Neuromuscular Fatigue at Task Failure and During Immediate Recovery after Isometric Knee Extension Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Procedures
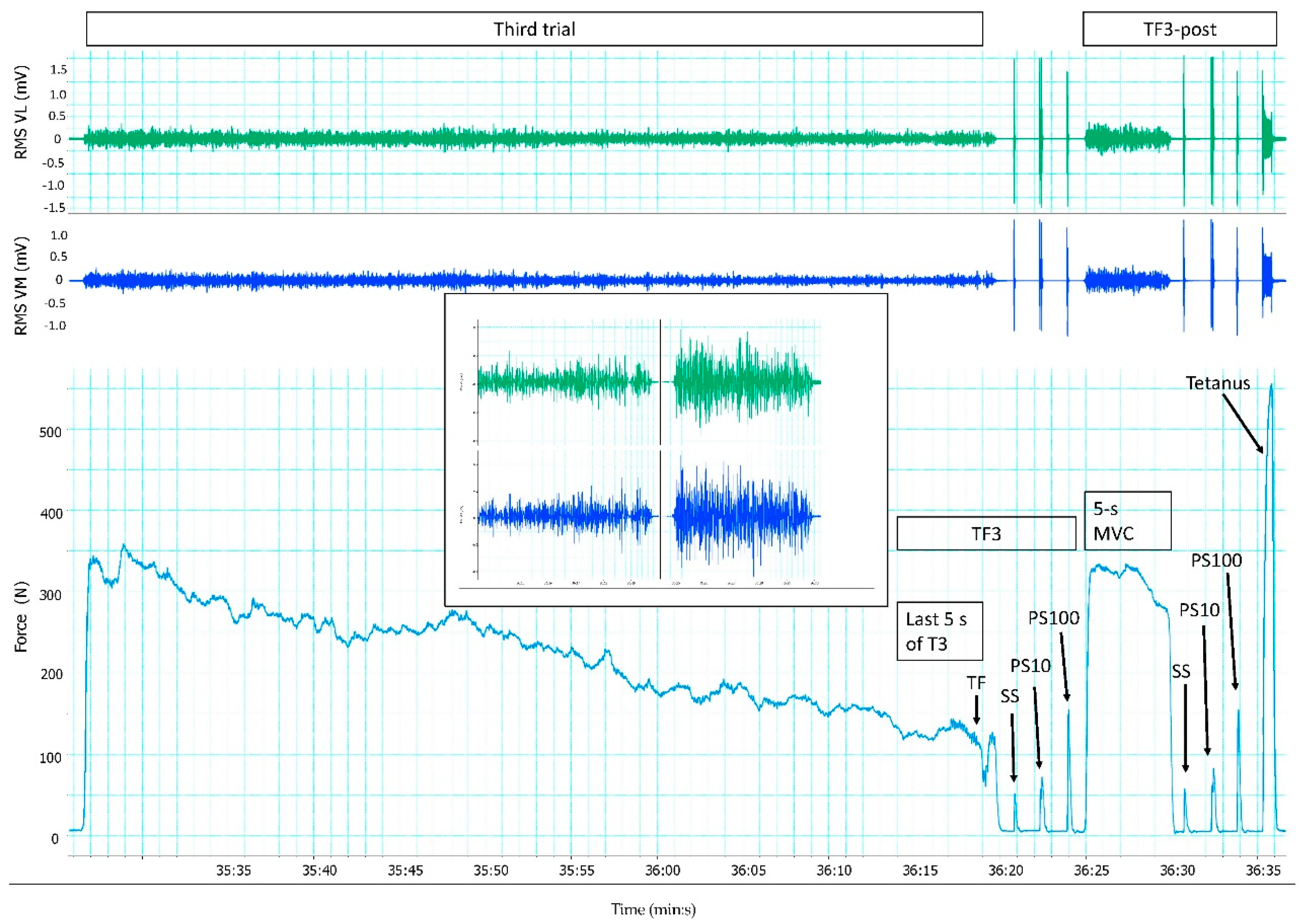
2.4. Data Collection
2.5. Data Analysis
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. Neuromuscular Activation and Peripheral Fatigue at Task Failure
4.2. Neuromuscular Activation at the End of the Third Trial and during the Immediate Post-Exercise Recovery Period
4.3. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Kayser, B. Exercise starts and ends in the brain. Eur. J. Appl. Physiol. 2003, 90, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Verges, S.; Maffiuletti, N.A.; Kerherve, H.; Decorte, N.; Wuyam, B.; Millet, G.Y. Comparison of electrical and magnetic stimulations to assess quadriceps muscle function. J. Appl. Physiol. 2009, 106, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.Y.; Martin, V.; Martin, A.; Verges, S. Electrical stimulation for testing neuromuscular function: from sport to pathology. Eur. J. Appl. Physiol. 2011, 111, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.E.; Gard, M.; Drinkwater, E.J. The limits to exercise performance and the future of fatigue research. Br. J. Sports Med. 2011, 45, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W. The limit to exercise tolerance in humans: mind over muscle? Eur. J. Appl. Physiol. 2010, 109, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Amann, M. Significance of group III and IV muscle afferents for the endurance exercising human. Clin. Exp. Pharmacol. Physiol. 2012, 39, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Amann, M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 2011, 43, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Dempsey, J.A. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J. Physiol. 2008, 586, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Kayser, B.; Place, N. Are There Critical Fatigue Thresholds? Aggregated vs. Individual Data. Front. Physiol. 2016, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Sharpe, G.R.; Williams, N.C.; Hannah, R. Locomotor muscle fatigue is not critically regulated after prior upper body exercise. J. Appl. Physiol. 2015, 119, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Froyd, C.; Beltrami, F.G.; Millet, G.Y.; Noakes, T.D. Central Regulation and Neuromuscular Fatigue during Exercise of Different Durations. Med. Sci. Sports Exerc. 2016, 48, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Froyd, C.; Beltrami, F.; Millet, G.; Noakes, T. No critical peripheral fatigue threshold during intermittent isometric time to task failure test with the knee extensors. Front. Physiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Christian, R.J.; Bishop, D.J.; Billaut, F.; Girard, O. Peripheral fatigue is not critically regulated during maximal, intermittent, dynamic leg extensions. J. Appl. Physiol. 2014, 117, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Elmeua, M.; Howatson, G.; Goodall, S. Intensity-Dependent Contribution of Neuromuscular Fatigue after Constant-Load Cycling. Med. Sci. Sports Exerc. 2016, 48, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Bigland-Ritchie, B.; Furbush, F.; Woods, J.J. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J. Appl. Physiol. 1986, 61, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Decorte, N.; Lafaix, P.A.; Millet, G.Y.; Wuyam, B.; Verges, S. Central and peripheral fatigue kinetics during exhaustive constant-load cycling. Scand. J. Med. Sci. Sports 2012, 22, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Bigland-Ritchie, B.; Jones, D.A.; Hosking, G.P.; Edwards, R.H. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin. Sci. Mol. Med. 1978, 54, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Maffiuletti, N.A.; Martin, A.; Lepers, R. Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve. 2007, 35, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, B.; Place, N.; Martin, A.; Lepers, R. Neuromuscular fatigue differs following unilateral vs bilateral sustained submaximal contractions. Scand. J. Med. Sci. Sports 2011, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Maffiuletti, N.A.; Kayser, B.; Place, N. Mechanisms of fatigue and task failure induced by sustained submaximal contractions. Med. Sci. Sports Exerc. 2012, 44, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, A.J.; Zackowski, K.M.; Huey, K.A.; Enoka, R.M. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J. Physiol. 1993, 460, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.N.; Cresswell, A.G.; Thorstensson, A. Central fatigue during a long-lasting submaximal contraction of the triceps surae. Exp. Brain Res. 1996, 108, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peralta, R.; Morales-Alamo, D.; Gonzalez-Izal, M.; Losa-Reyna, J.; Perez-Suarez, I.; Izquierdo, M.; Calbet, J.A. Task Failure during Exercise to Exhaustion in Normoxia and Hypoxia Is Due to Reduced Muscle Activation Caused by Central Mechanisms While Muscle Metaboreflex Does Not Limit Performance. Front. Physiol. 2016, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Losa-Reyna, J.; Torres-Peralta, R.; Martin-Rincon, M.; Perez-Valera, M.; Curtelin, D.; Ponce-Gonzalez, J.G.; Santana, A.; Calbet, J.A. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015, 593, 4631–4648. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Hermens, H. Introduction to the special issue on the SENIAM European Concerted Action. J. Electromyogr. Kinesiol. 2000, 10, 283–286. [Google Scholar] [CrossRef]
- Pageaux, B. Perception of effort in Exercise Science: Definition, measurement and perspectives. Eur. J. Sport Sci. 2016, 16, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Perceived exertion. Exerc. Sport Sci. Rev. 1974, 2, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Eldridge, M.W.; Lovering, A.T.; Stickland, M.K.; Pegelow, D.F.; Dempsey, J.A. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J. Physiol. 2006, 575, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Olivier, N.; Millet, G.Y.; Meste, O.; Blain, G.M. Exercise performance is regulated during repeated sprints to limit the development of peripheral fatigue beyond a critical threshold. Exp. Physiol. 2014, 99, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Froyd, C.; Millet, G.Y.; Noakes, T.D. The development of peripheral fatigue and short-term recovery during self-paced high-intensity exercise. J. Physiol. 2013, 591, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Cheung, S.S.; Jacobs, I. Variability of time to exhaustion during submaximal exercise. Can. J. Appl. Physiol. 1995, 20, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Richardson, R.S.; Amann, M. Less peripheral fatigue after prior exercise is not evidence against the regulation of the critical peripheral fatigue threshold. J. Appl. Physiol. 2015, 119, 1520. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Sharpe, G.R.; Williams, N.C.; Hannah, R. Reply to Broxterman, Richardson, and Amann. J. Appl. Physiol. 2015, 119, 1521. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Romer, L.M.; Amann, M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? Eur. J. Sport Sci. 2016, 1, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; St-Pierre, D.M. Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J. Appl. Physiol. 1997, 82, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Schlinder Delap, B.; Griffith, E.E.; Hunter, S.K. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve. 2007, 36, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.W.; Finn, H.T.; Siegler, J.C. The Magnitude of Peripheral Muscle Fatigue Induced by High and Low Intensity Single-Joint Exercise Does Not Lead to Central Motor Output Reductions in Resistance Trained Men. PloS ONE 2015, 10, e0140108. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J.Gen.Physiol 1991, 98, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Kreivenaite, L.; Streckis, V.; Mickeviciene, D.; Skurvydas, A. Effects of age and sex on fatigability and recovery from a sustained maximal isometric voluntary contraction. J. Electromyogr. Kinesiol. 2017, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C.; Allen, G.M.; Butler, J.E.; Taylor, J.L. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J. Physiol. 1996, 490, 529–536. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Martin, P.G.; Gandevia, S.C.; Taylor, J.L. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J. Physiol. 2009, 587, 5601–5612. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.S.; Fitzpatrick, S.C.; Gandevia, S.C.; Taylor, J.L. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J. Appl. Physiol. 2015, 118, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Fatigue is a Brain-Derived Emotion that Regulates the Exercise Behavior to Ensure the Protection of Whole Body Homeostasis. Front. Physiol. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Layec, G.; Rossman, M.J.; Richardson, R.S. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J. Appl. Physiol. 2013, 115, 355–364. [Google Scholar] [CrossRef] [PubMed]

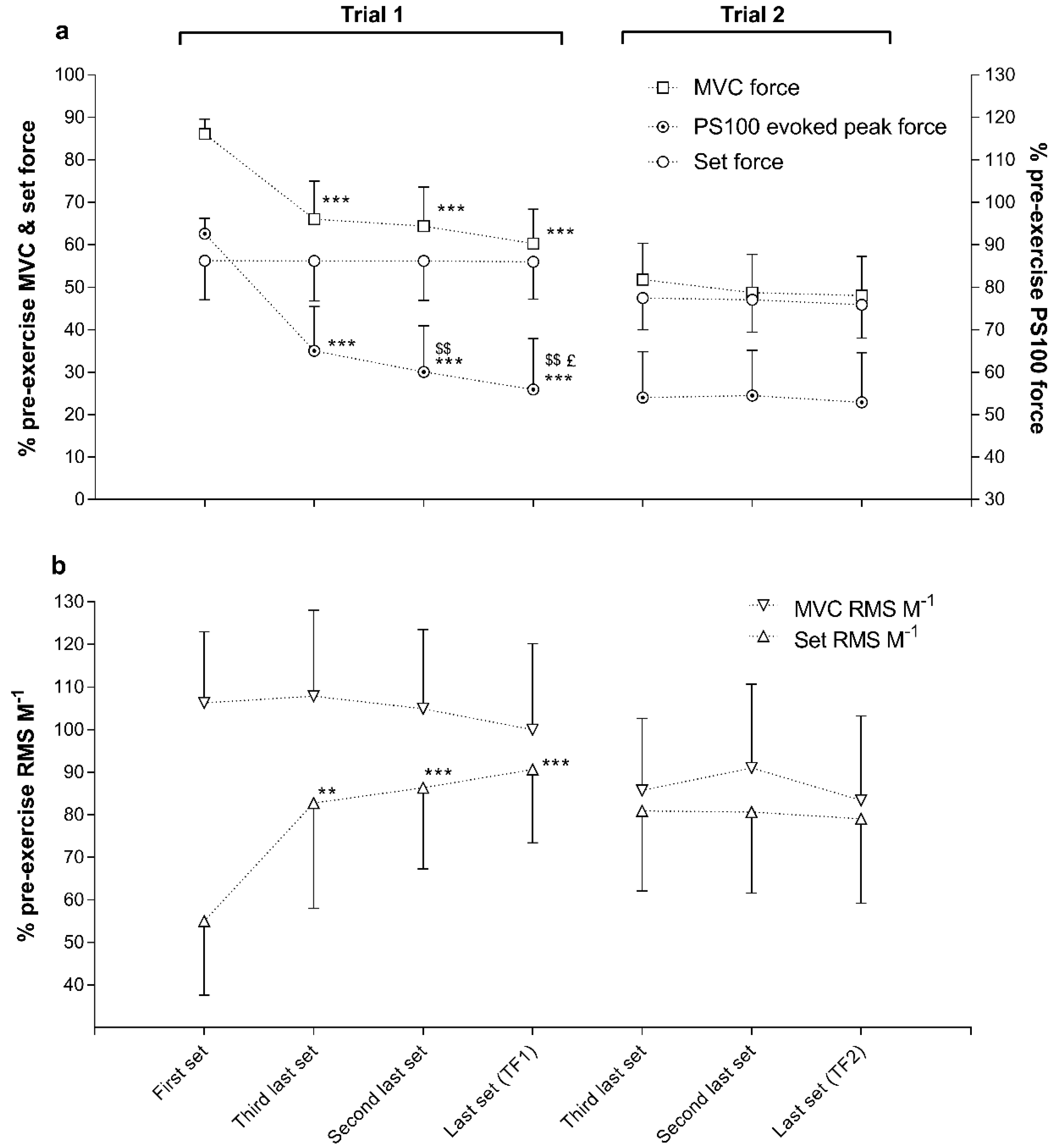
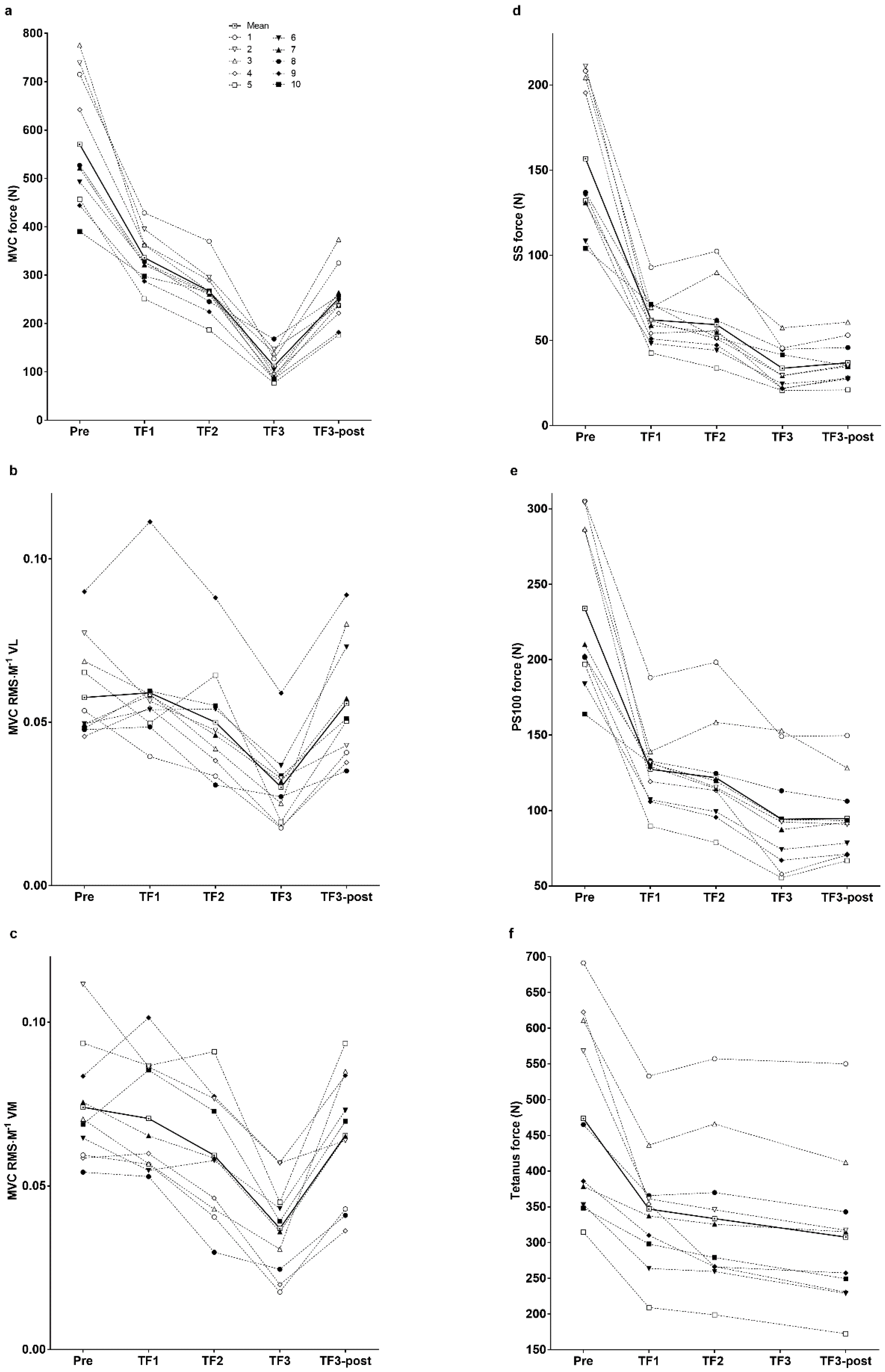
| Parameter | Pre-Exercise | Task Failure 1 | Task Failure 2 | Task Failure 3 | Task Failure 3-Post | Effect Size |
|---|---|---|---|---|---|---|
| MVC (N) | 571 ± 137 | 336 ± 53 *** | 267 ± 48 ***,$$$ | 112 ± 31 ***,$$$,£££ | 252 ± 60 ***,$$,&&& | η² = 0.914 |
| Δ% | −40 ± 8 | −52 ± 9 $$$ | −80 ± 5 $$$,£££ | −55 ± 9 $$,&&& | η² = 0.921 | |
| MVC RMS∙M−1 VL | 0.060 ± 0.015 | 0.059 ± 0.019 | 0.050 ± 0.017 | 0.030 ± 0.012 **,$$$,££ | 0.057 ± 0.019 && | η² = 0.611 |
| Δ% | 0 ± 21 | −15 ± 21 | −49 ± 17 $$$,£££ | −5 ± 27 &&& | η² = 0.729 | |
| MVC RMS∙M−1 VM | 0.074 ± 0.018 | 0.071 ± 0.018 | 0.059 ± 0.020 *,$ | 0.037 ± 0.014 ***,$$$,££ | 0.066 ± 0.020 && | η² = 0.708 |
| Δ% | −4 ± 16 | −21 ± 17 $ | −51 ± 13 $$$,£££ | −11 ± 22 &&& | η² = 0.722 | |
| SS (N) | 157 ± 43 | 62 ± 15 *** | 59 ± 21 *** | 34 ± 13 ***,$$$,££ | 37 ± 13 ***,$$$,££ | η² = 0.898 |
| Δ% | −58 ± 12 | −62 ± 10 | −78 ± 9 $$$,£££ | −76 ± 7 $$$,£££ | η² = 0.855 | |
| PS10 | 249 ± 66 | 95 ± 22 *** | 83 ± 26 ***,$ | 48 ± 21 ***,$$$,£££ | 53 ± 17 ***,$$$,£££ | η² = 0.909 |
| Δ% | −60 ± 13 | −66 ± 9 $ | −80 ± 9 $$$,£££ | −78 ± 7 $$$,£££ | η² = 0.873 | |
| PS100 (N) | 234 ± 55 | 127 ± 26 *** | 122 ± 34 *** | 94 ± 35 ***,$$,££ | 95 ± 27 ***,$$$,£££ | η² = 0.884 |
| Δ% | −44 ± 12 | −47 ± 12 | −59 ± 13 $$,£££ | −59 ± 10 $$$,£££ | η² = 0.826 | |
| Tetanus (N) | 474 ± 137 | 347 ± 90 ** | 334 ± 108 ** | NA | 308 ± 109 **,$,££ | η² = 0.747 |
| Δ% | −26 ± 10 | −29 ± 13 | NA | −35 ± 14 $$,£££ | η² = 0.618 | |
| PS10∙PS100−1 | 1.07 ± 0.08 | 0.75 ± 0.07 *** | 0.67 ± 0.05 ***,$$$ | 0.49 ± 0.09 ***,$$$,£££ | 0.55 ± 0.07 ***,$$$,£££,& | η² = 0.968 |
| Δ% | −30 ± 7 | −37 ± 5 $$ | −54 ± 7 $$$,£££ | −48 ± 6 $$$,£££,& | η² = 0.922 | |
| PPA VL (mV) | 3.88 ± 0.67 | 3.66 ± 0.67 | 3.25 ± 0.80 | 3.09 ± 0.76 * | 3.15 ± 0.72 * | η² = 0.572 |
| Δ% | −5 ± 13 | −16 ± 14 | −21 ± 14 $ | −19 ± 13 | η² = 0.503 | |
| PPA VM (mV) | 3.74 ± 0.78 | 3.58 ± 0.75 | 3.32 ± 0.82 | 3.35 ± 0.91 | 3.39 ± 0.90 | η² = 0.406 |
| Δ% | −4 ± 11 | −12 ± 12 | −11 ± 14 | −10 ± 13 | η² = 0.396 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froyd, C.; Beltrami, F.G.; Noakes, T.D. Neuromuscular Fatigue at Task Failure and During Immediate Recovery after Isometric Knee Extension Trials. Sports 2018, 6, 156. https://doi.org/10.3390/sports6040156
Froyd C, Beltrami FG, Noakes TD. Neuromuscular Fatigue at Task Failure and During Immediate Recovery after Isometric Knee Extension Trials. Sports. 2018; 6(4):156. https://doi.org/10.3390/sports6040156
Chicago/Turabian StyleFroyd, Christian, Fernando G. Beltrami, and Timothy D. Noakes. 2018. "Neuromuscular Fatigue at Task Failure and During Immediate Recovery after Isometric Knee Extension Trials" Sports 6, no. 4: 156. https://doi.org/10.3390/sports6040156
APA StyleFroyd, C., Beltrami, F. G., & Noakes, T. D. (2018). Neuromuscular Fatigue at Task Failure and During Immediate Recovery after Isometric Knee Extension Trials. Sports, 6(4), 156. https://doi.org/10.3390/sports6040156





