Effects of Alloying Elements Addition on Delayed Fracture Properties of Ultra High-Strength TRIP-Aided Martensitic Steels
Abstract
:1. Introduction
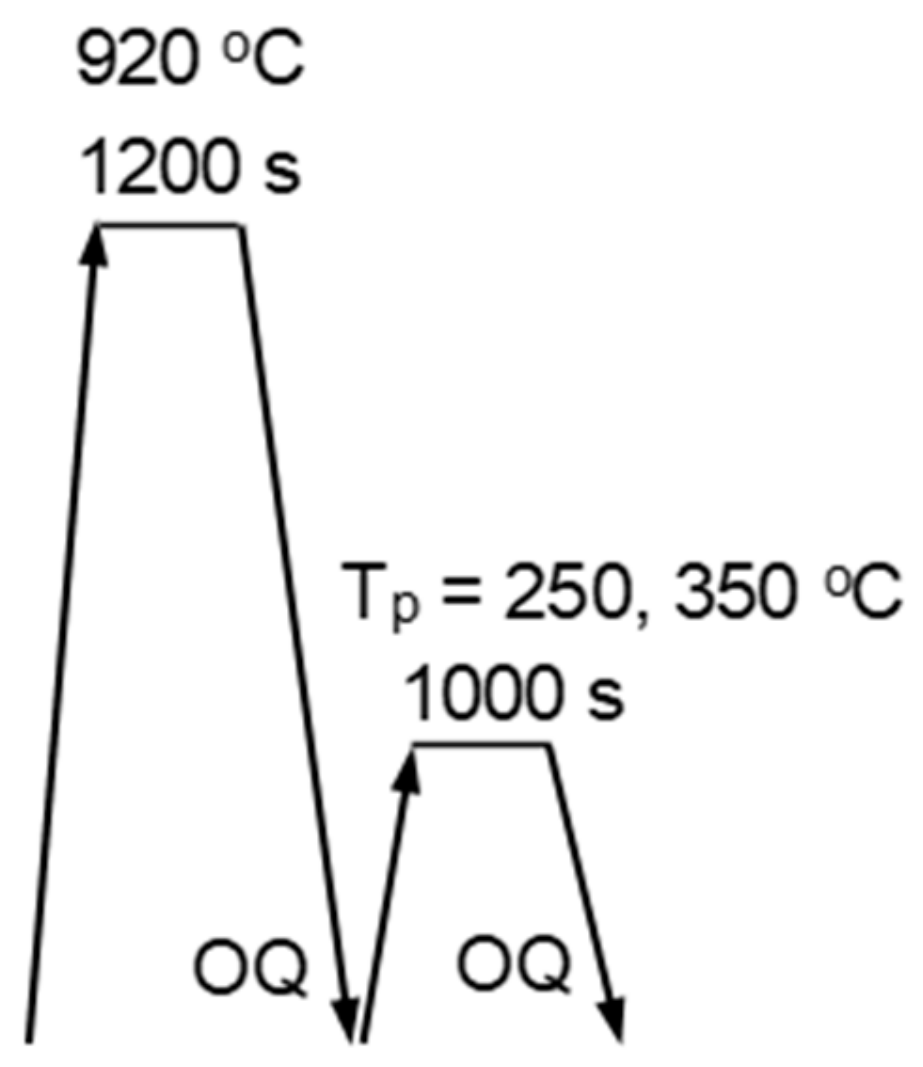
2. Materials and Methods
3. Results
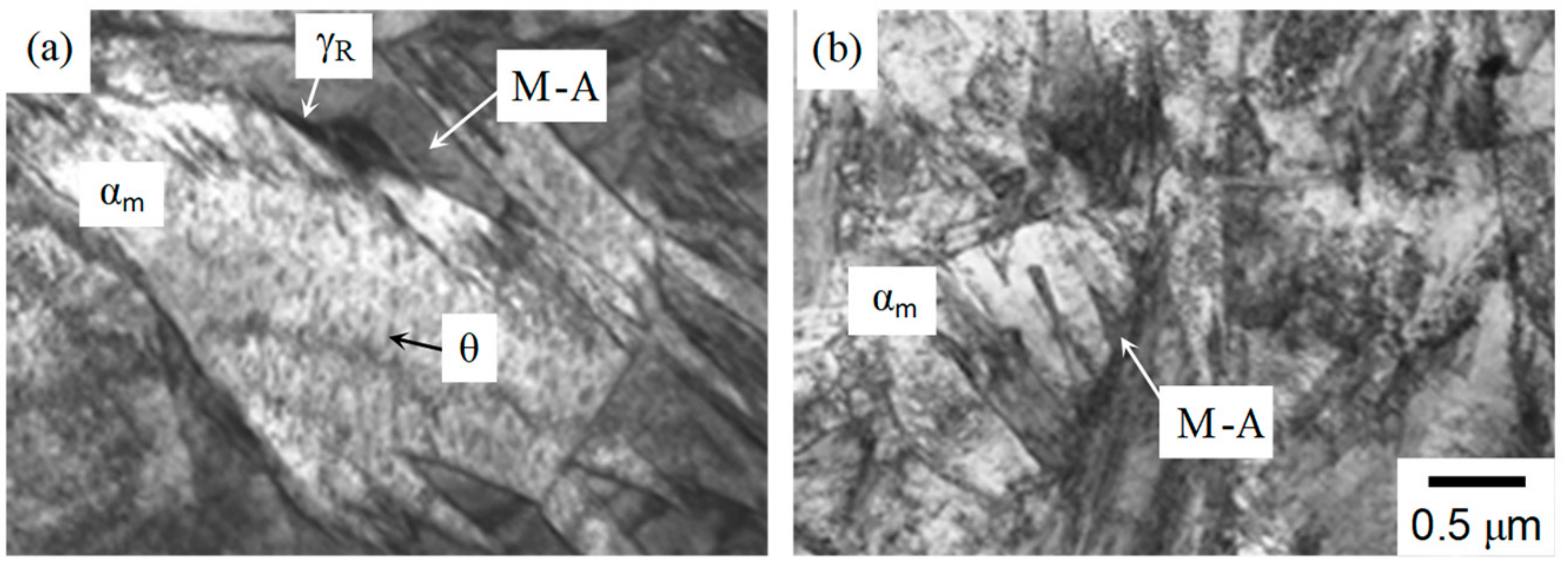
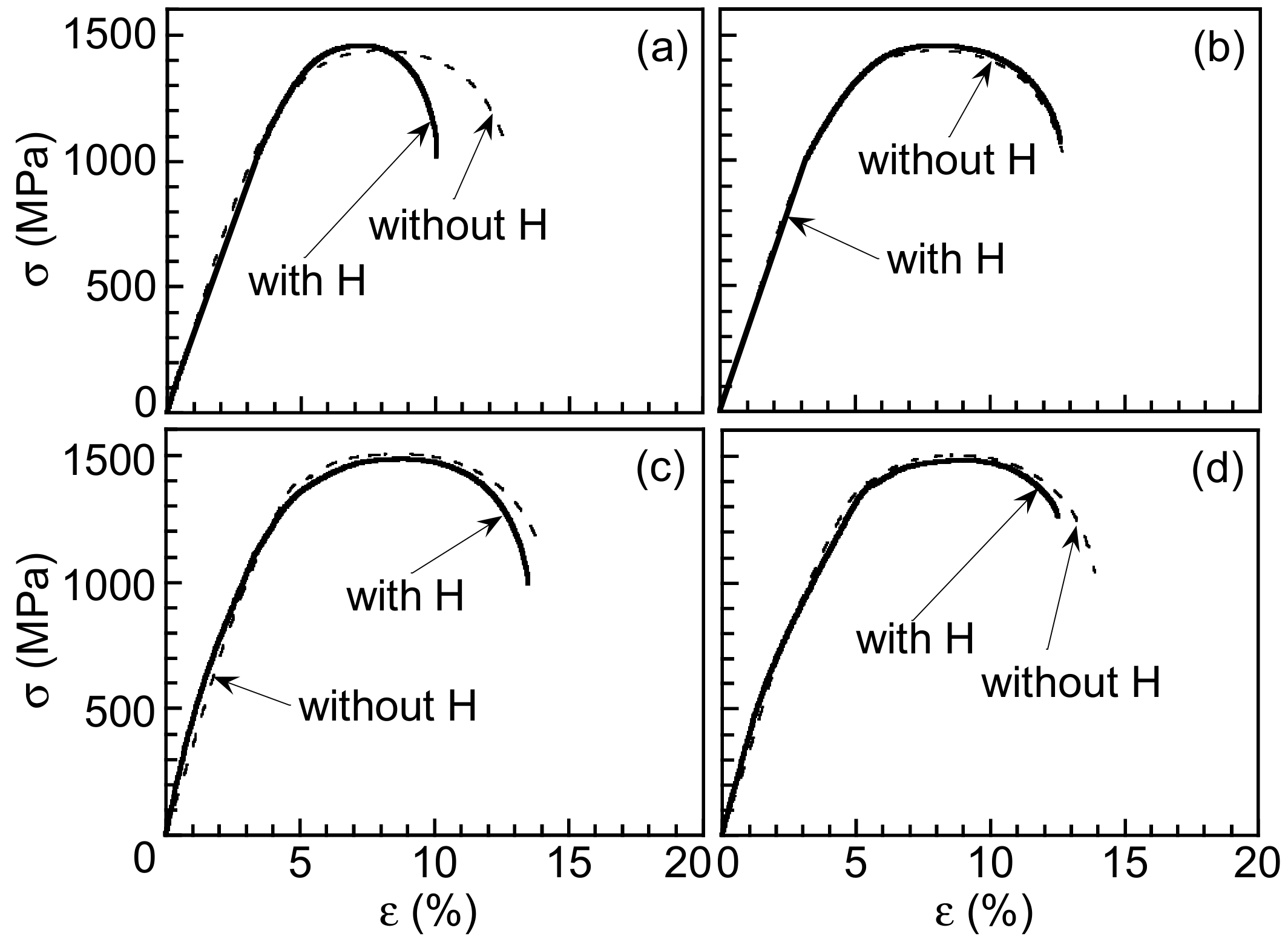
3.1. Microstructure and Tensile Properties
3.2. Hydrogen Enbrittlement Properties Evaluated by Four-Point Bending Test
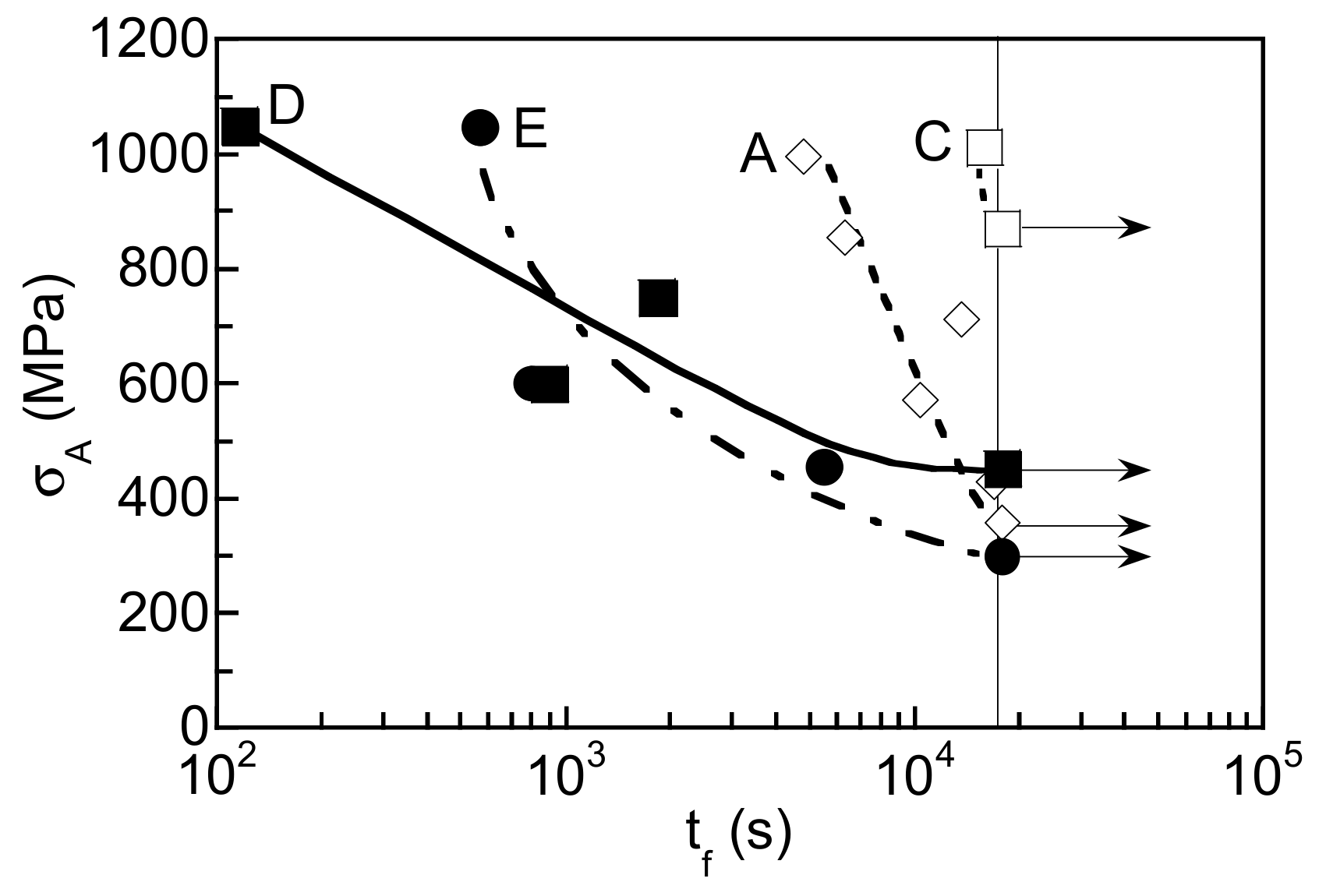
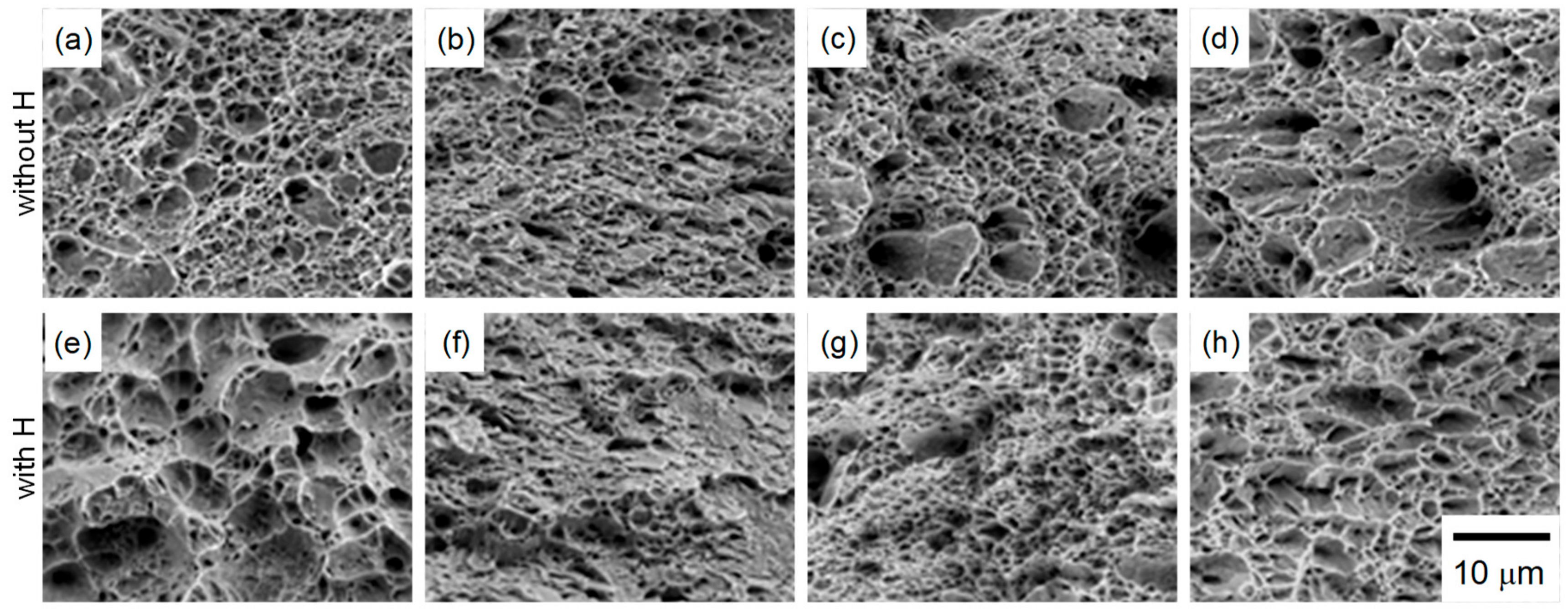
3.3. Hydrogen Embrittlement Properties Evaluated by Tensile Test
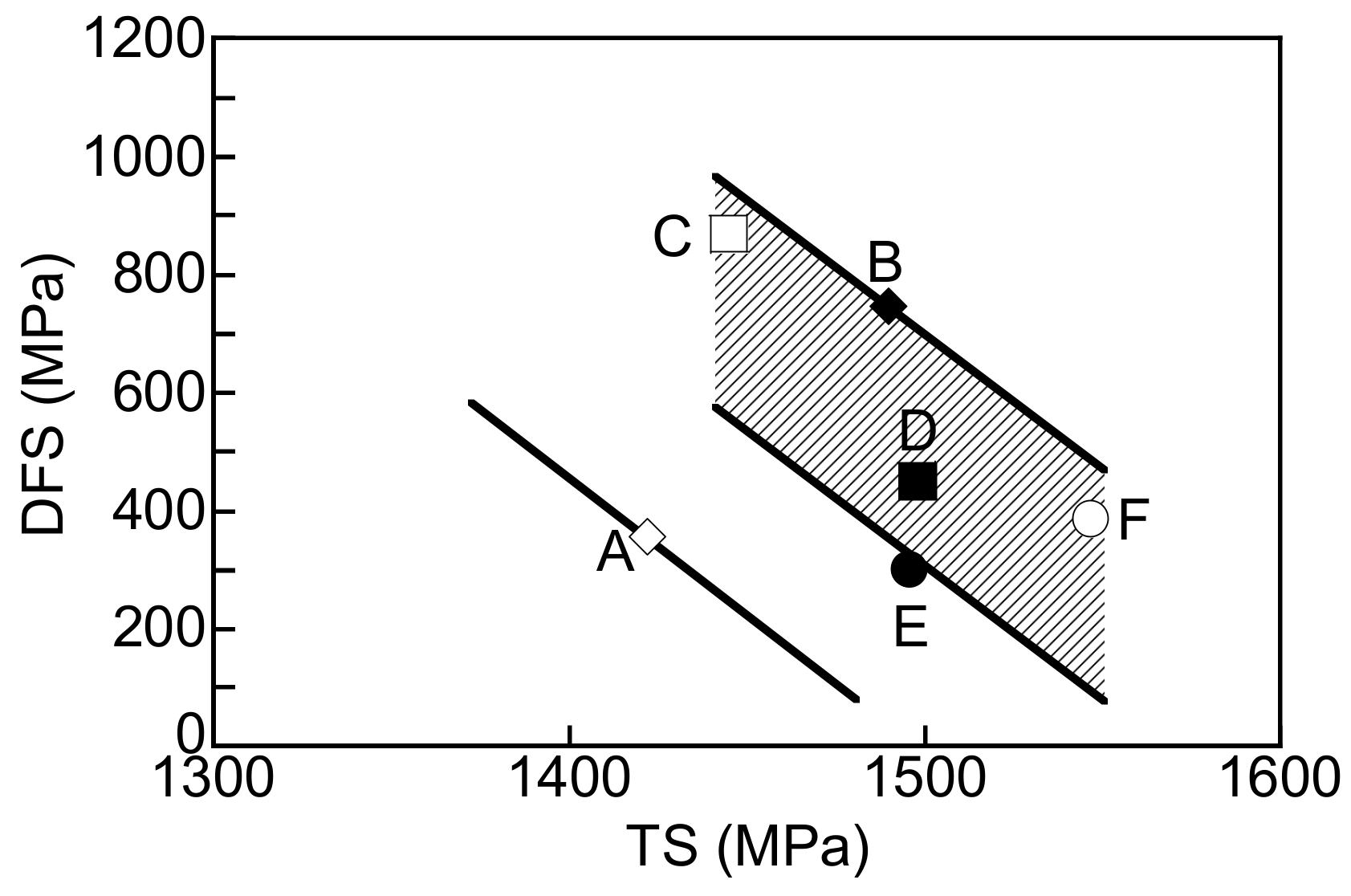
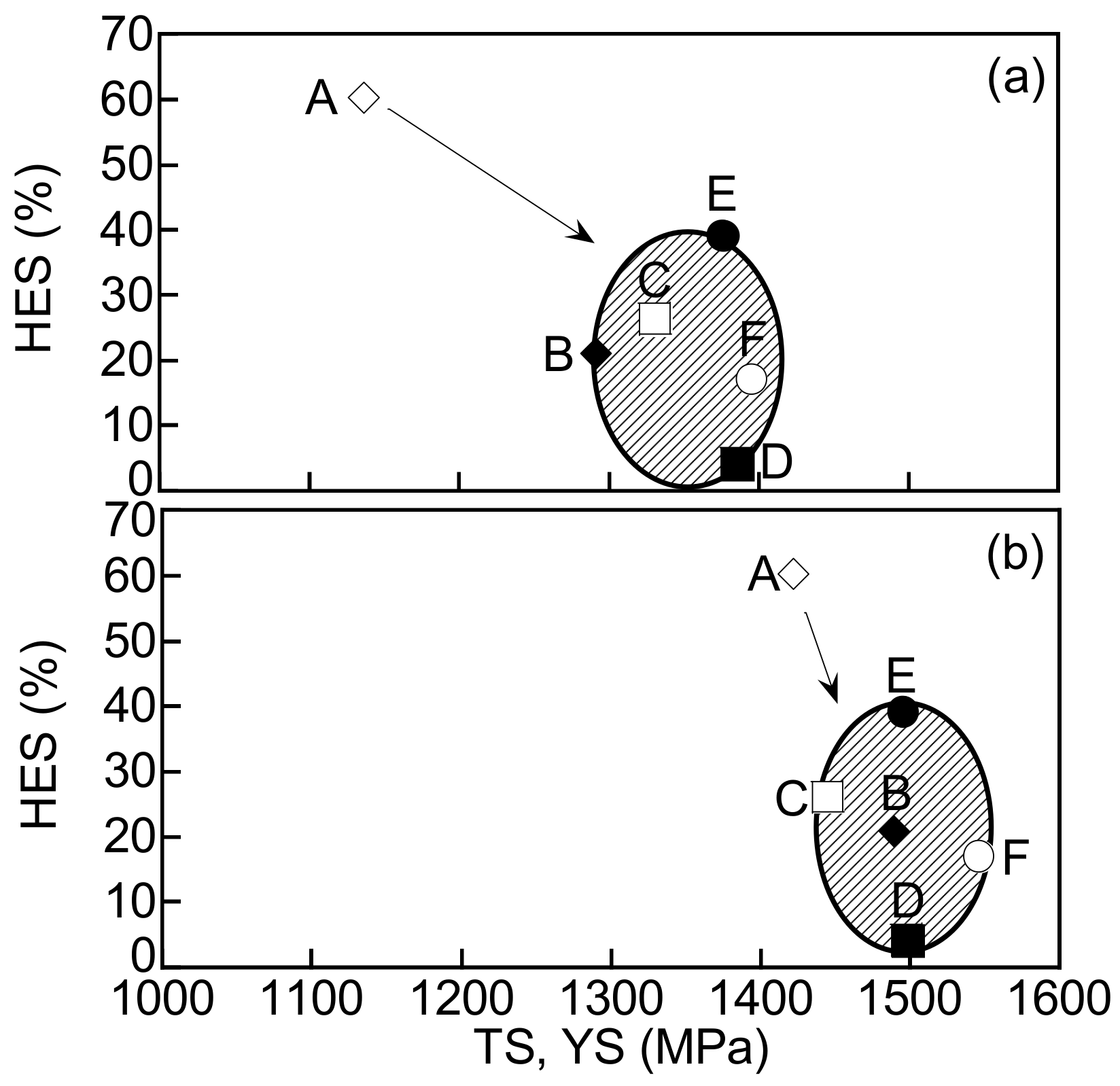
4. Discussion
4.1. Improvement of Hydrogen Embrittlement Properties by Refinement of Microstructure
4.2. Effects of Stability of Retained Austenite on Hydrogen Embrittlement Properties
4.3. Hydrogen Absorption Properties and Hydrogen Embrittlement Resistance
4.4. Mechanism of Hydrogen Embrittlement in TM steels
5. Conclusions
- (1)
- Hydrogen embrittlement resistance was improved by the addition of alloying elements, especially 1.0 mass% chromium addition was most effective to improve the hydrogen embrittlement resistance.
- (2)
- Higher hydrogen embrittlement resistance of the microalloyed TRIP-aided martensitic steels was obtained by (i) the suppression of initiation and propagation of voids and cracks at prior austenite grain, packet, block, and lath boundaries owing to the refinement of their sizes; (ii) the suppression of the hydrogen trapping at martensite matrix/cementite interfaces because of the hindered precipitation of cementite; and (iii) the restriction of martensitic transformation of retained austenite because of the high stability of retained austenite.
Author Contributions
Funding
Conflicts of Interest
References
- Zackay, V.F.; Parker, E.R.; Fahr, D.; Bush, R. The enhancement of ductility in high-strength steels. Trans. Am. Soc. Met. 1967, 60, 252–258. [Google Scholar]
- Sugimoto, K.; Nakano, K.; Song, S.M.; Kashima, T. Retained austenite characteristics and stretch-flangeability of high-strength low-alloy TRIP type bainitic sheet steels. ISIJ Int. 2002, 42, 450–455. [Google Scholar] [CrossRef]
- Hojo, T.; Kobayashi, J.; Sugimoto, K. Impact properties of low-alloy transformation-induced plasticity-steels with different matrix. Mater. Sci. Technol. 2016, 32, 1035–1042. [Google Scholar] [CrossRef]
- Song, S.M.; Sugimoto, K.; Kandaka, S.; Futamura, A.; Kobayashi, M.; Masuda, S. Effects of prestraining on high-cycle fatigue strength of high-strength low alloy TRIP-aided steels. Mater. Sci. Res. Int. 2003, 52, 223–229. [Google Scholar] [CrossRef]
- Hojo, T.; Sugimoto, K.; Mukai, Y.; Ikeda, S. Effects of aluminum on delayed fracture properties of ultra high strength low alloy TRIP-aided steels. ISIJ Int. 2008, 48, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, B.; Huang, M.-d.; Cui, D. Effect of hot stamping parameters on the mechanical properties and microstructure of cold-rolled 22MnB5 steel strips. Int. J. Miner. Metall. Mater. 2014, 21, 544–555. [Google Scholar] [CrossRef]
- Hagihara, Y. Evaluation of delayed fracture characteristics of high-strength bolt steels by CSRT. ISIJ Int. 2012, 52, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Takagi, S.; Hagihara, Y.; Hojo, T.; Urushihara, W.; Kawasaki, K. Comparison of hydrogen embrittlement resistance of high strength steel sheets evaluated by several methods. ISIJ Int. 2016, 56, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Chida, T.; Hagihara, Y.; Akiyama, E.; Iwanaga, K.; Takagi, S.; Hayakawa, M.; Ohishi, H.; Hirakami, D.; Tarui, T. Comparison of constant load, SSRT and CSRT methods for hydrogen embrittlement evaluation using round bar specimens of high strength steels. ISIJ Int. 2016, 56, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Tamura, I. Steel Material Study on the Strength; Nikkan-Kogyo Shinbun Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Grossmann, M.A. Hardenability calculated from chemical composition. Trans. AIME 1942, 150, 227–255. [Google Scholar]
- Kobayashi, J.; Ina, D.; Nakajima, Y.; Sugimoto, K. Effects of microalloying on the impact toughness of ultrahigh-strength TRIP-aided martensitic steels. Metall. Mater. Trans. A 2013, 44, 5006–5017. [Google Scholar] [CrossRef] [Green Version]
- Dyson, D.J.; Holmes, B. Effect of alloying additions on the lattice parameter of austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Valentini, R.; Tedesco, M.M.; Corsinovi, S.; Bacchi, L.; Villa, M. Investigation of mechanical tests for hydrogen embrittlement in automotive PHS steels. Metals 2019, 9, 934. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.P.M.; Paxton, H.W. Effect of prior-austenite grain-size on stress-corrosion cracking susceptibility of AISI-4340 steel. Asm Trans. Q. 1969, 62, 989. [Google Scholar]
- Nagao, A.; Hayashi, K.; Oi, K.; Mitao, S. Effect of uniform distribution of fine cementite on hydrogen embrittlement of low carbon martensitic steel plates. ISIJ Int. 2012, 52, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Cho, L.; Seo, E.J.; Sulistiyo, D.H.; Jo, K.R.; Kim, S.W.; Oh, J.K.; Cho, Y.R.; De Cooman, B.C. Influence of vanadium on the hydrogen embrittlement of aluminized ultra-high strength press hardening steel. Mater. Sci. Eng. A 2018, 735, 448–455. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y.; Tarui, T. The first direct observation of hydrogen trapping sites in TiC precipitation-hardening steel through atom probe tomography. Scr. Mater. 2010, 63, 261–264. [Google Scholar] [CrossRef]
- Sugimoto, K.; Murata, M.; Song, S.M. Formability of Al–Nb bearing ultra high-strength TRIP-aided sheet steels with bainitic ferrite and/or martensite matrix. ISIJ Int. 2010, 50, 162–168. [Google Scholar] [CrossRef]
- Van Pham, D.; Kobayashi, J.; Sugimoto, K. Effects of microalloying on stretch-flangeability of ultrahigh-strength TRIP-aided martensitic steel sheets. ISIJ Int. 2014, 54, 1943–1951. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Tonegawa, H.; Sugimoto, K. Cold formability of 22SiMnCrB TRIP-aided martensitic sheet steel. Procedia Eng. 2014, 81, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Hojo, T.; Koyama, M.; Terao, N.; Tsuzaki, K.; Akiyama, E. Transformation-assisted hydrogen desorption during deformation in steels: Examples of α’- and ε-martensite. Int. J. Hydrog. Energy 2019, 44, 30472–30477. [Google Scholar] [CrossRef]
- Hojo, T.; Kobayashi, J.; Kochi, T.; Sugimoto, K. Effects of thermomechanical processing on microstructure and shear properties of 22SiMnCrMoB TRIP-aided martensitic steel. Iron Steel Technol. 2015, 10, 102–110. [Google Scholar]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen and stress concentration on the notch tensile strength of AISI 4135 steel. Mater. Sci. Eng. A 2005, 398, 37–46. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of hydrogen embrittlement in steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of strain rate on hydrogen embrittlement in low-carbon martensitic steel. Int. J. Hydrog. Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Chan, S.L.I.; Lee, H.L.; Yang, J.R. Effect of retained austenite on the hydrogen content and effective diffusivity of martensitic structure. Metall. Trans. A 1991, 22, 2579–2586. [Google Scholar] [CrossRef]
- Gu, J.; Chang, K.D.; Fang, H.; Bai, B. Delayed fracture properties of 1 500 MPa bainite/martensite dual-phase high strength steel and its hydrogen traps. ISIJ Int. 2002, 42, 1560–1564. [Google Scholar] [CrossRef] [Green Version]







| Steel | C | Si | Mn | Ni | Cr | Mo | Al | Nb | Ti | B | MS | Πfi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.20 | 1.50 | 1.50 | - | - | - | 0.039 | - | - | - | 420 | 14.60 |
| B | 0.20 | 1.52 | 1.50 | - | - | - | 0.039 | 0.05 | 0.02 | 0.0018 | 420 | 29.21 |
| C | 0.21 | 1.49 | 1.50 | - | 0.50 | - | 0.04 | 0.05 | - | - | 407 | 30.55 |
| D | 0.20 | 1.49 | 1.50 | - | 1.00 | - | 0.04 | 0.05 | - | - | 401 | 46.99 |
| E | 0.18 | 1.48 | 1.49 | - | 1.02 | 0.20 | 0.043 | 0.05 | - | - | 407 | 76.82 |
| F | 0.21 | 1.49 | 1.49 | 1.52 | 1.00 | 0.20 | 0.034 | 0.049 | - | - | 370 | 135.8 |
| Steel | TS | YS | TEl | UEl | fγ0 | Cγ0 | fM–A | d |
|---|---|---|---|---|---|---|---|---|
| A | 1422 | 1137 | 12.6 | 4.2 | 2.1 | 1.20 | 20.0 | 22.6 |
| B | 1490 | 1292 | 13.9 | 5.6 | 2.0 | 1.32 | 21.4 | 16.0 |
| C | 1445 | 1331 | 12.6 | 4.7 | 1.8 | 0.75 | 22.3 | 14.3 |
| D | 1498 | 1386 | 14.2 | 5.1 | 2.2 | 1.19 | 20.7 | 14.8 |
| E | 1496 | 1376 | 15.3 | 5.1 | 2.2 | 0.52 | 24.9 | 11.3 |
| F | 1547 | 1396 | 14.1 | 4.3 | 1.9 | 0.96 | 26.7 | 12.7 |
| Steels | HD (wt. ppm) |
|---|---|
| A | 0.65 |
| B | 0.85 |
| C | 0.90 |
| D | 0.93 |
| E | 0.88 |
| F | 0.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojo, T.; Kobayashi, J.; Sugimoto, K.-i.; Nagasaka, A.; Akiyama, E. Effects of Alloying Elements Addition on Delayed Fracture Properties of Ultra High-Strength TRIP-Aided Martensitic Steels. Metals 2020, 10, 6. https://doi.org/10.3390/met10010006
Hojo T, Kobayashi J, Sugimoto K-i, Nagasaka A, Akiyama E. Effects of Alloying Elements Addition on Delayed Fracture Properties of Ultra High-Strength TRIP-Aided Martensitic Steels. Metals. 2020; 10(1):6. https://doi.org/10.3390/met10010006
Chicago/Turabian StyleHojo, Tomohiko, Junya Kobayashi, Koh-ichi Sugimoto, Akihiko Nagasaka, and Eiji Akiyama. 2020. "Effects of Alloying Elements Addition on Delayed Fracture Properties of Ultra High-Strength TRIP-Aided Martensitic Steels" Metals 10, no. 1: 6. https://doi.org/10.3390/met10010006






