Monitoring Effect of Fire on Ant Assemblages in Brazilian Rupestrian Grasslands: Contrasting Effects on Ground and Arboreal Fauna
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sample Areas and Fire History
2.3. Ant Sampling
2.4. Statistical Analysis
3. Results
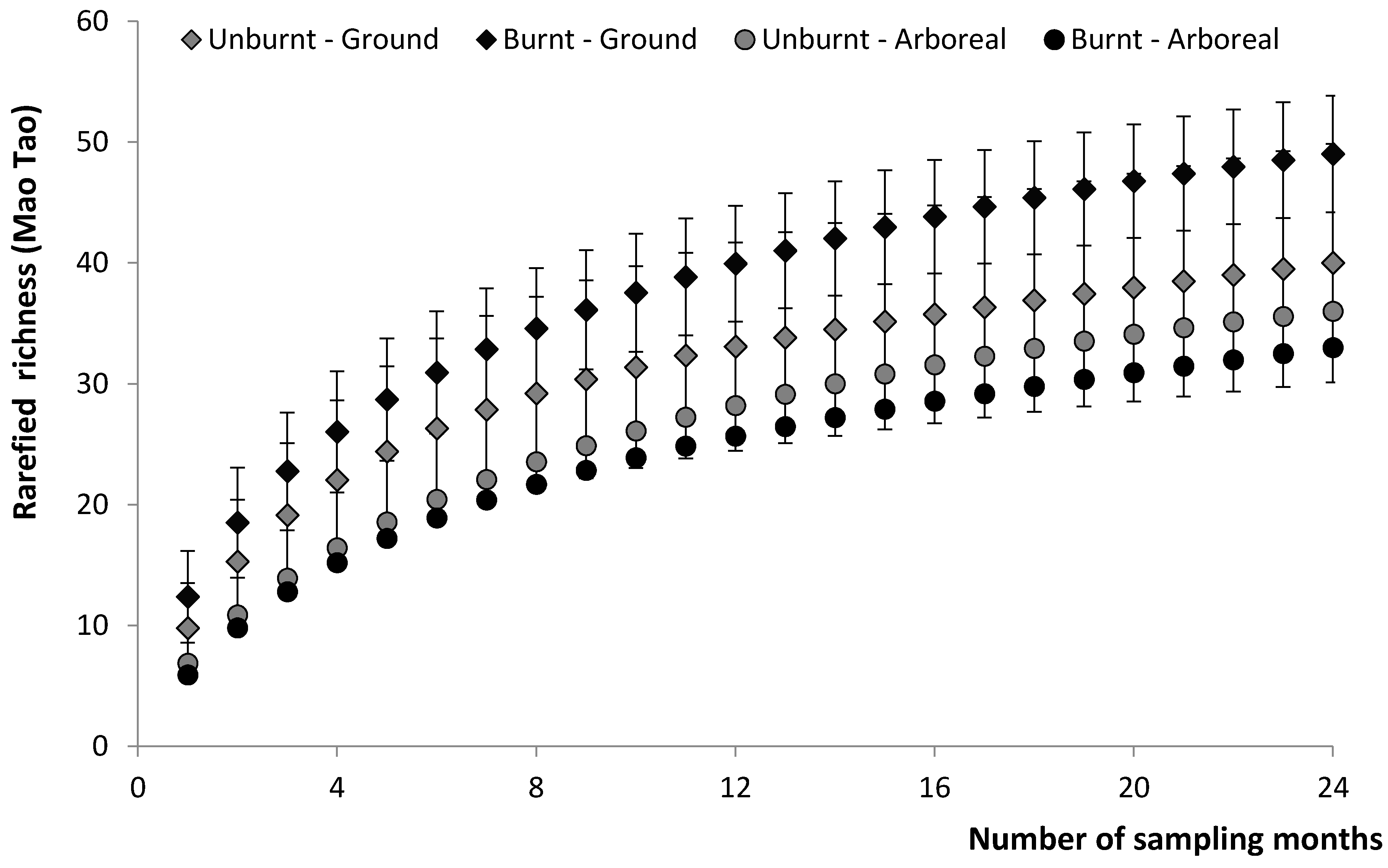
3.1. Cumulative Species Richness
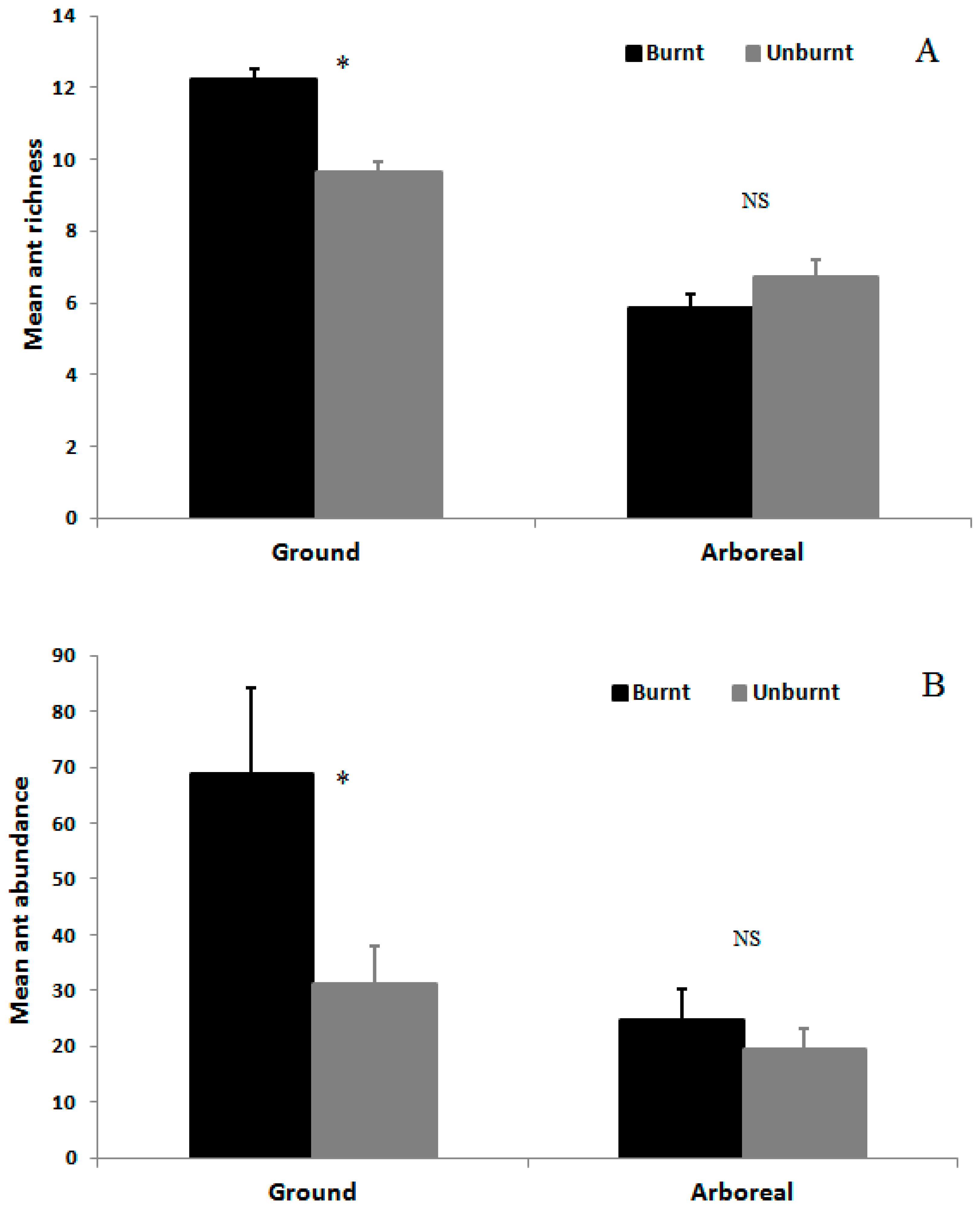
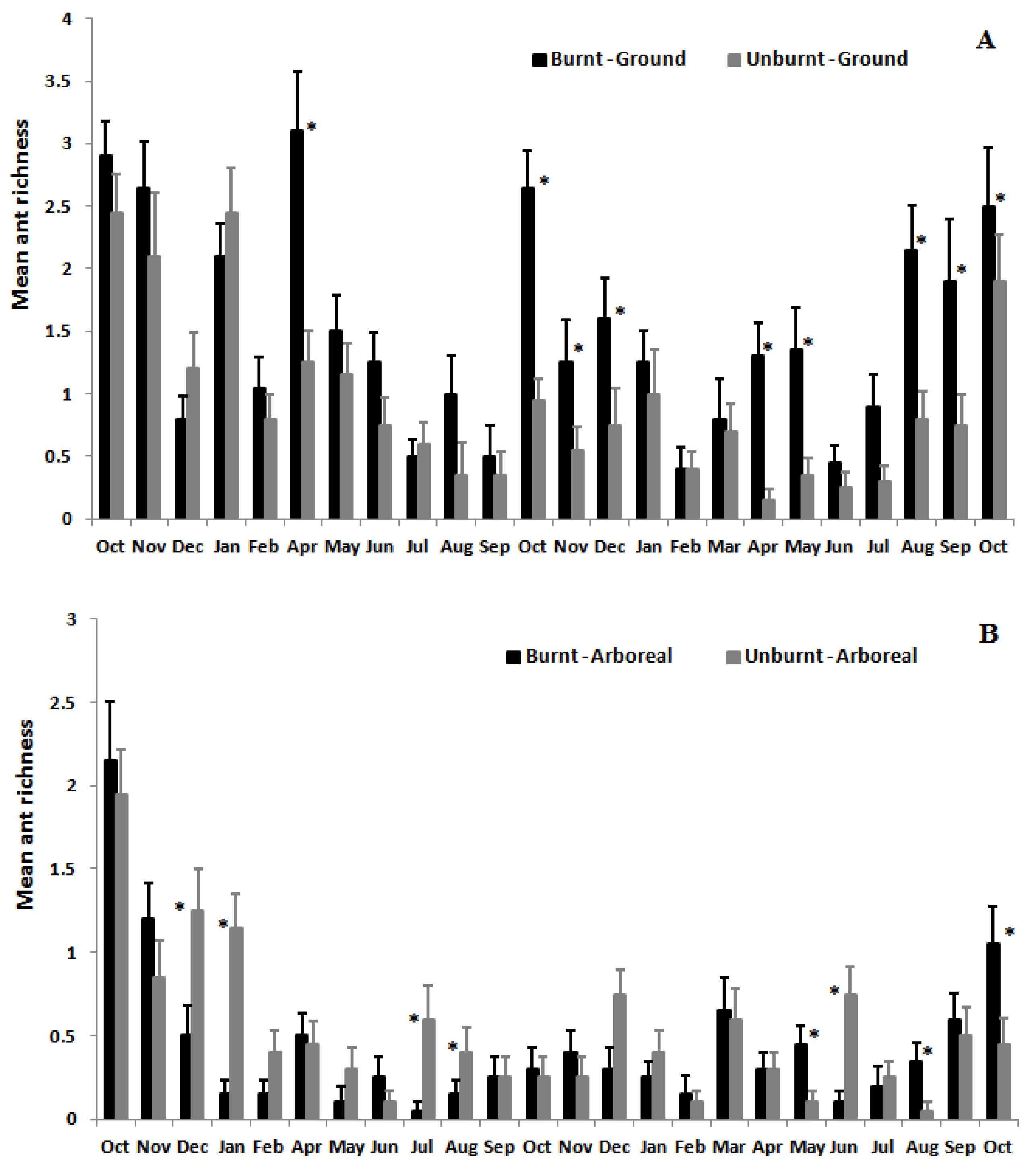
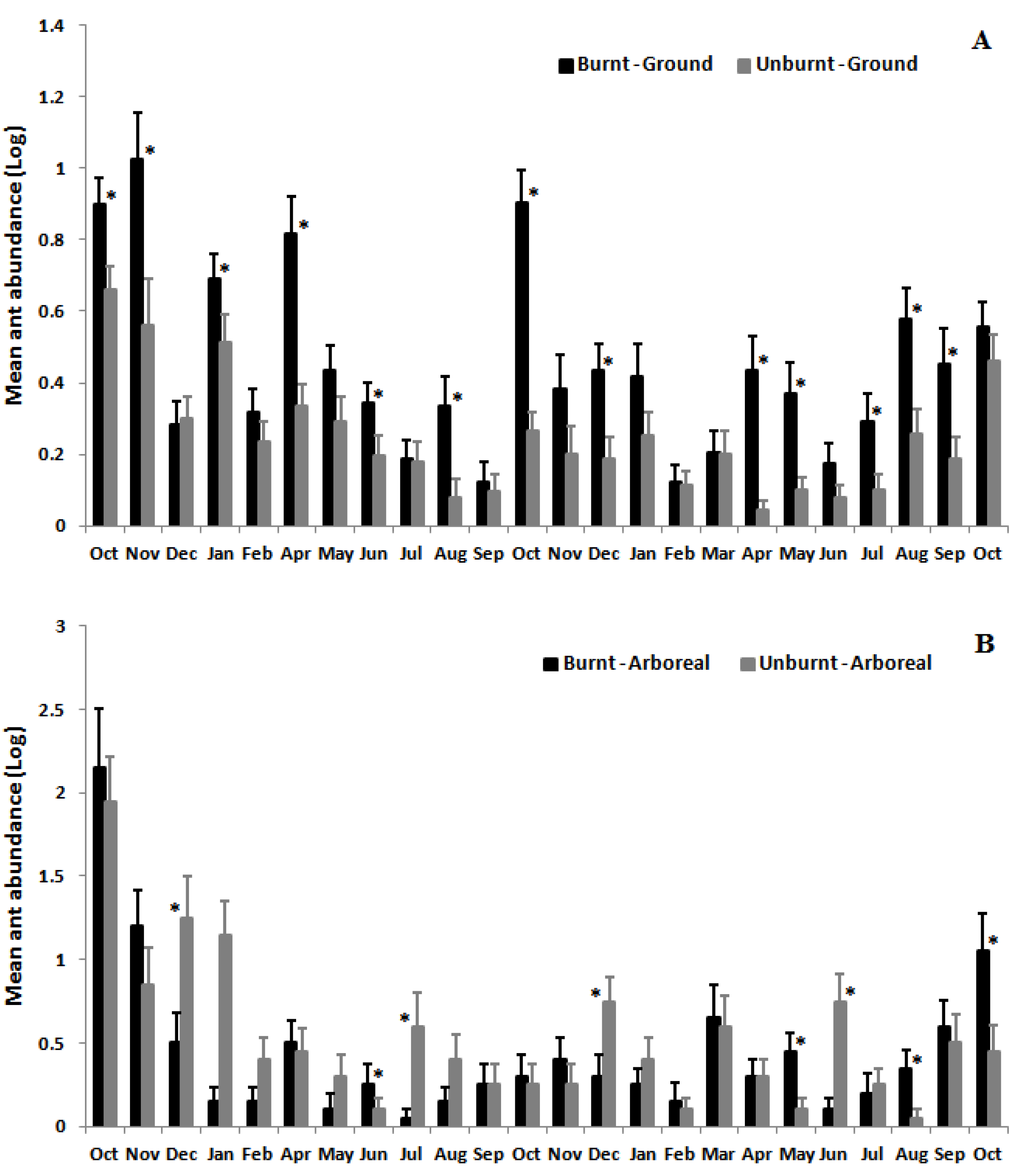
3.2. Ant Species Richness and Abundance Based on Mean Species Density per Sample per Month
3.3. Ant Species Composition
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandes, G.W. Ecology and Conservation of Mountaintop Grasslands in Brazil; Springer: Cham, Switzerland, 2016; pp. 1–561. [Google Scholar]
- Giulietti, A.M.; Pirani, J.R.; Harley, R.M. Espinhaço range region, eastern Brazil. In Centres of Plant Diversity: A Guide and Strategy for their Conservation; Davis, S.D., Ed.; Cambridge University Press: Cambridge, UK, 1997; Volume 3, pp. 397–404. [Google Scholar]
- Rapini, A.; Ribeiro, P.L.; Lambert, S.; Pirani, J.R. A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 2008, 4, 15–23. (In Portuguese) [Google Scholar]
- Silveira, F.A.O.; Negreiros, D.; Barbosa, N.P.; Lambers, H. Ecology and evolution of plant diversity in the endangered campo rupestre: A neglected conservation priority. Plant. Soil. 2016, 403, 129–152. [Google Scholar] [CrossRef]
- Le Stradic, S.; Silveira, F.A.O.; Buisson, E.; Cazelles, K.; Carvalho, V.; Fernandes, G.W. Diversity of germination strategies and seed dormancy in herbaceous species of campo rupestre grasslands. Austral Ecol. 2015, 40, 537–546. [Google Scholar] [CrossRef]
- Conceição, A.A.; Alencar, T.G.; Souza, J.M.; Moura, A.D.C.; Silva, G.A. Massive post-fire flowering events in a tropical mountain region of Brazil: High episodic supply of floral resources. Acta Bot. Bras. 2013, 27, 847–850. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Barbosa, N.P.U.; Negreiros, D.; Paglia, A.P. Challenges for the conservation of vanishing megadiverse rupestrian grasslands. Nat. Conserv. 2014, 2, 162–165. [Google Scholar] [CrossRef]
- Veldman, J.W.; Buisson, E.; Durigan, G.; Fernandes, G.W.; le Stradic, S.; Mahy, G.; Negreiros, D.; Overbeck, G.E.; Veldman, R.G.; Zaloumis, N.P.; et al. William J Bond Toward an oldgrowth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 2015, 13, 154–162. [Google Scholar] [CrossRef]
- Cochrane, M.A. Fire science for rainforests. Nature 2003, 421, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends. Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Anjos, D.; Alves-Silva, E.; Ribeiro, S.P. Do fire and seasonality affect the establishment and colonisation of litter arthropods? J. Insect. Conserv. 2016, 20, 653–661. [Google Scholar] [CrossRef]
- Frizzo, T.L.M.; Campos, R.I.; Vasconcelos, H.L. Contrasting effects of fire on arboreal and ground-dwelling ant communities of a Neotropical savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Andersen, A.N.; Ribbons, R.R.; Petti, M.; Parr, C.L. Burning for biodiversity: Highly resilient ant communities respond only to strongly contrasting fire regimes in Australias’s seasonal tropics. J. Appl. Ecol. 2014, 51, 1406–1413. [Google Scholar] [CrossRef]
- Maravalhas, J.; Vasconcelos, H.L. Revisiting the pyrodiversity-biodiversity hypothesis: Long term fire regimes and the structure of ant communities in a Neotropical savanna hotspot. J. Appl. Ecol. 2014, 51, 1–8. [Google Scholar] [CrossRef]
- Anjos, D.V.; Campos, R.B.F.; Ribeiro, S.P. Temporal turnover of species maintains ant diversity but transforms species assemblage. Sociobiology 2015, 62, 389–395. [Google Scholar] [CrossRef]
- Neves, F.S.; Lana, T.C.; Anjos, M.C.; Reis, A.C.; Fernandes, G.W. Ant Community in Burned and Unburned Sites in Campos Rupestres Ecosystem. Sociobiology 2016, 63, 628–636. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of ground-foraging ant communities to three experimental fire regimes in a savanna forest of tropical Australia. Biotropica 1991, 23, 575–585. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African savanna ants to long-term fire regimes. J. Appl. Ecol. 2004, 41, 630–642. [Google Scholar] [CrossRef]
- Silveira, J.M.; Barlow, J.; Andrade, R.B.; Mestre, L.A.M.; Lacau, S.; Cochrane, M.A. Responses of leaf-litter ant communities to tropical forest wildfires vary with season. J. Trop. Ecol. 2012, 28, 515–518. [Google Scholar] [CrossRef]
- Silveira, J.M.; Barlow, J.; Andrade, R.B.; Louzada, J.; Mestre, L.A.; Lacau, S.; Zanetti, R.; Numata, I.; Cochrane, M.A. The responses of leaf litter ant communities to wildfires in the Brazilian Amazon: A multi-region assessment. Biol. Conserv. 2013, 22, 513–529. [Google Scholar] [CrossRef]
- Paolucci, L.N.; Maia, M.L.; Solar, R.R.; Campos, R.I.; Schoereder, J.H.; Andersen, A.N. Fire in the Amazon: impact of experimental fuel addition on responses of ants and their interactions with myrmecochorous seeds. Oecologia 2016, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.M. Aspectos ecológicos do fogo no cerrado. I-A temperatura do solo durante as queimadas (1978). Rev. Bras. Bot. 1978, 1, 93–96. (In Portuguese) [Google Scholar]
- Miranda, A.C.; Miranda, H.S.; Dias, I.D.O.; Dias, B.F.D. Soil and air temperatures during prescribed cerrado fires in Central Brazil. J. Trop. Ecol. 1993, 9, 313–320. [Google Scholar] [CrossRef]
- Morais, H.C.; Benson, W.W. Recolonização de vegetação de Cerrado após queimada por formigas arborícolas. Rev. Bras. Biol. 1988, 48, 459–466. [Google Scholar]
- Semir, J. Revisão taxonômica de Lychnophora Mart. (Vernoniaceae: Compositae). Ph.D. Thesis, State University of Campinas, Campinas, Brazil, 1991. [Google Scholar]
- Fujaco, M.A.G.; Leite, M.G.P.; Messias, M.C.T. Análise multitemporal das mudanças no uso e ocupação do Parque Estadual do Itacolomi (MG) através de técnicas de geoprocessamento. Revista Escola de Minas 2010, 63, 695–701. (In Portuguese) [Google Scholar] [CrossRef]
- Gastauer, K.; Messias, M.C.T.; Neto, J.A.A.M. Floristic Composition, Species Richness and Diversity of Campo Rupestre Vegetation from the Itacolomi State Park, Minas Gerais. Environ. Nat. Resour. Res. 2012, 2, 115–128. [Google Scholar] [CrossRef]
- Ribeiro, S.P.; Department of Biodiversity, Evolution and Environment, Federal University of Ouro Preto. Ouro Preto, MG, Brazil. Personal communication, 2010.
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9.1.0. 2013. Available online: http://viceroy.eeb.uconn.edu/estimates (accessed on 20 April 2016).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 2010; pp. 1–944. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; Available online: http://www.R-project.org/ (accessed on 5 May 2016).
- Brower, J.C.; Kile, K.M. Sedation of an original data matrix as applied to paleoecology. Lethaia 1988, 21, 79–93. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-Palaeontological Statistics, ver. 1.89. Palaeontolog. Electron. 2001, 4, 1–9. [Google Scholar]
- Costa-Milanez, C.B.; Ribeiro, F.F.; Castro, P.T.A.; Ribeiro, S.P.; Majer, J.N. Effect of Fire on Ant Assemblages in Veredas of Brazilian Cerrado. Sociobiology 2015, 62, 494–505. [Google Scholar] [CrossRef]
- Simon, M.F.; Grether, R.; Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.B.; Sampaio, A.B.; Borghetti, F. Efeitos da época de queima sobre a reprodução sexuada e estrutura populacional de Heteropterys pteropetala (Adr. Juss.), Malpighiaceae, em áreas de Cerrado sensu stricto submetidas a queimas bienais. Acta Bot. Bras. 2005, 19, 927–934. (In Portuguese) [Google Scholar] [CrossRef]
- Alves-silva, E. Post fire resprouting of Banisteriopsis malifolia (Malpighiaceae) and the role of extrafloral nectaries on the associated ant fauna in a Brazilian Savanna. Sociobiology 2011, 58, 327–339. [Google Scholar]
- Alves-Silva, E.; Del-Claro, K. Effect of post-fire resprouting on leaf fluctuating asymmetry, extrafloral nectar quality, and ant–plant–herbivore interactions. Naturwissenschaften 2013, 100, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.L.; Pacheco, R.; Silva, R.C.; Vasconcelos, P.B.; Lopes, C.T.; Costa, N.A.; Bruna, E.M. Dynamics of the leafer litter arthropod fauna following fire in a Neotropical Woodland Savanna. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Yen, L. Immediate effects of fire on ants in the semi-arid mallee region of northwestern Victoria. Aust. J. Ecol. 1985, 10, 25–30. [Google Scholar] [CrossRef]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995; pp. 1–345. [Google Scholar]
- Andersen, A.N.; Muller, W.J. Arthropod responses to experimental fire regimes in a Australia tropical savanah: Ordinal-level analysis. Austral Ecol. 2000, 25, 199–209. [Google Scholar] [CrossRef]
- Byk, J.; Del-Claro, K. Ant-plant interaction in the Neotropical Savanna: Direct beneficial effects of extrafloral nectar on ant colony fitness. Popul. Ecol. 2011, 53, 327–332. [Google Scholar] [CrossRef]
- Fagundes, R.; Anjos, D.V.; Carvalho, R.L.; Del-Claro, K. Availability of food and nesting-sites as regulatory mechanisms for the recovery of ant diversity after fire disturbance. Sociobiology 2015, 62, 1–9. [Google Scholar]
- Cochrane, M.A.; Schulze, M.D. Fire as a recurrent event in Tropical Forests of the eastern amazon: Effects on forest structure, biomass, and species composition. Biotropica 1999, 31, 2–16. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Verssimo, A.; Alencar, A.; Nobre, C.; Lima, E.; Lefebvre, P.; Schlesinger, P.; Potter, C.; Moutinho, P.; Mendoza, E.; et al. Large-scale impoverishment of Amazonian forests by logging and fire. Nature 1999, 398, 505–508. [Google Scholar] [CrossRef]
- Wilson, E.O. Pheidole in the New World: A Dominant, Hyperdiverse Ant Genus; Harvard University Press: Cambridge, MA, USA, 2003; pp. 1–791. [Google Scholar]
- De Andrade, M.L.; Baroni-Urbani, C. Diversity and adaptation in the ant genus Cephalotes, past and present. Stuttg. Beitr. Naturkunde Ser. B (Geol. Paläontologie) 1999, 271, 1–889. [Google Scholar]
- Powell, S. Ecological specialization and the evolution of a specialized caste in Cephalotes ants. Funct. Ecol. 2008, 22, 902–911. [Google Scholar] [CrossRef]
- Ribas, C.R.; Shoereder, J.H.; Mireille, P.; Soares, S.M. Tree heterogeneity, resource availability, and larger scale processes regulating arboreal ant species richness. Austral Ecol. 2003, 28, 305–314. [Google Scholar] [CrossRef]
- Silva, R.R.; Brandão, C.R.F.; Silvestre, R. Similarity between Cerrado localities in central and southeastern Brazil based on the dry season bait visitors ant fauna. Stud. Neotrop. Fauna Environ. 2004, 39, 191–199. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Leite, M.F.; Vilhena, J.M.S.; Lima, A.P.; Magnusson, W.E. Ant diversity in a Amazonia Savanna: Relationship with vegetation structure, disturbance by fire, and dominant ants. Austral Ecol. 2008, 33, 221–231. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T.A. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Campos, R.B.F.; Schoereder, J.H.; Speber, C.F. Small-scale patch dynamics after disturbance in litter ant communities. Basic. Appl. Ecol. 2007, 8, 36–43. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjos, D.; Campos, R.; Campos, R.; Ribeiro, S. Monitoring Effect of Fire on Ant Assemblages in Brazilian Rupestrian Grasslands: Contrasting Effects on Ground and Arboreal Fauna. Insects 2017, 8, 64. https://doi.org/10.3390/insects8030064
Anjos D, Campos R, Campos R, Ribeiro S. Monitoring Effect of Fire on Ant Assemblages in Brazilian Rupestrian Grasslands: Contrasting Effects on Ground and Arboreal Fauna. Insects. 2017; 8(3):64. https://doi.org/10.3390/insects8030064
Chicago/Turabian StyleAnjos, Diego, Ricardo Campos, Renata Campos, and Sérvio Ribeiro. 2017. "Monitoring Effect of Fire on Ant Assemblages in Brazilian Rupestrian Grasslands: Contrasting Effects on Ground and Arboreal Fauna" Insects 8, no. 3: 64. https://doi.org/10.3390/insects8030064





