Behavioral Response of Leptoglossus zonatus (Heteroptera: Coreidae) to Stimuli Based on Colors and its Aggregation Pheromone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. The Response of Nymphs and Adults of Leptoglossus zonatus to Different Colors
2.3. Sexual Activity of Leptoglossus zonatus
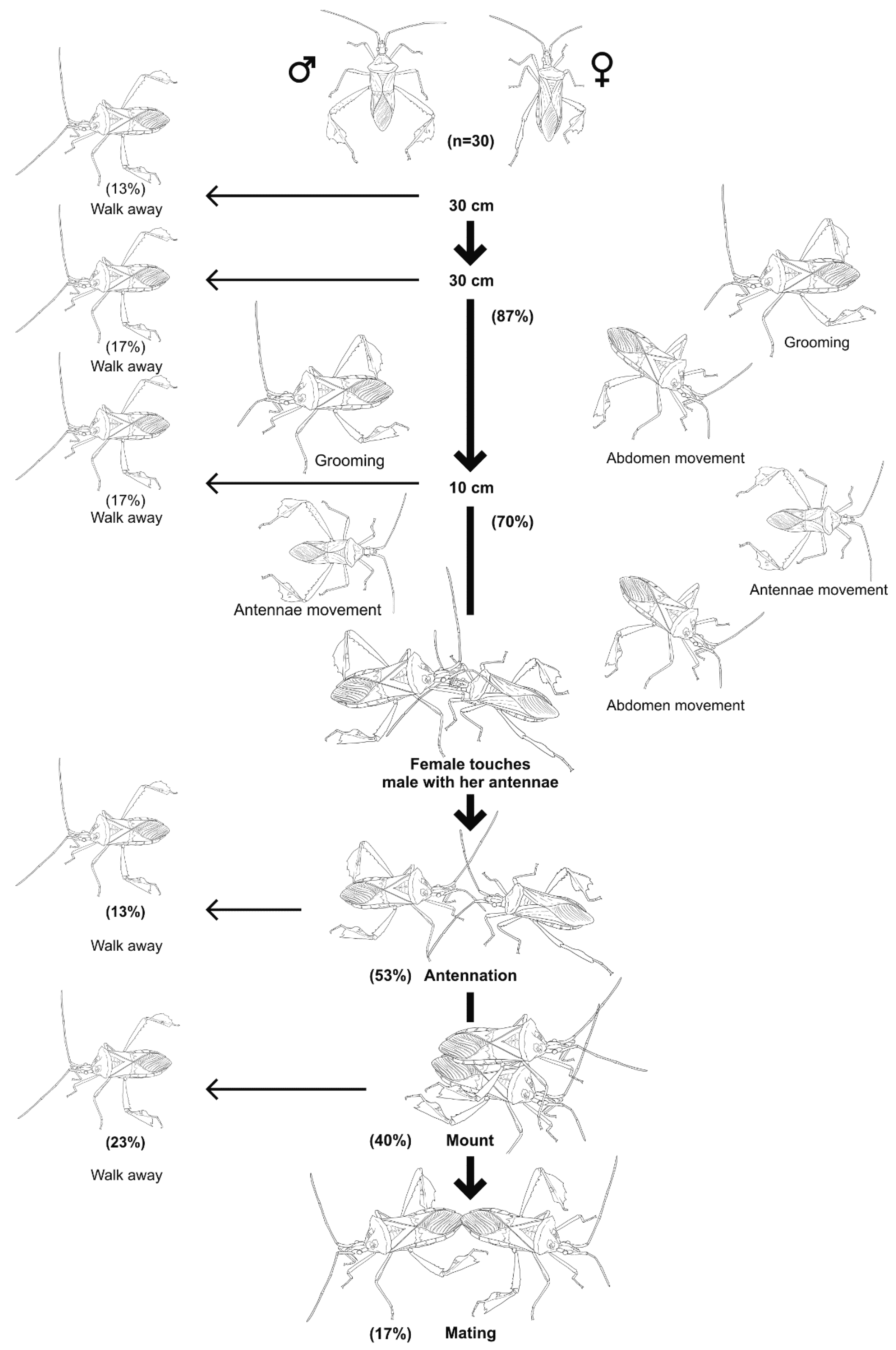
2.4. Mating Behavior of Leptoglossus zonatus
2.5. Active Volatile Collection from Conspecifics
2.6. Behavioral Response of Leptoglossus zonatus to Conspecific Volatiles
2.7. Statistical Analysis
3. Results
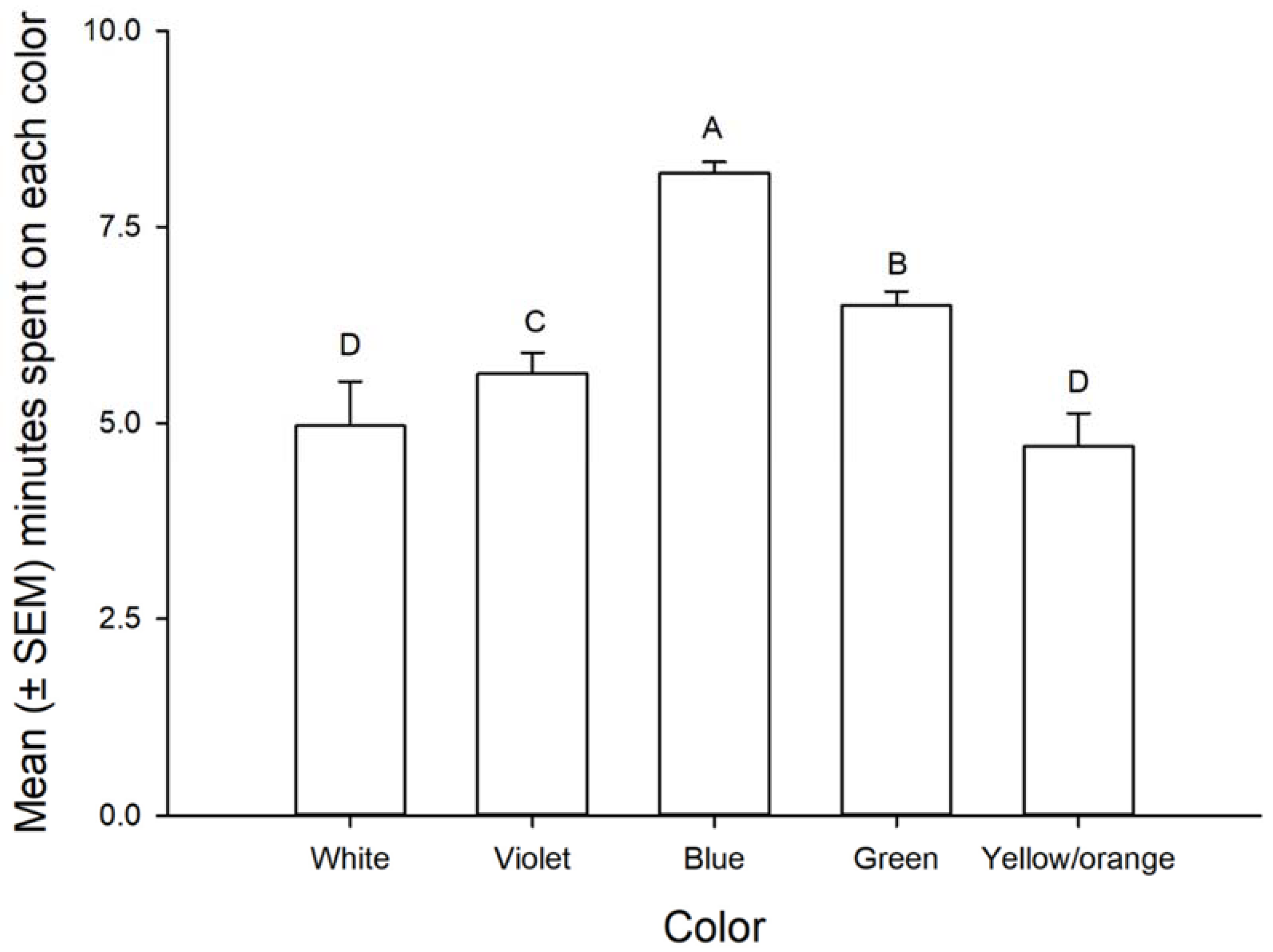
3.1. Color Preference
3.2. The Response of Nymphs and Adults of Leptoglossus zonatus to Different Colors
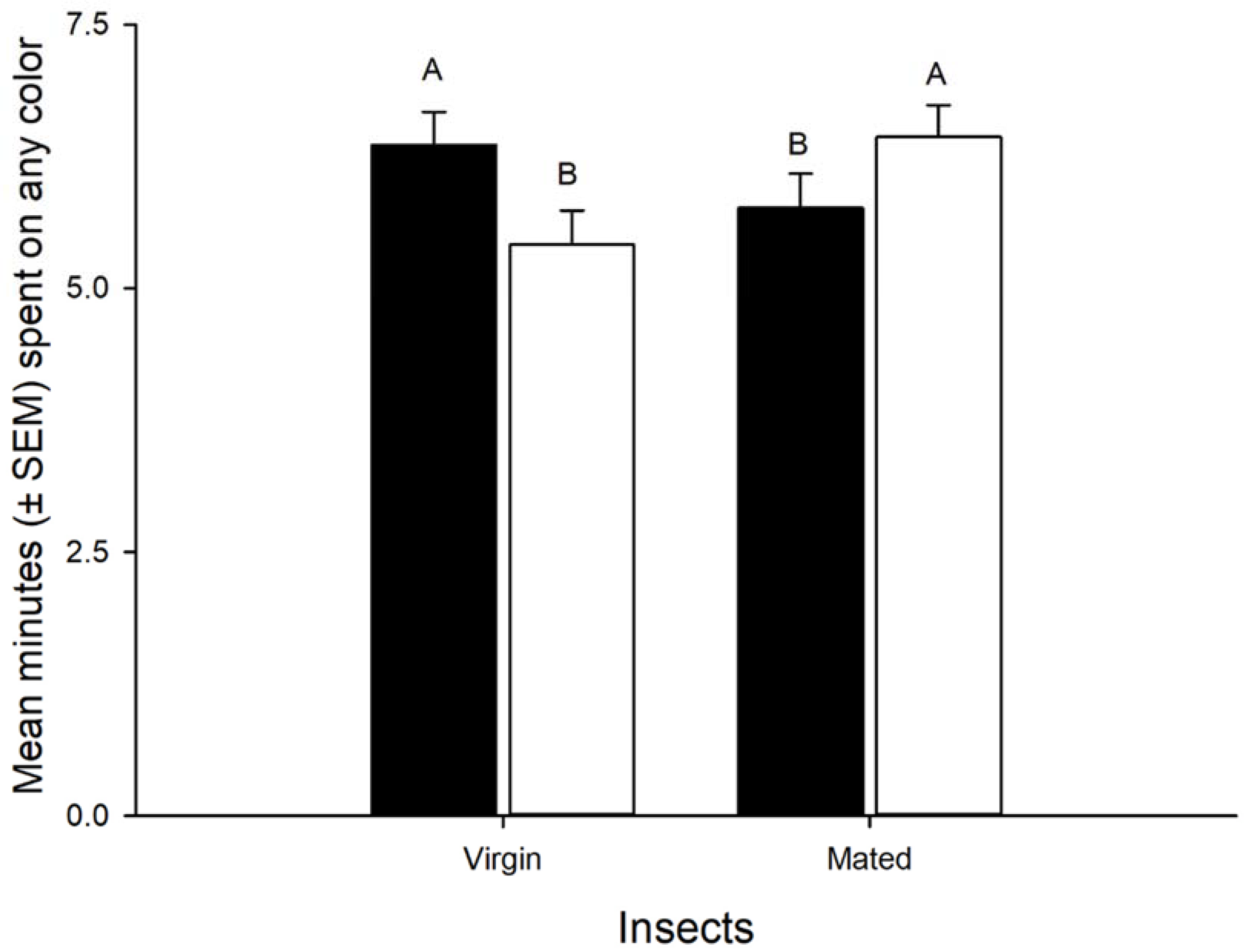
3.3. Mating Activity of Leptoglossus zonatus
3.4. Mating Behavior of Leptoglossus zonatus
3.5. Response to Conspecifics Volatiles
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miklas, N.; Lasnier, T.; Renou, M. Male bugs modulate pheromone emission in response to vibratory signals from conspecifics. J. Chem. Ecol. 2003, 29, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Bagwell, G.J.; Čokl, A.; Millar, J.G. Characterization and comparison of substrate-borne vibrational signals of Chlorochroa uhleri, Chlorochroa ligata, and Chlorochroa sayi (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2008, 101, 235–246. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Cañas, L.A. Visual cues enhance the response of Lygus hesperus (Heteroptera: Miridae) to volatiles from host plants. Environ. Entomol. 2005, 34, 1524–1533. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, Y. Morphology and chemical analysis of the metathoracic scent glands of Coreus marginatus (Linnaeus, 1758) (Heteroptera: Coreidae) from Turkey. Entomol. News 2007, 118, 227–234. [Google Scholar] [CrossRef]
- Millar, J.G.H. Pheromone of true bugs. Top. Curr. Chem. 2005, 240, 37–84. [Google Scholar]
- Leal, W.S.; Kuwahara, S.; Shi, X.; Higuchi, H.; Marino, C.E.B.; Ono, M.; Meinwald, J. Male-released sex pheromone of the stink bug Piezodorus hybneri. J. Chem. Ecol. 1998, 24, 1817–1829. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Oliver, J.E.; Waite, G.K.; Moore, C.J.; Waters, R.M. Identification of the presumed pheromone blend from the Australasian predaceous bug, Oechalia schellenbergii (Heteroptera: Pentatomidae). J. Chem. Ecol. 1996, 22, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R. Chemical ecology of the Heteroptera. Annu. Rev. Entomol. 1988, 33, 211–238. [Google Scholar] [CrossRef]
- Romani, R.; Rossi, M.V.S. Mapping and ultrastructure of antennal chemosensilla of the wheat bug Eurygaster maura. Insect Sci. 2009, 16, 193–203. [Google Scholar] [CrossRef]
- Mainali, B.P.; Lim, U.T. Circular yellow sticky trap with black background enhances attraction of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2010, 45, 207–213. [Google Scholar] [CrossRef]
- Esker, P.D.; Obrycki, J.; Nutter, F.W. Trap height and orientation of yellow sticky traps affect capture of Chaetocnema pulicaria (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2004, 97, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Tokumaru, S.; Higuchi, T.; Taguchi, Y. Control of the whiteflies, Trialeurodes vaporariorum and Bemisia tabaci, in tomato greenhouse by yellow sticky long film. Ann. Rept. Kansai Plant. Prot. 2009, 51, 87–88. [Google Scholar] [CrossRef]
- Gu, X.S.; Bu, W.J.; Xu, W.H.; Bai, Y.C.; Liu, B.M.; Liu, T.X. Population suppression of Bemisia tabaci (Hemiptera: Aleyrodidae) using yellow sticky traps and Eretmocerus nr. rajasthanicus (Hymenoptera: Aphelinidae) on tomate plants in greenhouse. Insect Sci. 2008, 15, 263–270. [Google Scholar]
- Vaishampayan, S.M.; Kogan, M.; Waldbauer, G.P.; Wooley, J.T. Spectral specific responses in the visual behaviour of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleurodidae). Entomol. Exp. Appl. 1975, 18, 344–356. [Google Scholar] [CrossRef]
- Jang, Y.; Hyon-Gyong, A.N.; Hyojoong, K.; Kwang-Ho, K. Spectral preferences of Lycorma delicatula (Hemiptera: Fulgoridae). Entomol. Res. 2013, 43, 115–122. [Google Scholar] [CrossRef]
- Brødsgaard, H.F. Coloured sticky traps for Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in glasshouses. J. Appl. Entomol. 1989, 107, 136–140. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Khrimian, A.; Chen, X.; Camp, M.J. Semiochemically based monitoring of the invasion of the brown marmorated stink bug and unexpected attraction of the native green stink bug (Heteroptera: Pentatomidae) in Maryland. Fla. Entomol. 2009, 92, 483–491. [Google Scholar] [CrossRef]
- Khrimian, A.; Shearer, P.W.; Zhang, A.; Hamilton, G.C.; Aldrich, J.R. Field trapping of the invasive brown marmorated stink bug, Halyomorpha halys, with geometric isomers of methyl 2,4,6-Decatrienoate. J. Agric. Food Chem. 2008, 56, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K. Seasonal fluctuations and physiological status of Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) adults captured in traps baited with synthetic aggregation pheromone of Plautia crossota stali Scott (Heteroptera: Pentatomidae). Jpn. J. Appl. Entomol. Zool. 2008, 52, 69–75. [Google Scholar] [CrossRef]
- Adachi, I.; Uchino, K.; Mochizuki, F. Development of a pyramidal trap for monitoring fruit-piercing stink bugs baited with Plautia crossota stali (Hemiptera: Pentatomidae) aggregation pheromone. Appl. Entomol. Zool. 2007, 42, 425–431. [Google Scholar] [CrossRef]
- Katase, M.; Shimizu, K.; Nagasaki, H.; Adachi, I. Application of synthetic aggregation pheromone of Plautia crossota stali Scott to the monitoring and mass trapping of fruit-piercing stink bugs. Bull. Chiba Prefectural Agric. Res. Cent. 2005, 4, 135–144. [Google Scholar]
- Matrangolo, W.J.R.; Waquil, J.M. Biologia de Leptoglossus zonatus (Dallas, 1852) (Hemiptera: Coreidae) alimentados com milho e sorgo. An. Soc. Entomol. Bras. 1994, 23, 419–423. [Google Scholar]
- Henne, D.C.; Johnson, S.; Bourgeois, W.J. Pest status of the leaf-footed bugs (Heteroptera: Coreidae) on citrus in Louisiana. Proc. Fla. State Hort. Soc. 2003, 116, 240–241. [Google Scholar]
- Raga, A.; Piza, C.T., Jr.; Souza, M.F. Ocorrência e danos de Leptoglossus zonatus (Dallas) (Heteroptera: Coreidae) em romã, Punica granatum L., em Campinas, São Paulo. An. Soc. Entomol. Bras. 1995, 24, 183–185. [Google Scholar]
- Xiao, Y.F.; Fadamiro, H.Y. Evaluation of damage to satsuma mandarin (Citrus unshiu) by the leaffooted bug, Leptoglossus zonatus (Hemiptera: Coreidae). J. App. Entomol. 2010, 134, 694–703. [Google Scholar] [CrossRef]
- Kubo, R.K.; Batista, A.F. Ocorrência e danos provocados por Leptoglossus zonatus (Dallas, 1852) (Hemiptera: Coreidae) em citros. An. Soc. Entomol. Bras. 1992, 21, 467–470. [Google Scholar]
- Morales, C.J.; Aguilar, E.A.; Quiroga, R.R.M.; Rosales, M.A.E. Insectos asociados al fruto del piñón (Jatropha curcas L.) en los municipios de Villaflores y Villa Corzo, Chiapas, México. Dugesiana 2011, 18, 85–89. [Google Scholar]
- Grimm, C.; Fuhrer, E. Population dynamics of true bugs (Heteroptera) in physic nut (Jatropha curcas) plantations in Nicaragua. J. App. Entomol. 1998, 122, 515–521. [Google Scholar] [CrossRef]
- Gonzaga-Segura, J.; Valdez-Carrasco, J.; Castrejón-Gómez, V.R. The metathoracic scent gland of the leaf-footed bug, Leptoglossus zonatus. J. Insect Sci. 2013, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S.; Panizzi, A.R.; Niva, C.C. Alarm pheromone system of leaf-footed bug Leptoglossus zonatus (Heteroptera: Coreidae). J. Chem. Ecol. 1994, 20, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Oliver, J.E.; Lusby, W.R.; Kochansky, J.P.; Lockwood, J.A. Pheromone strains of the cosmopolitan pest, Nezara viridula (Heteroptera: Pentatomidae). J. Exp. Zool. 1987, 244, 171–175. [Google Scholar] [CrossRef]
- Leal, W.S.; Kadosawa, T. (E)-2-Hexenyl hexanoate, the alarm pheromone of the bean bug Riptortus clavatus (Heteroptera: Alydidae). Biosci. Biotechnol. Biochem. 1992, 56, 1004–1005. [Google Scholar] [CrossRef] [PubMed]
- Brailovsky, H.; Barrera, E. A review of the Costa Rican species of Leptoglossus Guerin, with descriptions of two new species (Hemiptera: Heteroptera: Coreidae: Coreinae: Anisoscelini). Proc. Calif. Acad. Sci. 1998, 50, 167–184. [Google Scholar]
- Fagen, M.R.; Young, D.Y. Temporal patterns of behavior: durations, intervals, latencies and sequences. In Quantitative Ethology; Colgan, P.W., Ed.; John Wiley and Sons: New York, NY, USA, 1978; pp. 79–114. ISBN 9780471022367. [Google Scholar]
- Blackmer, J.L.; Cañas, L.A.; Byers, J.A. Behavioral response of Lygus hesperus conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J. Chem. Ecol. 2004, 30, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, J.E.; Francoeur, L.; Hamilton, G.C. Brown marmorated stink bug (Hemiptera: Pentatomidae) attraction to various light stimuli. Fla. Entomol. 2017, 100, 583–588. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Ann. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Mourão, A.P.M. Mating ovipositional rhythm and fecundity of Nezara viridula (L.) (Heteroptera: Pentatomidae) fed on privet, Ligustrum lucidum Thunb. and on soybean, Glycine max (L.) merrill fruits. An. Soc. Entomol. Bras. 1999, 28, 35–40. [Google Scholar] [CrossRef]
- Groot, A.T.; Van der Wal, E.; Schuurman, A.; Visser, J.H.; Blommers, L.H.M.; Van Beek, T.A. Copulation behaviour of Lygocoris pabulinus under laboratory conditions. Entomol. Exp. Appl. 1998, 88, 219–228. [Google Scholar] [CrossRef]
- Wang, Q.; Millar, J.G. Mating behavior and evidence for male-produced sex pheromones in Leptoglossus clypealis (Heteroptera: Coreidae). Ann. Entomol. Soc. Am. 2000, 93, 972–976. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.L. Mating frequency, duration, and circadian mating rhythm of New Zealand wheat bug Nysius huttoni White (Heteroptera: Lygaeidae). N. Z. Entomol. 2004, 27, 113–117. [Google Scholar] [CrossRef]
- Hebbalkar, D.S.; Sharma, R.N. Photoperiod linked patterns of daily mating behaviour in the bug Dysdercus koenigii F. (Hemiptera: Pyrrhocoridae). Curr. Sci. 1988, 57, 472–474. [Google Scholar]
- Zahn, D.K.; Girling, R.B.; Mcelfresh, J.S.; Carde, R.T.; Millar, J.G. Biology and reproductive behavior of Murgantia histrionica (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2008, 10, 215–228. [Google Scholar] [CrossRef]
- McBrien, H.L.; Millar, J.G. Substrate-borne vibrational signals of the consperse stink bug (Hemiptera: Pentatomidae). Can. Entomol. 2003, 135, 555–567. [Google Scholar] [CrossRef]
- Borges, M.; Jepson, P.C.; Howse, P.E. Long-range mate location and close-range courtship behaviour of the green stink bug, Nezara viridula and its mediation by sex pheromones. Entomol. Exp. Appl. 1987, 44, 205–212. [Google Scholar] [CrossRef]
- Guarino, S.; De Pasquele, C.; Peri, E.; Alonzo, G.; Colazza, S. Role of volatile and contact pheromones in the mating behavior of Bagrada hilaris (Heteroptera: Pentatomidae). Eur. J. Entomol. 2008, 105, 613–617. [Google Scholar] [CrossRef]
- Gillot, C. Nervous and Chemical Integrations. In Fundamentals of Insects Physiology; Blum, M.S., Ed.; John Wiley and Sons: New York, NY, USA; London, UK, 1995; pp. 287–356. ISBN 978-0471054689. [Google Scholar]
- Blatt, S.E.; Borden, J.H. Evidence for a male-produced aggregation pheromone in the western conifer seeds bug, Leptoglossus occidentalis H. (Hemiptera: Coreidae). Can. Entomol. 1996, 128, 777–778. [Google Scholar] [CrossRef]
- Yasuda, K. Ecology of the leaf footed plant bug, Leptoglossus australis Fabricius (Heteroptera: Coreidae), in the sub-tropical region of Japan. Trop. Agric. Res. Serie. 1990, 23, 229–238. [Google Scholar]
- Aldrich, J.R.; Lusby, W.R.; Kochansky, J.P.; Abrams, C.B. Volatile compounds from the predatory insect Podisus maculivenhis (Hemiptera: Pentatomidae): Male and female metathoracic scent gland and female dorsal abdominal gland secretions. J. Chem. Ecol. 1984, 10, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Kochansky, J.P.; Abrams, C.B. Attractant for a Beneficial Insect and Its Parasitoids: Pheromone of the Predatory Spined Soldier Bug, Podisus maculiventris (Hemiptera: Pentatomidae). Environ. Entomol. 1984, 13, 1031–1036. [Google Scholar] [CrossRef]
- Aller, T.; Caldwell, R.L. An investigation of the possible presence of an aggregation pheromone in the milkweed bugs, Oncopeltus fasciatus and Lygaeus kalmii. Physiol. Entomol. 1979, 4, 287–290. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Oliver, J.E.; Taghizadeh, T.; Ferreira, J.T.B.; Liewehr, D. Pheromones and colonization: reassessment of the milkweed bug migration model (Heteroptera: Lygaeidae: Lygaeinae). Chemoecology 1999, 9, 63–71. [Google Scholar] [CrossRef]
- Khrimian, A.; Harry, A.C.F.; Guzman, F.; Chauhan, K.; Moore, C.; Aldrich, J.R. Pheromone of the banana-spotting bug, Amblypelta lutescens lutescens Distant (Heteroptera: Coreidae): identification, synthesis, and field bioassay. Psyche 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Ishiwatari, T. Studies on the scent of stink bugs (Hemiptera: Pentatomidae). II Aggregation pheromone activity. Appl. Entomol. Zool. 1976, 11, 38–44. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Story, R.N. Bifunctional pheromone in the first instar of the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatomidae): Its characterization and interaction with other stimuli. Ann. Entomol. Soc. Am. 1985, 78, 474–479. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Archundia, S.L.; Gonzaga-Segura, A.J.; Jiménez-Pérez, A.; Castrejón-Gómez, V.R. Behavioral Response of Leptoglossus zonatus (Heteroptera: Coreidae) to Stimuli Based on Colors and its Aggregation Pheromone. Insects 2018, 9, 91. https://doi.org/10.3390/insects9030091
Franco-Archundia SL, Gonzaga-Segura AJ, Jiménez-Pérez A, Castrejón-Gómez VR. Behavioral Response of Leptoglossus zonatus (Heteroptera: Coreidae) to Stimuli Based on Colors and its Aggregation Pheromone. Insects. 2018; 9(3):91. https://doi.org/10.3390/insects9030091
Chicago/Turabian StyleFranco-Archundia, Sandra Lisbeth, Agustín Jesús Gonzaga-Segura, Alfredo Jiménez-Pérez, and Víctor Rogelio Castrejón-Gómez. 2018. "Behavioral Response of Leptoglossus zonatus (Heteroptera: Coreidae) to Stimuli Based on Colors and its Aggregation Pheromone" Insects 9, no. 3: 91. https://doi.org/10.3390/insects9030091




