Trap Nesting Wasps and Bees in Agriculture: A Comparison of Sown Wildflower and Fallow Plots in Florida
Abstract
1. Introduction
2. Experimental Section
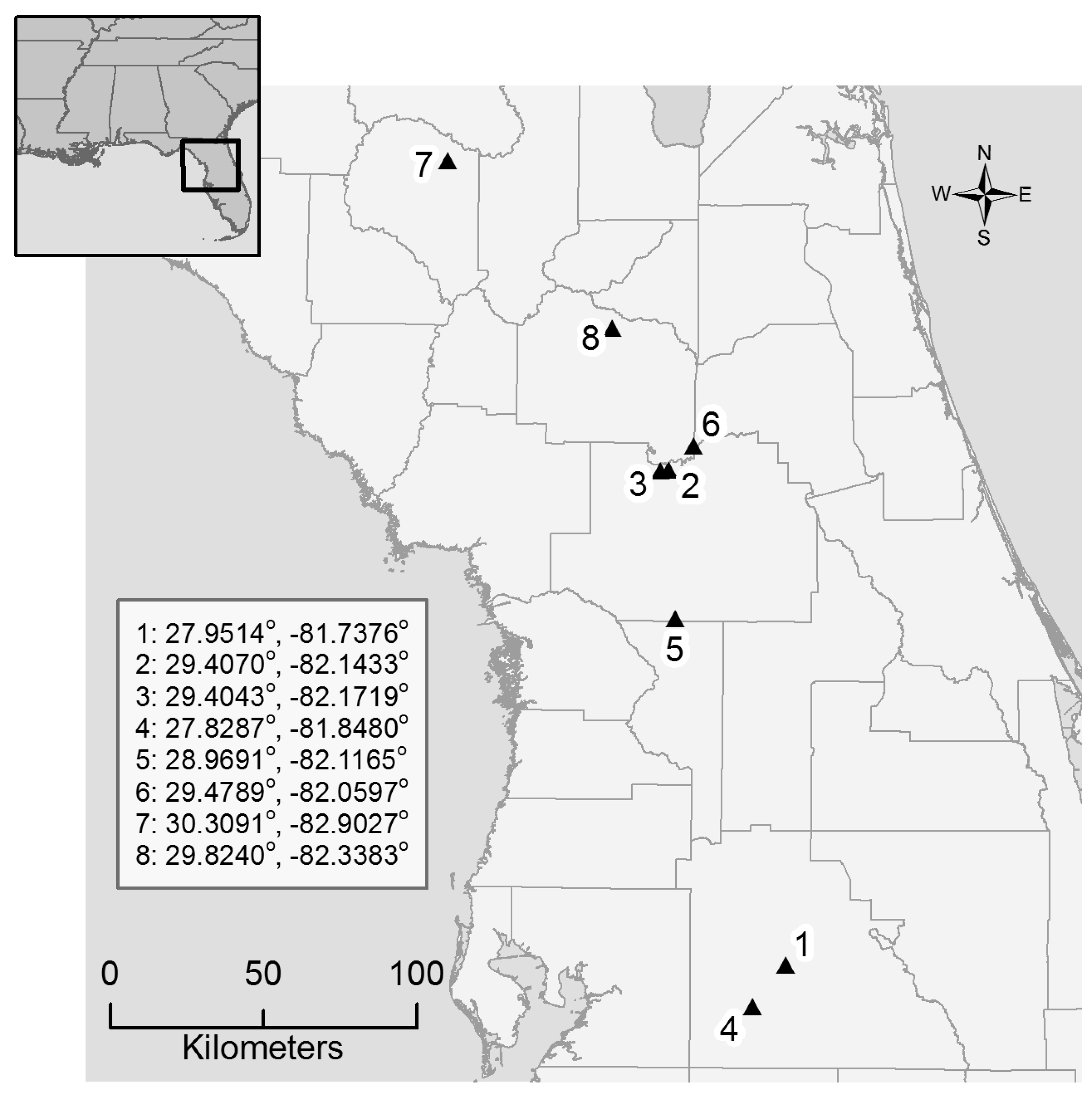
2.1. Study Sites
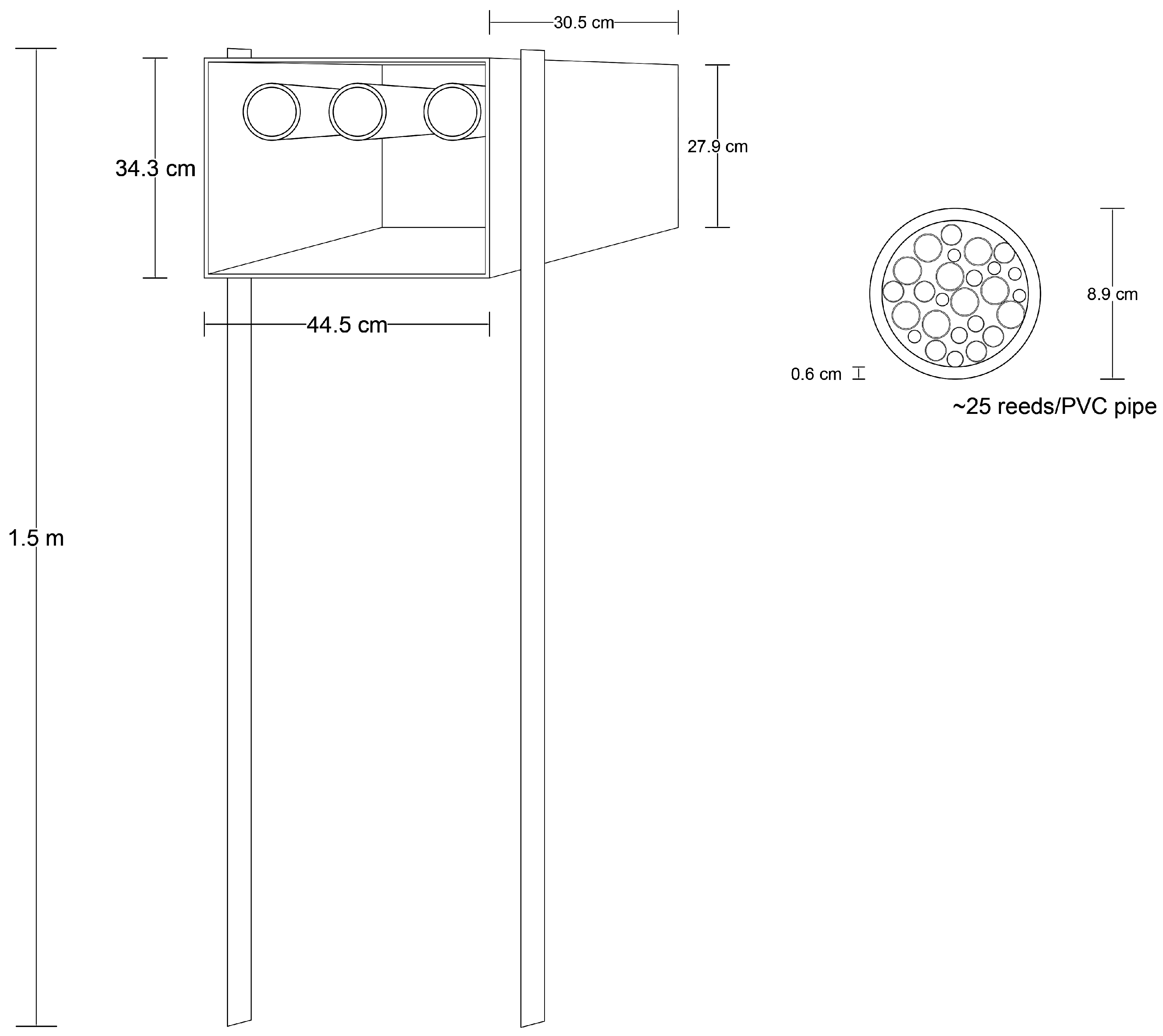
2.2. Trap-Nests
2.3. Data Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C. Ancistrocerus gazella (Hymenoptera: Vespoidea: Eumenidae): A potentially useful biological control agent for leafrollers Planotortrix octo, P. excessana, Ctenopseustis oblinquana, C. herana and Epiphyas postvittana (Lepidoptera: Tortricidae) in New Zealand. N. Z. J. Crop Hortic. Sci. 1994, 22, 235–238. [Google Scholar] [CrossRef]
- Holzschuh, A.; Steffan-Dewenter, I.; Tscharntke, T. How do landscape composition and configuration, organic farming and fallow strips affect the diversity of bees, wasps and their parasitoids? J. Anim. Ecol. 2010, 79, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I. Landscape context affects trap-nesting bees, wasps, and their natural enemies. Ecol. Entomol. 2002, 27, 631–637. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Evol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- McLaughlin, A.; Mineau, P. The impact of agricultural practices on biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Carreck, N.L.; Williams, I.H. Food for insect pollinators on farmland: Insect visits to flowers of annual seed mixtures. J. Insect Conserv. 2002, 6, 13–23. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Augmenting flower trait diversity in wildflower strips to optimize the conservation of arthropod functional groups for multiple agroecosystem services. J. Insect Conserv. 2014, 18, 713–728. [Google Scholar] [CrossRef]
- Feltham, H.; Park, K.; Minderman, J.; Goulson, D. Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol. Evol. 2015, 5, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Haaland, C.; Bersier, L.F. What can sown wildflower strips contribute to butterfly conservation? An example from a Swiss lowland agricultural landscape. J. Insect Conserv. 2011, 15, 301–309. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Moonen, A.C. Field margins in northern Europe: Their functions and interactions with agriculture. Agric. Ecosyst. Environ. 2002, 89, 5–21. [Google Scholar] [CrossRef]
- Krombein, K.V. Trap-Nesting Wasps and Bees: Life Histories, Nests and Associates; Smithsonian Press: Washington, DC, USA, 1967. [Google Scholar]
- Jenkins, D.A.; Matthews, R.W. Cavity-nesting Hymenoptera in disturbed habitats of Georgia and South Carolina: Nest architecture and seasonal occurrence. J. Kans. Entomol. Soc. 2004, 77, 203–214. [Google Scholar] [CrossRef]
- Tscharntke, T.; Gathmann, A.; Steffan-Dewenter, I. Bioindication using trap-nesting bees and wasps and their natural enemies: Community structure and interactions. J. Appl. Ecol. 1998, 35, 708–719. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W. Pollinators, flowering plants, and conservation biology. BioScience 1997, 47, 297–307. [Google Scholar] [CrossRef]
- Matsumura, C.; Washitani, I. Effects of population size and pollinator limitation on seed-set of Primula sieboldii populations in a fragmented landscape. Ecol. Res. 2000, 15, 307–322. [Google Scholar] [CrossRef]
- Graham, J.R.; Tan, Q.; Jones, L.C.; Ellis, J.D. Native buzz: Citizen scientists creating nesting habitat for solitary bees and wasps. Fla. Sci. 2014, 77, 204–218. [Google Scholar]
- Graham, J.R. Monitoring Tunnel Nesting, Solitary Bees and Wasps in North Central Florida USING Artificial Nesting Habitats. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2014. [Google Scholar]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullmann, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.B. Bees of the Eastern United States: Vol II; North Carolina Agricultural Experiment Station: Salisbury, NC, USA, 1962. [Google Scholar]
- O’Neill, K.M. Solitary Wasps: Behavior and Natural History; Comstock Publishing Associates, Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Korpela, E.L.; Hyvöenen, T.; Lindgren, S.; Kuussaari, M. Can pollination services, species diversity and conservation be simultaneously promoted by sown wildflower strips on farmland? Agric. Ecosyst. Environ. 2013, 179, 18–24. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Wäckers, F.L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol. Control 2008, 46, 400–408. [Google Scholar] [CrossRef]
- Balmer, O.; Gѐneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower companion plants increase pest parasitation and yield in cabbage fields: Experimental demonstration and call for caution. Biol. Control 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Hunt, J.H.; Brown, P.A.; Sago, K.M.; Kerker, J.A. Vespid wasps eat pollen (Hymenoptera: Vespidae). J. Kans. Entomol. Soc. 1991, 64, 127–130. [Google Scholar]
- Gathmann, A.; Greiler, H.J.; Tscharntke, T. Trap-nesting bees and wasps colonizing set-aside fields: Succession and body size, management by cutting and sowing. Oecologia 1994, 98, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pfiffner, L.; Luka, H.; Schlatter, C.; Juen, A.; Traugott, M. Impact of wildflower strips on biological control of cabbage lepidopterans. Agric. Ecosyst. Environ. 2009, 129, 310–314. [Google Scholar] [CrossRef]
- Fricke, J.M. Trap-nest bore diameter preferences among sympatric Passaloecus spp. (Hymenoptera: Sphecidae). Gt. Lakes Entomol. 1991, 24, 123–125. [Google Scholar]
- Bohart, G.E. How to manage the alfalfa leaf-cutting bee (Megachile rotundata Fabr.) for alfalfa pollination. Utah Agric. Exp. Stn. Circ. 1962, 144, 1–7. [Google Scholar]
- Bosch, J.; Kemp, W.P. Developing and establishing bee species as crop pollinators: The example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar] [PubMed]
- Smith, S. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Genaro, J.A. Origins, composition and distribution of the bees of Cuba (Hymenoptera: Apoidea: Anthophila). Insecta Mundi 2008, 0052, 1–16. [Google Scholar]
- Krueger, R.; Wang, K.H.; McSorley, R.; Gallaher, R.N. Artificial and natural pollination of sunn hemp in Florida. Proc. Fla. State Hort. Soc. 2008, 121, 234–237. [Google Scholar]
- Holzschuh, A.; Steffan-Dewenter, I.; Kleijn, D.; Tscharntke, T. Diversity of flower-visiting bees in cereal fields: Effects of farming system, landscape composition and regional context. J. Appl. Ecol. 2007, 44, 41–49. [Google Scholar] [CrossRef]
- Benjamin, F.E.; Reilly, J.R.; Winfree, R. Pollinator body size mediates the scale at which land use drives crop pollination services. J. Appl. Ecol. 2014, 51, 440–449. [Google Scholar] [CrossRef]
- Fortel, L.; Henry, M.; Guilbaud, L.; Mouret, H.; Vaissière, B.E. Use of human-made nesting structures by wild bees in an urban environment. J. Insect Conserv. 2016, 20, 239–253. [Google Scholar] [CrossRef]

| Plant List | Type | Seed Rate (kg/ha) |
|---|---|---|
| Partridge Pea (Chamaecrista fasciculata) | Annual | 1.12 * |
| Goldenmane Tickseed (Coreopsis basalis) | Annual | 1.01 |
| Lanceleaf Tickseed (Coreopsis lanceolata) | Perennial | 0.19 |
| Leavenworth's Coreopsis (Coreopsis leavenworthii) | Perennial | 0.19 |
| Indian Blanket (Gaillardia pulchella) | Annual | 3.03 |
| Swamp Sunflower (Helianthus angustifolius) | Perennial | 0.37 |
| Spotted Beebalm (Monarda punctata) | Perennial | 0.15 |
| Blackeyed Susan (Rudbeckia hirta) | Annual | 0.59 |
| Tall Ironweed (Vernonia angustifolia) | Perennial | 0.69 |
| Order | Family | Species | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Total | Food/Food Breadth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hymenoptera (Wasps) | Chrysididae | C. inaequidens/wasbaueri | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | Wasp/Bee |
| Chrysis inaequidens | 0 | 1 | 0 | 4 | 5 | 3 | 5 | 1 | 19 | Wasp/Bee | ||
| Chrysis nisseri | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 7 | Wasp/Bee | ||
| Chrysis pellucidula/remissa | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | Wasp/Bee | ||
| Chrysis remissa | 0 | 4 | 0 | 0 | 0 | 14 | 1 | 0 | 19 | Wasp/Bee | ||
| Chrysis tripartita | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | Wasp/Bee | ||
| Chrysis wasbaueri | 0 | 3 | 0 | 1 | 0 | 1 | 1 | 4 | 10 | Wasp/Bee | ||
| Leucospididae | Leucospis affinis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | Bee | |
| Crabronidae | Trypoxylon collinum | 0 | 8 | 1 | 0 | 8 | 1 | 0 | 1 | 19 | Araneae | |
| Trypoxylon lacitarse | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | Araneae | ||
| Sphecidae | Isodontia auripes | 0 | 14 | 14 | 1 | 6 | 0 | 0 | 9 | 44 | Orthoptera | |
| Isodontia Mexicana | 1 | 15 | 2 | 0 | 2 | 0 | 14 | 2 | 36 | Orthoptera | ||
| Vespidae | Euodynerus annulatus | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 0 | 6 | Lepidoptera | |
| Euodynerus hildalgo | 0 | 8 | 0 | 3 | 0 | 3 | 2 | 0 | 16 | Lepidoptera | ||
| Euodynerus megaera | 0 | 6 | 0 | 0 | 30 | 18 | 13 | 36 | 103 | Lepidoptera | ||
| Monobia quadridens | 0 | 11 | 0 | 0 | 3 | 3 | 0 | 12 | 29 | Lepidoptera | ||
| Pachodynerus erynnis | 2 | 36 | 22 | 24 | 5 | 40 | 37 | 3 | 169 | Lepidoptera | ||
| Pachodynerus nasidens | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 7 | Lepidoptera | ||
| Parancistrocerus pedestris | 3 | 29 | 7 | 0 | 1 | 5 | 29 | 6 | 80 | Lepidoptera | ||
| Parancistrocerus fulvipes | 0 | 23 | 13 | 0 | 0 | 4 | 14 | 10 | 64 | Lepidoptera | ||
| Hymenoptera (Bees) | Megachilidae | Megachile lanata | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | Polylectic |
| Megachile parallela | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | Polylectic | ||
| Megachile policaris | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | Polylectic | ||
| Megachile xylocopoides | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | Polylectic | ||
| Diptera | Bombyliidae | Toxophora amphitea | 1 | 2 | 0 | 1 | 1 | 1 | 3 | 0 | 9 | Wasp/Bee |
| Sarcophagidae | Miltogramminae | 1 | 3 | 0 | 1 | 1 | 1 | 2 | 1 | 10 | Wasp/Bee | |
| (incl. Amobia sp., Senotainia sp., Phrosinella sp.) | ||||||||||||
| Coleoptera | Ripiphoridae | Macrosiagon cruenta | 1 | 5 | 1 | 2 | 2 | 1 | 1 | 3 | 16 | Wasp |
| Macrosiagon sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | Wasp | ||
| Total | 9 | 170 | 60 | 56 | 65 | 101 | 130 | 95 | 686 |
| Avg. #/Reed | Range | |
|---|---|---|
| Isodontia auripes | 3.0 (0.3) | 1–8 |
| Isodontia mexicana | 3.7 (0.3) | 1–7 |
| Trypoxylon spp. | 3.9 (0.5) | 1–10 |
| Euodynerus annulatus | 2.2 (0.5) | 1–3 |
| Euodynerus hildalgo | 3.1 (0.7) | 1–11 |
| Euodynerus megaera | 2.0 (0.4) | 1–8 |
| Monobia quadridens | 1.0 (0.3) | 1–4 |
| Pachodynerus erynnis | 2.8 (0.2) | 1–7 |
| Pachodynerus nasidens | 1.0 (0.4) | 1–4 |
| Parancistrocerus pedestris | 1.1 (0.1) | 1–6 |
| Parancistrocerus fulvipes | 1.0 (0.2) | 1–3 |
| Megachile spp. | 2.7 (0.6) | 1–6 |
| Fallow Controls | Wildflower Plots | Ptrt (df = 7) | |
|---|---|---|---|
| Euodynerus megaera | 1.9 (1.7) | 1.6 (1.0) | 0.85 |
| Isodontia auripes | 1.8 (1.6) | 2.0 (1.1) | 0.56 |
| Isodontia mexicana | 1.1 (0.7) | 3.1 (1.5) | 0.08 |
| Pachodynerus erynnis | 8.0 (3.3) | 6.1 (2.3) | 0.90 |
| Parancistrocerus pedestris | 4.6 (2.2) | 4.6 (1.9) | 0.90 |
| Parancistrocerus fulvipes | 2.5 (1.0) | 1.8 (0.9) | 0.55 |
| Total parasites | 3.1 (1.5) | 2.3 (1.0) | 0.66 |
| Species richness | 4.4 (0.8) | 5.2 (0.7) | 0.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, J.W.; Smithers, C.; Irvin, A.; Kimmel, C.B.; Stanley-Stahr, C.; Daniels, J.C.; Ellis, J.D. Trap Nesting Wasps and Bees in Agriculture: A Comparison of Sown Wildflower and Fallow Plots in Florida. Insects 2017, 8, 107. https://doi.org/10.3390/insects8040107
Campbell JW, Smithers C, Irvin A, Kimmel CB, Stanley-Stahr C, Daniels JC, Ellis JD. Trap Nesting Wasps and Bees in Agriculture: A Comparison of Sown Wildflower and Fallow Plots in Florida. Insects. 2017; 8(4):107. https://doi.org/10.3390/insects8040107
Chicago/Turabian StyleCampbell, Joshua W., Cherice Smithers, Allyn Irvin, Chase B. Kimmel, Cory Stanley-Stahr, Jaret C. Daniels, and James D. Ellis. 2017. "Trap Nesting Wasps and Bees in Agriculture: A Comparison of Sown Wildflower and Fallow Plots in Florida" Insects 8, no. 4: 107. https://doi.org/10.3390/insects8040107
APA StyleCampbell, J. W., Smithers, C., Irvin, A., Kimmel, C. B., Stanley-Stahr, C., Daniels, J. C., & Ellis, J. D. (2017). Trap Nesting Wasps and Bees in Agriculture: A Comparison of Sown Wildflower and Fallow Plots in Florida. Insects, 8(4), 107. https://doi.org/10.3390/insects8040107







