A.; Anderson, G.S. Spectral Signatures of Immature Lucilia sericata (Meigen) (Diptera: Calliphoridae). Insects 2017, 8, 34. https://doi.org/10.3390/insects8020034
Warren J-A, Ratnasekera TDP, Campbell DA, Anderson GS. Spectral Signatures of Immature Lucilia sericata (Meigen) (Diptera: Calliphoridae). Insects. 2017; 8(2):34. https://doi.org/10.3390/insects8020034
Chicago/Turabian StyleWarren, Jodie-A., T. D. Pulindu Ratnasekera, David A. Campbell, and Gail S. Anderson. 2017. "Spectral Signatures of Immature Lucilia sericata (Meigen) (Diptera: Calliphoridae)" Insects 8, no. 2: 34. https://doi.org/10.3390/insects8020034




