Impact of Subolesin and Cystatin Knockdown by RNA Interference in Adult Female Haemaphysalis longicornis (Acari: Ixodidae) on Blood Engorgement and Reproduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ticks and Animals
2.2. Salivary Glands
2.3. Total RNA Extraction and Synthesis of Complementary DNA from Tick Salivary Glands
2.4. RT-PCR for Detecting Salivary Cystatin and Subolesin
2.5. Purification of PCR Product and Sequencing
2.6. Synthesis of Double-Stranded RNA
2.7. Injection of Double-Stranded RNA
2.8. Analysis of Gene Silencing at Messenger RNA-Level by Real-Time Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
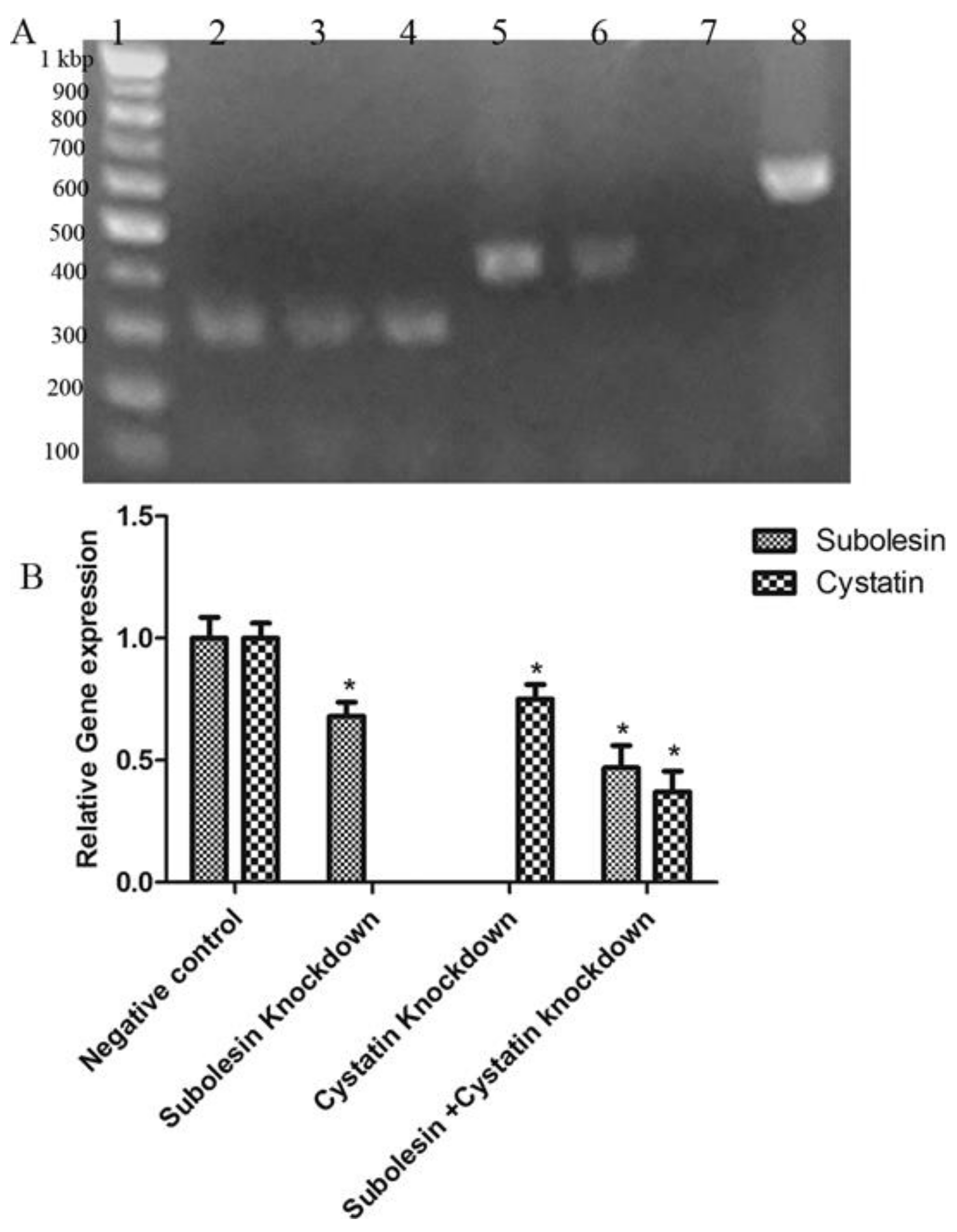
3.1. Detection of Salivary Cystatin and Subolesin by Real-Time Polymerase Chain Reaction and Sequencing
3.2. Silencing of Subolesin and Cystatin by RNA Interference
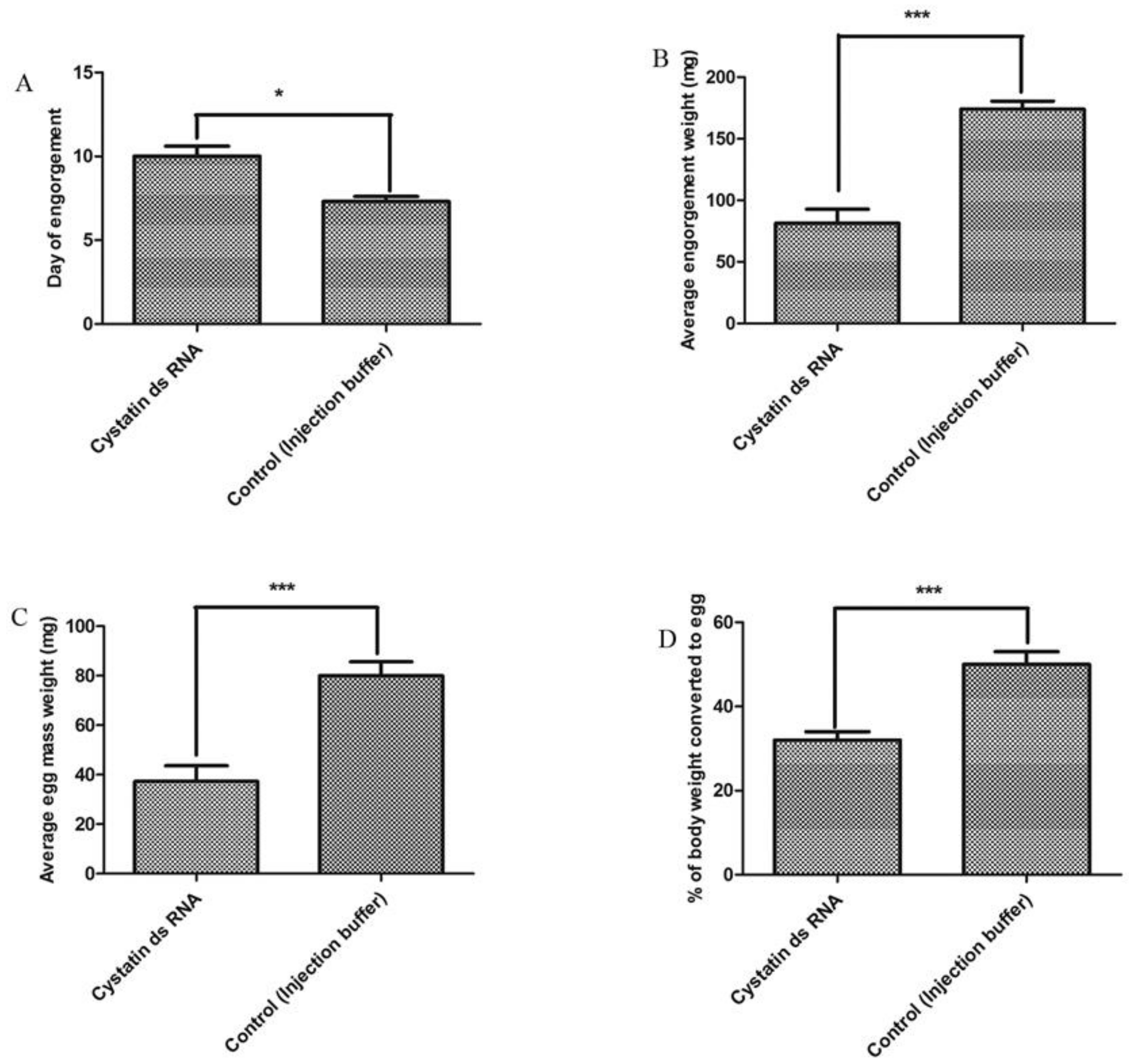
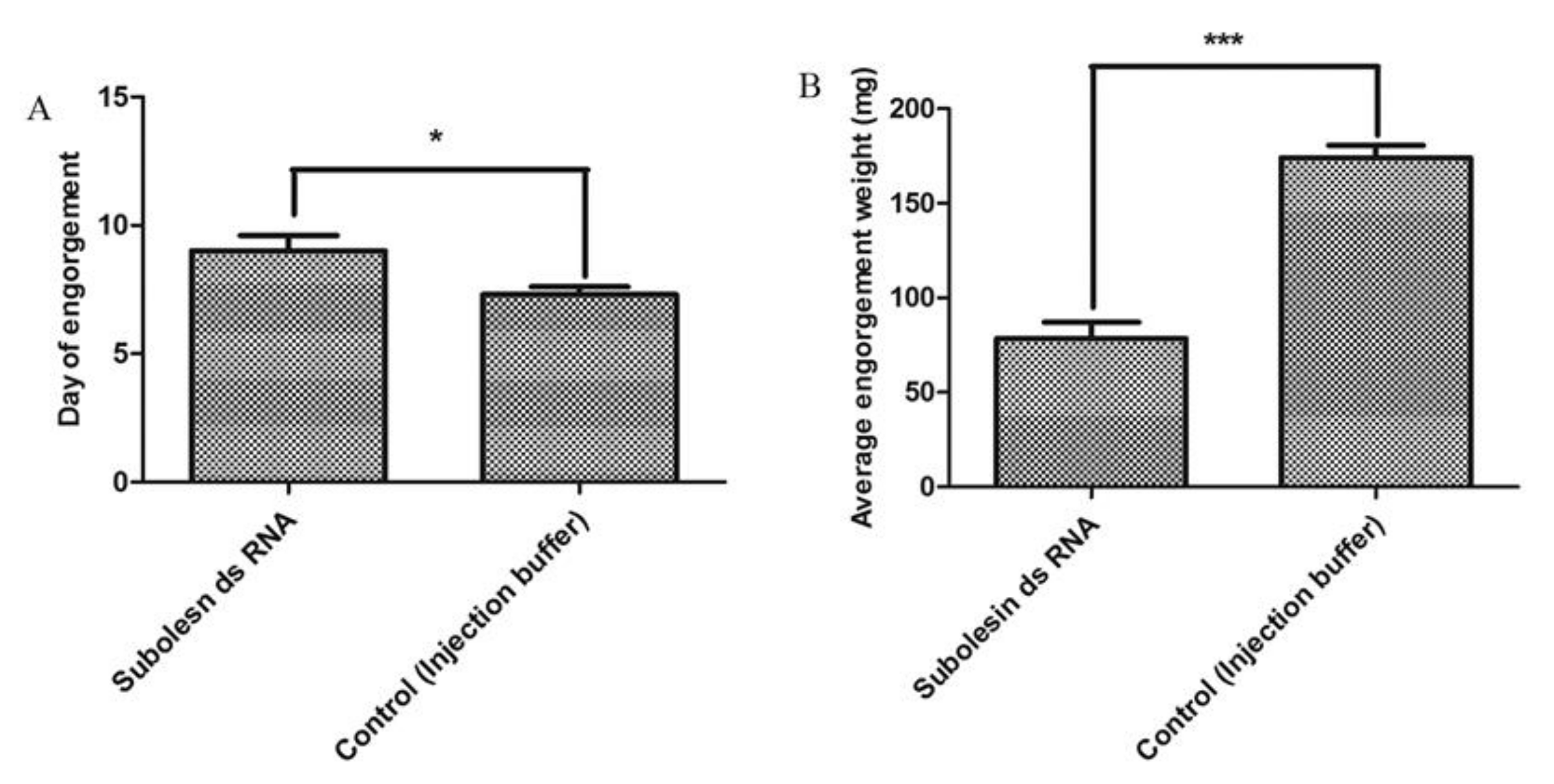
3.3. Effects on Feeding Duration and Engorgement
3.4. Combined Effects on Feeding Duration and Engorgement
3.5. Effects on Reproduction
3.6. Combined Effects on Reproduction
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Duggan, M.F. Management of arthropod vector data—Social and ecological dynamics facing the One Health perspective. Acta Trop. 2018, 182, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Canale, A.; Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases? Parasitol. Res. 2016, 115, 2545–2560. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Divya, M.; Abhinaya, M.; Gobi, N.; Bhattacharyya, A.; Balashanmugam, N.; Surmistha, D.; Murugan, K.; et al. Ecotoxicity of Musa paradisiaca leaf extract-coated Zno nanoparticles to the freshwater microcrustacean Ceriodaphnia cornuta. Limnologica 2017, 67, 1–6. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lupi, E.; Hatz, C.; Schlagenhauf, P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.—A literature review. Travel Med. Infect. Dis. 2013, 11, 374–411. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Moreau, E.; Liu, J.; Hao, X.; Ma, M.; Luo, J.; Chauvin, A.; Yin, H. Babesia sp. Bq1 (lintan): Molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 2010, 59, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.S.; Jang, W.J.; Koh, S.E.; Park, T.K.; Kang, S.S.; Kim, B.J.; Kook, Y.H.; Park, K.H.; Lee, S.H. Identification of the Coxiella sp. Detected from Haemaphysalis longicornis ticks in Korea. Microbiol. Immunol. 2004, 48, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.S.; Jung, K.D.; Jang, W.J.; Koh, S.E.; Kang, S.S.; Lee, I.Y.; Lee, W.J.; Kim, B.J.; Kook, Y.H.; et al. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol. Immunol. 2003, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chae, J.S. Molecular detection of Ehrlichia chaffeensis and Anaplasma bovis in the salivary glands from Haemaphysalis longicornis ticks. Vector Borne Zoonotic Dis. 2010, 10, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, J.; Guan, G.; Ma, M.; Liu, A.; Liu, J.; Ren, Q.; Niu, Q.; Lu, B.; Gao, J.; et al. Experimental transmission of Theileria uilenbergi infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol. Res. 2009, 104, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Q.; Lu, L.; Ding, G.; Guo, J.; Fu, G.; Zhang, J.; Meng, F.; Wu, H.; Song, X.; et al. Coinfection with four genera of bacteria (Borrelia, Bartonella, Anaplasma, and Ehrlichia) in Haemaphysalis longicornis and Ixodes sinensis ticks from China. Vector Borne Zoonotic Dis. 2008, 8, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, J.; Liu, S.; Shen, H.; Zhu, Y.; Wu, J.; Zhang, X.; Zhou, X.; Wang, C.; Qu, J.; et al. A reported death case of a novel bunyavirus in Shanghai, China. Virol. J. 2013, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Valdes, J.J.; Kotsyfakis, M. The role of cystatins in tick physiology and blood feeding. Ticks Tick-Borne Dis. 2012, 3, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef] [PubMed]
- Vray, B.; Hartmann, S.; Hoebeke, J. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci. 2002, 59, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, M.; Alvarez-Fernandez, M.; Nathanson, C.M. Cystatins. Biochem. Soc. Symp. 2003, 70, 179–199. [Google Scholar] [CrossRef]
- Valenzuela, J.G.; Francischetti, I.M.; Pham, V.M.; Garfield, M.K.; Mather, T.N.; Ribeiro, J.M. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2002, 205, 2843–2864. [Google Scholar] [PubMed]
- Zhou, J.; Liao, M.; Ueda, M.; Gong, H.; Xuan, X.; Fujisaki, K. Characterization of an intracellular cystatin homolog from the tick Haemaphysalis longicornis. Vet. Parasitol. 2009, 160, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ueda, M.; Umemiya, R.; Battsetseg, B.; Boldbaatar, D.; Xuan, X.; Fujisaki, K. A secreted cystatin from the tick Haemaphysalis longicornis and its distinct expression patterns in relation to innate immunity. Insect Biochem. Mol. Biol. 2006, 36, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S.; Gough, J.M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [PubMed]
- Gough, J.M.; Kemp, D.H. Localization of a low abundance membrane protein (bm86) on the gut cells of the cattle tick boophilus microplus by immunogold labeling. J. Parasitol. 1993, 79, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Jarmey, J.M.; Riding, G.A.; Pearson, R.D.; McKenna, R.V.; Willadsen, P. Carboxydipeptidase from boophilus microplus: A "concealed" antigen with similarity to angiotensin-converting enzyme. Insect Biochem. Mol. Biol. 1995, 25, 969–974. [Google Scholar] [CrossRef]
- De la Fuente, J.; Almazan, C.; Blas-Machado, U.; Naranjo, V.; Mangold, A.J.; Blouin, E.F.; Gortazar, C.; Kocan, K.M. The tick protective antigen, 4D8, is a conserved protein involved in modulation of tick blood ingestion and reproduction. Vaccine 2006, 24, 4082–4095. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Moreno-Cid, J.A.; Canales, M.; Villar, M.; de la Lastra, J.M.; Kocan, K.M.; Galindo, R.C.; Almazan, C.; Blouin, E.F. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 2011, 181, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.C.; Doncel-Perez, E.; Zivkovic, Z.; Naranjo, V.; Gortazar, C.; Mangold, A.J.; Martin-Hernando, M.P.; Kocan, K.M.; de la Fuente, J. Tick subolesin is an ortholog of the akirins described in insects and vertebrates. Dev. Comp. Immunol. 2009, 33, 612–617. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Almazan, C.; Naranjo, V.; Blouin, E.F.; Kocan, K.M. Synergistic effect of silencing the expression of tick protective antigens 4D8 and Rs86 in Rhipicephalus sanguineus by RNA interference. Parasitol. Res. 2006, 99, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; Manzano-Roman, R.; de la Fuente, J. Transovarial silencing of the subolesin gene in three-host Ixodid tick species after injection of replete females with subolesin dsRNA. Parasitol. Res. 2007, 100, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Taoufik, A.; de la Fuente, J.; Kocan, K.M.; de Vries, E.; Jongejan, F. Gene silencing of the tick protective antigens, Bm86, Bm91 and subolesin, in the one-host tick Boophilus microplus by RNA interference. Int. J. Parasitol. 2007, 37, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhou, Y.; Yu, Y.; Cao, J.; Zhang, H.; Gong, H.; Li, G.; Zhou, J. RNA interference and the vaccine effect of a subolesin homolog from the tick Rhipicephalus haemaphysaloides. Exp. Appl. Acarol. 2016, 68, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P. Anti-tick vaccines. Parasitology 2004, 129, S367–S387. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Guo, X.; de la Fuente, J.; Naranjo, V.; Kocan, K.M.; Kaufman, W.R. The impact of RNA interference of the subolesin and voraxin genes in male Amblyomma hebraeum (Acari: Ixodidae) on female engorgement and oviposition. Exp. Appl. Acarol. 2009, 47, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, L.; Islam, M.S.; Kim, T.K.; Diedrich, J.K.; Yates, J.R., 3rd; Pinto, A.F.; Mulenga, A.; You, M.J.; Da Silva Vaz, I., Jr. Saliva from nymph and adult females of Haemaphysalis longicornis: A proteomic study. Parasites Vectors 2015, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.G.; Dietrich, G.; Brandt, K.; Dolan, M.C.; Piesman, J.; Gilmore, R.D., Jr. Saliva, salivary gland, and hemolymph collection from Ixodes scapularis ticks. J. Vis. Exp. 2012, 3894. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.L.; Williams, J.; Karim, S. Temporal gene expression analysis and RNA silencing of single and multiple members of gene family in the lone star tick Amblyomma americanum. PLoS ONE 2016, 11, e0147966. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; Blouin, E.; de la Fuente, J. RNA interference in ticks. J. Vis. Exp. 2011, 2474. [Google Scholar] [CrossRef] [PubMed]
- Haimes, J.; Kelley, M.; Dharmacon, Now Part of GE Healthcare, Lafayette, CO, USA. Demonstration of a δδcq Calculation Method to Compute Thermo Scientific Relative Gene Expression from qPCR Data; GE Healthcare: Little Chalfont, UK, 2014. [Google Scholar]
- Karim, S.; Essenberg, R.C.; Dillwith, J.W.; Tucker, J.S.; Bowman, A.S.; Sauer, J.R. Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyomma americanum (L.). Insect Biochem. Mol. Biol. 2002, 32, 1711–1721. [Google Scholar] [CrossRef]
- Karim, S.; Ramakrishnan, V.G.; Tucker, J.S.; Essenberg, R.C.; Sauer, J.R. Amblyomma americanum salivary glands: Double-stranded RNA-mediated gene silencing of synaptobrevin homologue and inhibition of PGE2 stimulated protein secretion. Insect Biochem. Mol. Biol. 2004, 34, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, D343–D350. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Maekawa, Y.; Ishii, K.; Zhang, T.; Nashed, B.F.; Sakai, T.; Takashima, M.; Himeno, K. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun. 2001, 69, 7380–7386. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Almazan, C.; Naranjo, V.; Blouin, E.F.; Meyer, J.M.; Kocan, K.M. Autocidal control of ticks by silencing of a single gene by RNA interference. Biochem. Biophys. Res. Commun. 2006, 344, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P. Antigen cocktails: Valid hypothesis or unsubstantiated hope? Trends Parasitol. 2008, 24, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Parizi, L.F.; Reck, J., Jr.; Oldiges, D.P.; Guizzo, M.G.; Seixas, A.; Logullo, C.; de Oliveira, P.L.; Termignoni, C.; Martins, J.R.; Vaz Ida, S., Jr. Multi-antigenic vaccine against the cattle tick Rhipicephalus (boophilus) microplus: A field evaluation. Vaccine 2012, 30, 6912–6917. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.R.; Labbe, J.C.; Goldstein, B. Using RNA interference to identify genes required for RNA interference. Proc. Natl. Acad. Sci. USA 2002, 99, 4191–4196. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Sequence (5′-3′) | Product Size | Use |
|---|---|---|---|
| HlCF | TTCTAGCAACACGCGTCAAC | 300 bp | Cystatin-identifying primer |
| HlCR | TCACTCCCATTACCCAGAGC | ||
| HlSF | TTAAAGCGGACACACGATTG | 396 bp | Subolesin-identifying primer |
| HlSR | GCTCTCTCGCTCCTTCATCA | ||
| HlAF | CCAACAGGGAGAAGATGACG | 540 bp | Actin-identifying primer |
| HlAR | ACAGGTCCTTACGGATGTCC | ||
| Sub-1 F | TAATACGACTCACTATAGGGTACT ACGTCCCACCGAAGTTGA | 218 bp | Subolesin ds RNA synthesis |
| Sub-1 R | TAATACGACTCACTATAGGGTACT CTGGCGGAAGGTGAACAG | ||
| Sub-2 F | ACGTCCCACCGAAGTTGA | 218 bp | Subolesin real-time PCR |
| Sub-2 R | CTGGCGGAAGGTGAACAG | ||
| Cys-1 F | TAATACGACTCACTATAGGGTACTGACACCAAAACCCTTTGAGC | 194 bp | Cystatin ds RNA synthesis |
| Cys-1 R | TAATACGACTCACTATAGGGTACTGGGAACCTTGTTAGAAGAGC | ||
| Cys-2 F | GACACCAAAACCCTTTGAGC | 194 bp | Cystatin real-time PCR |
| Cys-2 R | GGGAACCTTGTTAGAAGAGC | ||
| Actin-2 F | AGCGTGGCTACTCTTTCACC | 229 bp | Actin real-time PCR |
| Actin-2 R | GATTCCATACCCAGGAACGA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.K.; Saiful Islam, M.; You, M. Impact of Subolesin and Cystatin Knockdown by RNA Interference in Adult Female Haemaphysalis longicornis (Acari: Ixodidae) on Blood Engorgement and Reproduction. Insects 2018, 9, 39. https://doi.org/10.3390/insects9020039
Rahman MK, Saiful Islam M, You M. Impact of Subolesin and Cystatin Knockdown by RNA Interference in Adult Female Haemaphysalis longicornis (Acari: Ixodidae) on Blood Engorgement and Reproduction. Insects. 2018; 9(2):39. https://doi.org/10.3390/insects9020039
Chicago/Turabian StyleRahman, Md. Khalesur, Mohammad Saiful Islam, and Myungjo You. 2018. "Impact of Subolesin and Cystatin Knockdown by RNA Interference in Adult Female Haemaphysalis longicornis (Acari: Ixodidae) on Blood Engorgement and Reproduction" Insects 9, no. 2: 39. https://doi.org/10.3390/insects9020039





