Demographic Variation of Wolbachia Infection in the Endangered Mitchell’s Satyr Butterfly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Acquisition
2.2. Sample Preparation
2.3. Wolbachia Screens
2.4. Wolbachia Confirmation
3. Results and Discussion
3.1. Wolbachia Presence
3.2. Wolbachia Demographics in N. mitchellii
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parshall, D.K.; Kral, T.W. A new subspecies of Neonympha mitchellii (French) (Satyridae) from North Carolina. J. Lepid. Soc. 1989, 42, 114–119. [Google Scholar]
- Michigan Department of Natural Resources. Mitchell’s Satyr. Available online: http://www.michigan.gov/dnr/0,1607,7-153-10370_12145_12204-33013--,00.html (accessed on 25 October 2011).
- Mitchell’s Satyr Range wide Habitat Conservation Plan. Unites States Fish and Wildlife Service. Available online: http://www.fws.gov/midwest/endangered/permits/hcp/mitchellsatyr/index.html (accessed on 25 October 2011).
- French, G.H. A new species of Neonympha. Can. Entomol. 1889, 21, 25–27. [Google Scholar] [CrossRef]
- Casebere, L.; Denny, G.; Metzler, E.; Reznicek, T.; Schweitzer, D.; Shuey, J.; Sferra, N.; Walker, S.; Wilsmann, L.; Rabe, M. Mitchell’s Satyr Butterfly Neonympa mitchellii mitchellii Recovery Plan; U.S. Department of the Interior Fish and Wildlife Service: Falls Church, VA, USA, 1998.
- Hart, B. A Survey for the Mitchell’s Satyr (Neonympha mitchellii French) in the National Forests of Alabama Final Report; Report for the US Fish and Wildlife Service: Falls Church, VA, USA, 2004.
- Goldstein, P.Z.; Hall, S.; Hart, B.; Roble, S.M.; Shuey, J. Evaluation of Relationships and Conservation Status Within the Neonympha mitchellii Complex (Lepidoptera: Nymphalidae); Report for US Fish and Wildlife Service: Falls Church, VA, USA, 2004.
- Hamm, C.A. Development of polymorphic anonymous nuclear DNA markers for the endangered Mitchell’s satyr butterfly, Neonympha mitchellii mitchellii (Lepidoptera: Nymphalidae). Conserv. Genet. Resour. 2012, 4, 127–128. [Google Scholar] [CrossRef]
- McAlpine, W.S.; Hubbell, S.P.; Pliske, T.E. The distribution, habits, and life history of Euptychia mitchellii (Satyridae). J. Lep. Soc. 1960, 14, 209–226. [Google Scholar]
- Kuefler, D.; Haddad, N.M.; Hall, S.; Hudgens, B.; Bartel, B.; Hoffman, E. Distribution, population structure and habitat use of the endangered Saint Francis Satyr butterfly, Neonympha mitchellii francisci. Am. Midl. Nat. 2008, 159, 298–320. [Google Scholar] [CrossRef]
- Hamm, C.A.; Rademacher, V.; Landis, D.A.; Williams, B.L. Conservation genetics and the implication for recovery of the endangered Mitchell’s Satyr butterfly, Neonympha mitchellii mitchellii. J. Hered. 2013, 105, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Crone, E.E.; Pickering, D.; Schultz, C.B. Can captive rearing promote recovery of endangered butterflies? An assessment in the face of uncertainty. Biol. Conserv. 2007, 139, 103–112. [Google Scholar] [CrossRef]
- De Roode, J.C.; Yates, A.J.; Altizer, S. Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. PNAS 2008, 105, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.; Hoy, M.A. Long PCR improves Wolbachia amplification: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000, 9, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. Lon. B Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Nice, C.C.; Gompert, Z.; Forister, M.L.; Fordyce, J.A. An unseen foe in arthropod conservation efforts: The case of Wolbachia infections in the Karner blue butterfly. Biol. Conserv. 2009, 142, 3137–3146. [Google Scholar] [CrossRef]
- Tolson, P. Rearing Mitchell’s satyr at the Toldeo Zoo-a first step towards eventual reintroduction in secure habitats. News J. Lepid. Soc. 2008, 50, 42–43. [Google Scholar]
- Hamm, C.A.; Handley, C.A.; Pike, A.; Forister, M.L.; Fordyce, J.A.; Nice, C.C. Wolbachia infection and Lepidoptera of conservation concern. J. Insect Sci. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Rose, O.C.; Brookes, M.I.; Mallet, J.L.B. A quick and simple nonlethal method for extracting DNA from butterfly wings. Mol. Ecol. 1994, 3, 275. [Google Scholar] [CrossRef]
- Morse, J.G.; Rugman-Jones, P.F.; Watson, G.W.; Robinson, L.J.; Bi, J.L.; Stouthamer, R. High levels of exotic armored scales on imported avocados raise concerns regarding USDA-APHIS’ phytosanitary risk assessment. J. Econ. Entomol. 2009, 102, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Simões, P.; Mialdea, G.; Reiss, D.; Sagot, M.F.; Charlat, S. Wolbachia detection: An assessment of standard PCR Protocols. Mol. Ecol. Resour. 2011, 11, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Baldo, L.; Hotopp, J.C.D.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
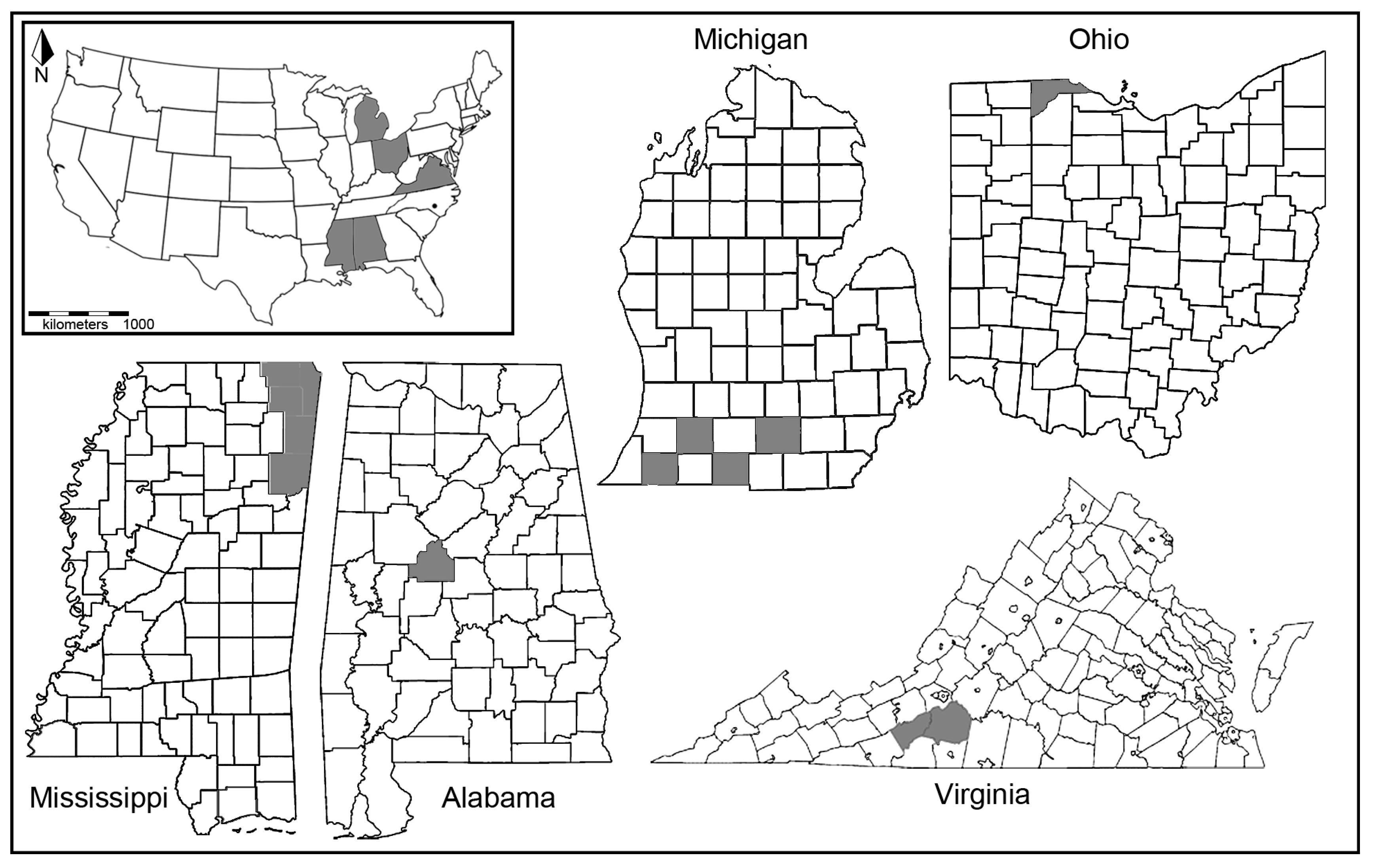
| Taxa | State | County | Number Tested | Number Positive for Wolbachia |
|---|---|---|---|---|
| Neonympha mitchelli mitchelli | AL | Bibb | 11 | - |
| MS | Alcorn | 1 | - | |
| MS | Prentis | 4 | 1 | |
| MS | Tishomingo | 6 | - | |
| MS | Itawamba | 1 | - | |
| MS | Monroe | 8 | 4 | |
| VA | Floyd | 2 | - | |
| VA | Franklin | 2 | - | |
| MI | Branch | 2 | - | |
| MI | Cass | 1 | 1 | |
| MI | Jackson | 3 | 1 | |
| MI | Kalamazoo | 2 | - | |
| OH | Toledo Zoo | 19 | - | |
| Neonympha mitchelli francisci | NC | Fort Bragg | 4 | - |
| Megisto cymela | AL | Baldwin | 2 | 1 |
| MS | Harrison | 2 | 1 | |
| MS | Tishomingo | 3 | 1 | |
| MS | Wilkininson | 1 | - | |
| TX | Blanco | 1 | 1 | |
| VA | Franklin | 1 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenner, J.; Seltzer, J.; Peyton, S.; Sullivan, H.; Tolson, P.; Walsh, R.P.; Hill, J.; Counterman, B.A. Demographic Variation of Wolbachia Infection in the Endangered Mitchell’s Satyr Butterfly. Insects 2017, 8, 50. https://doi.org/10.3390/insects8020050
Fenner J, Seltzer J, Peyton S, Sullivan H, Tolson P, Walsh RP, Hill J, Counterman BA. Demographic Variation of Wolbachia Infection in the Endangered Mitchell’s Satyr Butterfly. Insects. 2017; 8(2):50. https://doi.org/10.3390/insects8020050
Chicago/Turabian StyleFenner, Jennifer, Jennifer Seltzer, Scott Peyton, Heather Sullivan, Peter Tolson, Ryan P. Walsh, JoVonn Hill, and Brian A. Counterman. 2017. "Demographic Variation of Wolbachia Infection in the Endangered Mitchell’s Satyr Butterfly" Insects 8, no. 2: 50. https://doi.org/10.3390/insects8020050
APA StyleFenner, J., Seltzer, J., Peyton, S., Sullivan, H., Tolson, P., Walsh, R. P., Hill, J., & Counterman, B. A. (2017). Demographic Variation of Wolbachia Infection in the Endangered Mitchell’s Satyr Butterfly. Insects, 8(2), 50. https://doi.org/10.3390/insects8020050





