Are We Compensating for the Lack of Physical Activity in Our Diabetic Patients with Treatment Intensification?
Abstract
:1. Introduction
2. Patients and Methods
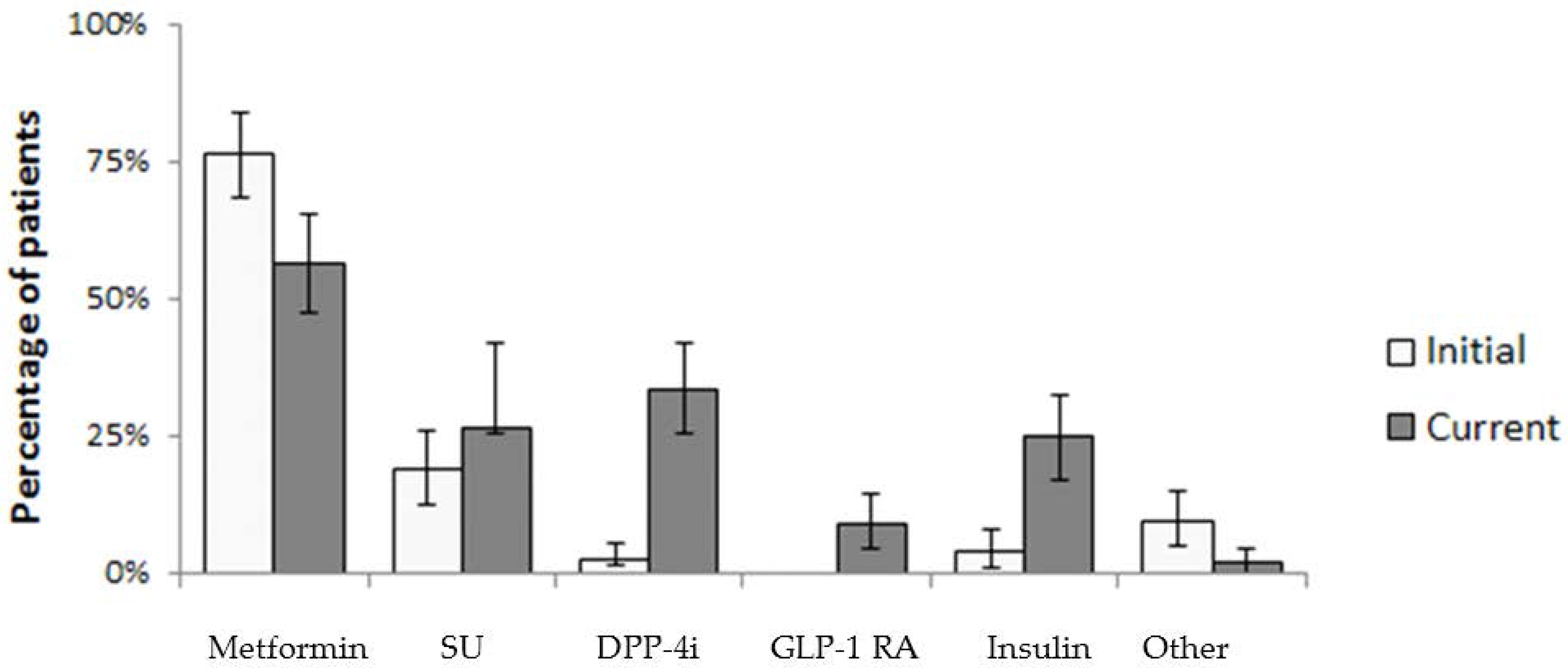
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Nichols, G.; Perry, A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004, 75, 1535–1540. [Google Scholar] [CrossRef]
- Khunti, K.; Wolden, M.L.; Thorsted, B.L.; Andersen, M.; Davies, M.J. Clinical inertia in people with type 2 diabetes: A retrospective cohort study of more than 80,000 people. Diabetes Care 2013, 36, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 6th ed. 2013. Available online: www.idf.org/diabetesatlas (accessed on 27 May 2016).
- Cuddihy, R.M.; Philis-Tsimikas, A.; Nazeri, A. Type 2 diabetes care and insulin intensification: Is a more multidisciplinary approach needed? Results from the MODIFY survey. Diabetes Educ. 2011, 37, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.; Kriska, A. Role of physical activity in diabetes management and prevention. J. Am. Diet Assoc. 2008, 108, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Hawley, J.A. The erosion of physical activity in Western societies: An economic death march. Diabetologia 2015, 58, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, S.M.; Soedamah-Muthu, S.S.; De Neve, M.; Toeller, M.; Chaturvedi, N.; Fuller, J.H.; Stamatakis, E. Association of physical activity with all-cause mortality and incident and prevalent cardiovascular disease among patients with type 1 diabetes: The EURODIAB Prospective Complications Study. Diabetologia 2013, 56, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ilanne-Parikka, P.; Laaksonen, D.E.; Eriksson, J.G.; Lakka, T.A.; Lindstr, J.; Peltonen, M.; Aunola, S.; Keinánen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J.; et al. Finnish Diabetes Prevention Study Group. Leisure-time physical activity and the metabolic syndrome in the Finnish diabetes prevention study. Diabetes Care 2010, 33, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- The 2011 Compendium of Physical Activities. Available online: https://sites.google.com/site/compendiumofphysicalactivities/corrected-mets (accessed on 14 January 2016).
- Wadén, J.; Tikkanen, H.; Forsblom, C.; Fagerudd, J.; Pettersson-Fernholm, K.; Lakka, T.; Riska, M.; Groop, P.H.; FinnDiane Study Group. Leisure time physical activity is associated with poor glycemic control in type 1 diabetic women: The FinnDiane study. Diabetes Care 2005, 28, 777–782. [Google Scholar] [PubMed]
- Wadén, J.; Tikkanen, H.K.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Saraheimo, M.; Tolonen, N.; Rosengård-Bärlund, M.; Gordin, D.; Tikkanen, H.O. Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: The FinnDiane Study. Diabetologia 2015, 58, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Redmon, B.J.; Bertoni, A.G.; Connelly, S.; Feeney, P.A.; Glasser, S.P.; Glick, H.; Greenway, F.; Hesson, L.A.; Lawlor, M.S.; Montez, M.; et al. Effect of the Look AHEAD Study Intervention on Medication Use and Related Cost to Treat Cardiovascular Disease Risk Factors in Individuals with Type 2 Diabetes. Diabetes Care 2010, 33, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Chang, H.Y.; Hsu, C.C.; Lu, J.F.; Fang, H.L. Joint predictability of health related quality of life and leisure time physical activity on mortality risk in people with diabetes. BMC Public Health 2013, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Huai, P.; Han, H.; Reilly, K.H.; Guo, X.; Zhang, J.; Xu, A. Leisure-time physical activity and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Endocrine 2016, 52, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; De Feo, P.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Fallucca, F.; Pugliese, G.; et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: A randomized controlled trial: The Italian Diabetes and Exercise Study (IDES). Arch. Intern. Med. 2010, 170, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Kaizu, S.; Kishimoto, H.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Jodai, T.; Kikuchi, Y.; Idewaki, Y.; Hirakawa, Y.; et al. Impact of leisure-time physical activity on glycemic control and cardiovascular risk factors in Japanese patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. PLoS ONE 2014, 9, e98768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iughetti, L.; Gavioli, S.; Bonetti, A.; Predieri, B. Effects of Exercise in Children and Adolescent with Type 1 Diabetes Mellitus. Health 2015, 7, 1357–1365. [Google Scholar] [CrossRef]
- Herbst, A.; Bachran, R.; Kapellen, T.; Holl, R.W. Effects of Regular Physical Activity on Glycemic Control in Children with Diabetes Mellitus Type 1. Arch. Pediatr. Adolesc. Med. 2006, 160, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.; Wilson, D.P.; Endres, R.K.; Goldstein, D.E. Improved Glycemic Control after Supervised 8-wk Exercise Program in Insulin-Dependent Diabetic Adolescents. Diabetes Care 1987, 10, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Jones, T.W.; Fournier, P.A. Exercise Training and Glycemic Control in Adolescents with Poorly Controlled Type 1 Diabetes Mellitus. J. Pediatr. Endocrinol. Metab. 2002, 15, 621–627. [Google Scholar] [CrossRef]
- Tanasecu, M.; Leitzmann, M.F.; Rimm, E.B.; Hu, F.B. Physical activity in relation to cardiovascular disease and total mortality among men withy type 2 diabetes. Circulation 2003, 107, 2435–2439. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Tanaka, S.; Tanaka, S.; Suzuki, S.; Seino, H.; Hanyu, O.; Sato, A.; Toyonaga, T.; Okita, K.; Ishibashi, S.; et al. Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: Analysis from the Japan Diabetes Complications Study (JDCS). Diabetologia 2013, 56, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham heart study. Neurobiol. Aging 2005, 26 (Suppl. 1), 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Dahl, A.K.; Pedersen, N.L.; Johansson, B. Overweight in midlife is related to lower cognitive function 30 years later: A prospective study with longitudinal assessments. Dement. Geriatr. Cogn. Disord. 2010, 29, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.K.; Nam, H.S.; Son, M.H.; Son, E.J.; Cho, K.H. Interactive effect of obesity indexes on cognition. Dement. Geriatr. Cogn. Disord. 2005, 19, 91–96. [Google Scholar] [CrossRef] [PubMed]
| Variable | DM Type 1 (n = 55) | DM Type 2 (n = 126) | p | Effect Size | ||
|---|---|---|---|---|---|---|
| Age (years) | 37 | (26–44) | 62 | (54–69) | <0.001 | 0.93 * |
| Age at diagnosis (years) | 23 | (13–29) | 51 | (45–59) | <0.001 | 0.97 * |
| Duration of diabetes (years) | 11 | (5–19) | 4 | (8–14) | 0.040 | 0.61 * |
| HbA1c (%) | ||||||
| at diagnosis | 9.7 | (6.9–12.1) | 8.0 | (7.2–9.9) | 0.104 | 0.60 * |
| at the time of enrolment | 7.0 | (6.3–7.6) | 6.6 | (6.1–7.2) | 0.020 | 0.65 * |
| Body mass index (kg/m2) | ||||||
| normal (≤24.99) | 32 | (84.2%) | 16 | (15.0%) | <0.001 | 0.65 † |
| overweight (25.00–29.99) | 2 | (5.3%) | 32 | (29.9%) | ||
| obese (≥30.00) | 4 | (10.5%) | 59 | (55.1%) | ||
| Hypoglycemia | 49 | (92.5%) | 45 | (37.2%) | <0.001 | 0.51 † |
| Blood glucose level at hypoglycemia (mmol/L) | 3.2 | (2.8–3.8) | 3.2 | (3.0–3.8) | 0.757 | 0.52 * |
| At the Time of Diagnosis | n = 126 | (%) | At the Time of Survey | n = 126 | (%) |
|---|---|---|---|---|---|
| Metformin | 96 | (76.2) | Metformin | 71 | (56.3) |
| Sulphonylurea | 24 | (19.0) | SU | 33 | (26.2) |
| DPP-4i | 3 | (2.4) | DPP-4i | 42 | (33.3) |
| GLP-1 | 0 | (0.0) | GLP-1 RA | 11 | (8.7) |
| Insulin | 5 | (4.0) | Insulin | 31 | (24.6) |
| Other | 12 | (9.5) | Other | 2 | (1.6) |
| Complete Therapy Structure | Complete Therapy Structure | ||||
| MET | 77 | (61.1) | MET | 22 | (17.4) |
| MET + SU | 13 | (10.3) | MET + SU | 7 | (5.6) |
| SU | 5 | (3.9) | SU | 2 | (1.5) |
| MET + TZD | 1 | (0.7) | MET + DPP-4i | 20 | (15.9) |
| SU + MET + DPP-4i | 3 | (2.3) | SU + MET + DPP-4i | 14 | (11.1) |
| Insulin | 3 | (2.3) | Insulin | 29 | (23.0) |
| AGI | 1 | (0.7) | SU + DPP-4i | 4 | (3.1) |
| MET + SU + insulin | 2 | (1.5) | DPP-4i | 3 | (2.3) |
| Repaglinide | 2 | (1.5) | OAD + GLP-1 RA | 9 | (6.8) |
| Not specified OAD | 3 | (2.3) | Not specified OAD | 2 | (1.5) |
| SU + MET + TZD | 1 | (0.7) | GLP-1 RA + insulin | 2 | (1.5) |
| Type of PA | Type 1 n = 55 (%) | Type 2 n = 126 (%) | p | Effect Size | ||
|---|---|---|---|---|---|---|
| Change of PA during the last 10 years | ||||||
| Decrease | 20 | (37.0) | 65 | (51.6) | <0.001 | 0.30 † |
| No change | 7 | (13.0) | 35 | (27.8) | ||
| Increase | 27 | (50.0) | 26 | (20.6) | ||
| Low intensity PA | 35 | (63.6) | 101 | (80.2) | 0.018 | 0.18 † |
| At least 3 times a week ≥ 60 min of moderate-to-vigorous PA | 20 | (36.4) | 25 | (19.8) | ||
| Total | 55 | (100.0) | 126 | (100.0) | ||
| Leisure Time Physical Activity | |||||||
|---|---|---|---|---|---|---|---|
| Variable | At Least 3 Times a Week ≥60 min of Moderate-to-Vigorous PA | Less PA | OR | (95% CI) | p | ||
| Average age (years) | 31 | (26–43) | 38 | (26–45) | 0.97 | (0.92–1.03) | 0.282 |
| Age at diagnosis (years) | 21 | (13–29) | 23 | (12–29) | 1.01 | (0.96–1.06) | 0.771 |
| Average duration of diabetes | 11 | (6–15) | 10 | (5–22) | 0.99 | (0.93–1.06) | 0.809 |
| HbA1c (%) | |||||||
| at diagnosis | 11.2 | (8.5–13.4) | 8.1 | (6.5–11.8) | 1.27 | (0.97–1.65) | 0.085 |
| at the time of enrolment | 7.3 | (6.7–8.6) | 7.0 | (6.2–7.5) | 1.87 | (1.00–3.45) | 0.048 |
| Average body mass index (kg/m2) | 19 | (16–21) | 21 | (18–24) | 0.95 | (0.85–1.06) | 0.359 |
| Number of patients experiencing hypoglycemia | |||||||
| no | 1 | (25.0) | 3 | (75.0) | 1 | ||
| yes | 19 | (38.8) | 30 | (61.2) | 1.90 | (0.18–19.6) | 0.590 |
| Blood glucose level at hypoglycemia (mmol/L) | 3.6 | (2.8–3.9) | 2.9 | (2.9–3.6) | 1.72 | (0.56–5.26) | 0.344 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maja Cigrovski Berkovic, M.C.; Bilic-Curcic, I.; Gradiser, M.; Herman-Mahecic, D.; Cigrovski, V.; Ivandic, M. Are We Compensating for the Lack of Physical Activity in Our Diabetic Patients with Treatment Intensification? Sports 2017, 5, 58. https://doi.org/10.3390/sports5030058
Maja Cigrovski Berkovic MC, Bilic-Curcic I, Gradiser M, Herman-Mahecic D, Cigrovski V, Ivandic M. Are We Compensating for the Lack of Physical Activity in Our Diabetic Patients with Treatment Intensification? Sports. 2017; 5(3):58. https://doi.org/10.3390/sports5030058
Chicago/Turabian StyleMaja Cigrovski Berkovic, Maja Cigrovski, Ines Bilic-Curcic, Marina Gradiser, Davorka Herman-Mahecic, Vjekoslav Cigrovski, and Marul Ivandic. 2017. "Are We Compensating for the Lack of Physical Activity in Our Diabetic Patients with Treatment Intensification?" Sports 5, no. 3: 58. https://doi.org/10.3390/sports5030058






