The Influence of Test-Panel Orientation and Exposure Angle on the Corrosion Rate of Carbon Steel. Mathematical Modelling
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Concentrations of Pollutants and Measurement of Corrosion Rates
3.2. Corrosion Rates
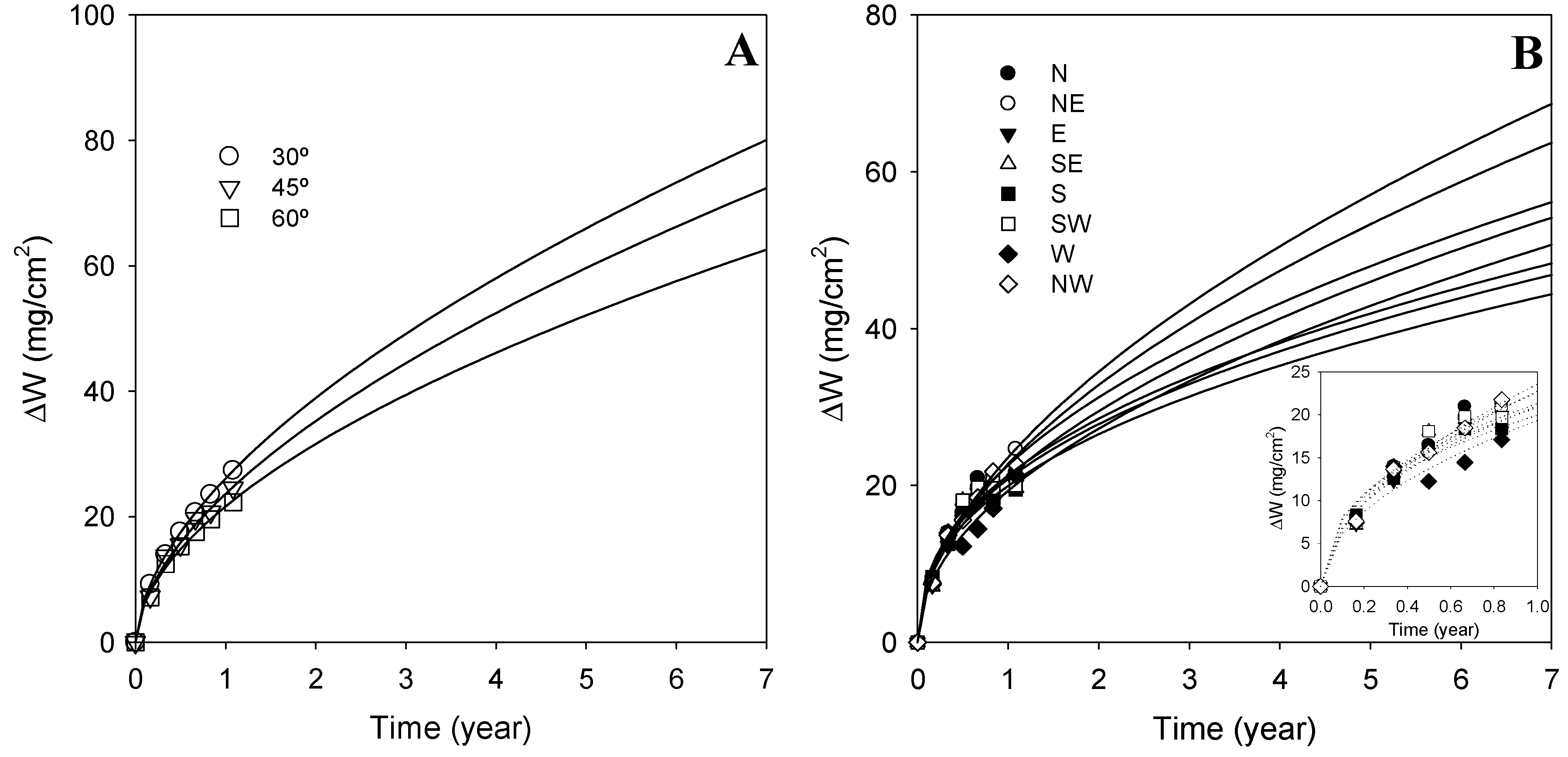
3.3. Mathematical Models
3.3.1. Exposure Angle
3.3.2. Orientation Angle
3.3.3. Global Mode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ghali, E.; Sastri, V.S.; Elboujdaini, M. Corrosion Prevention and Protection. Practical Solutions; John Wiley & Sons, Ltd.: Chichester, UK, 2007; ISBN 9780470024546. [Google Scholar]
- Cramer, S.D.; Covino, B.S., Jr. Corrosion, Fundamentals, Testing and Applications, ASM Handbook Series, 1st ed.; American Society for Testing Materials Int.: Columbus, OH, USA, 2003; Volume 13A, ISBN 978-1-62708-182-5. [Google Scholar]
- Schweitzer, P.A. Fundamentals of Metallic Corrosion; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429127137. [Google Scholar]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118762134. [Google Scholar]
- Schweitzer, P.A. Atmospheric Degradation and Corrosion Control; CRC Press: New York, NY, USA, 1999; ISBN 9780824777098. [Google Scholar]
- Veleva, L.; Kane, R.D. Atmospheric corrosion. In ASM Handbook Volume 13A: Corrosion: Fundamentals, Testing, and Protection; Cramer, S.D., Covino, B.S., Jr., Eds.; American Society for Testing Materials Int.: Columbus, OH, USA, 2003; Volume 13A, pp. 196–209. ISBN 978-0-87170-705-5. [Google Scholar]
- Giardina, M.; Buffa, P. A new approach for modeling dry deposition velocity of particles. Atmos. Environ. 2018, 180, 11–22. [Google Scholar] [CrossRef]
- Spence, J.W.; Lipfert, F.W.; Katz, S. The effect of specimen size, shape, and orientation on dry deposition to galvanized steel surfaces. Atmos. Environ. Part A. Gen. Top. 1993, 27, 2327–2336. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Vera, R.; Rosales, B.M.; Tapia, C. Effect of the exposure angle in the corrosion rate of plain carbon steel in a marine atmosphere. Corros. Sci. 2003, 45, 321–337. [Google Scholar] [CrossRef]
- Edney, E.O.; Cheek, S.F.; Stiles, D.C.; Corse, E.W. Field study investigations of the impact of shape, size and orientation on dry deposition induced corrosion of galvanized steel. Atmos. Environ. Part A. Gen. Top. 1992, 26, 2353–2363. [Google Scholar] [CrossRef]
- Coburn, S.K.; Komp, M.E.; Lore, S.C. Atmospheric corrosion rates of weathering steels at test sites in the Eastern United States—Effect of environment and test-panel orientation. In Atmospheric Corrosion; Kirk, W., Lawson, H., Eds.; ASTM STP 1239; American Society for Testing Materials Int.: West Conshocken, PA, USA, 1995; pp. 100–113. [Google Scholar]
- Vasconcelos, H.C.; Fernández-Pérez, B.M.; Morales, J.; Souto, R.M.; González, S.; Cano, V.; Santana, J.J. Development of mathematical models to predict the atmospheric corrosion rate of carbon steel in fragmented subtropical environments. Int. J. Electrochem. Sci. 2014, 9, 6514–6528. [Google Scholar]
- Spence, J.W.; McHenry, J.N. Development of regional corrosion maps for galvanized steel by linking the RADM engineering model with an atmospheric corrosion model. Atmos. Environ. 1994, 28, 3033–3046. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M.; Feliu, S. The prediction of atmospheric corrosion from meteorological and pollution parameters—II. Long-term forecasts. Corros. Sci. 1993, 34, 415–422. [Google Scholar] [CrossRef]
- Tidblad, J.; Mikhailov, A.; Kucera, V. Application of a model for prediction of atmospheric corrosion in tropical environments. In Marine Corrosion in Tropical Environments; Dean, S., Delgadillo, G.-D., Bushman, J., Eds.; ASTM International: West Conshohocken, PA, USA, 2000; pp. 250–285. ISBN 978-0-8031-2873-6. [Google Scholar]
- Tidblad, J.; Kucera, V.; Mikhailov, A.; Knotkova, D. Improvement of the ISO classification system based on dose-response functions describing the corrosivity of outdoor atmospheres. In Outdoor Atmospheric Corrosion; Townsend, H.E., Ed.; ASTM International: West Conshohocken, PA, USA, 2002; pp. 73–87. ISBN 978-0-8031-5467-4. [Google Scholar]
- Spence, J.W.; Haynie, F.H.; Lipfert, F.W.; Cramer, S.D.; McDonald, L.G. Atmospheric corrosion model for galvanized steel structures. Corrosion 1992, 48, 1009–1019. [Google Scholar] [CrossRef]
- Pourbaix, M. The linear bilogarithmic law for atmospheric corrosion. In Atmospheric Corrosion; Ailor, W.H., Ed.; J. Wiley & Sons: New York, NY, USA, 1982; pp. 107–121. [Google Scholar]
- Panchenko, Y.M.; Strekalov, P.V. Correlation between corrosion mass losses and corrosion product quantity retained on metals in a cold, moderate and tropical climate. Prot. Met. 1994, 30, 459–467. [Google Scholar]
- Morcillo, M.; Feliu, S.; Simancas, J. Deviation from bilogarithmic law for atmospheric corrosion of steel. Br. Corros. J. 1993, 28, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Almeida, E.; Morcillo, M.; Rosales, B.; Marrocos, M. Atmospheric corrosion of mild steel. Part I—Rural and urban atmospheres. Mater. Corros. 2000, 51, 859–864. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B. Atmospheric corrosion of mild steel. Part II—Marine atmospheres. Mater. Corros. 2000, 51, 865–874. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion mechanisms for iron and low alloy steels exposed to the atmosphere. J. Electrochem. Soc. 1990, 137, 2385. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Santana Rodríguez, J.J.; Santana Hernández, F.J.; González González, J.E. Mathematical and electro-chemical characterisation of the layer of corrosion products on carbon steel in various environments. Corros. Sci. 2002, 44, 2597–2610. [Google Scholar] [CrossRef]
- Zoccola, J.C.; Permoda, A.J.; Oehler, L.T.; Horton, J.B. Performance of Mayari R Weathering Steel (ASTM A242) in Bridges at the Eight Mile Road and John Lodge Expressway in Detroit, Michigan. Bethlehem Steel Corporation: Bethlehem, PA, USA. Available online: https://www.michigan.gov/documents/mdot/RR213CON_67_534507_7.pdf (accessed on 28 January 2020).
- Santana, J.J.; Santana, F.J.; González, J.E.; de la Fuente, D.; Chico, B.; Morcillo, M. Atmospheric corrosivity map for steel in Canary Isles. Br. Corros. J. 2001, 36, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Santana, J.J.; Ramos, A.; Rodriguez-Gonzalez, A.; Vasconcelos, H.C.; Mena, V.; Fernández-Pérez, B.M.; Souto, R.M. Shortcomings of International Standard ISO 9223 for the classification, determination, and estimation of atmosphere corrosivities in subtropical archipelagic conditions—The case of the Canary Islands (Spain). Metals 2019, 9, 1105. [Google Scholar] [CrossRef] [Green Version]
- ASTM D2010/D2010M-98(2017), Standard Test Methods for Evaluation of Total Sulfation Activity in the Atmosphere by the Lead Dioxide Technique; ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 9225:2012, Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Measurement of Environmental Parameters Affecting Corrosivity of Atmospheres, 2nd ed.; International Organization for Standardization: Geneva, The Switzerland, 2012.
- ASTM G1-90, Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; American Society for Testing Materials: Philadelphia, PA, USA, 1990.
- ISO 9223:2012, Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Clasification, Determination and Estimation, 2nd ed.; International Organization for Standardization: Geneva, The Switzerland, 2012.
- Larrabee, C.P. Corrosion resistance of high-strength low-alloy steels as influenced by composition and environment. Corrosion 1953, 9, 259–271. [Google Scholar] [CrossRef]
- LaQue, F.L. Corrosion testing. In ASTM Proceeding 1951—Volume 51; American Society for Testing and Materials: Philadelphia, PA, USA, 1951; Volume 51, pp. 495–582. [Google Scholar]
- Binh, D.T.; Strekalov, P.V.; Van Khuong, N. Effects of seasonal conditions and the slope of a specimen on the atmospheric corrosion of steel in the tropics of Viet-Nam. Prot. Met. 2003, 39, 278–287. [Google Scholar] [CrossRef]
- Veleva, L.; Maldonado, L. Classification of atmospheric corrosivity in humid tropical climates. Br. Corros. J. 1998, 33, 53–58. [Google Scholar] [CrossRef]
- Rostron, P.; Belbarak, C. Atmospheric corrosion issues in Abu Dhabi. Mater. Perform. 2015, 54, 1–7. [Google Scholar]
- Porro, A.; Otero, T.F.; Elola, A.S. Gravimetric corrosion monitoring and mathematical fitting of atmospheric corrosion data for carbon steel. Br. Corros. J. 1992, 27, 231–235. [Google Scholar] [CrossRef]
- Santana Rodríguez, J.J.; Santana Hernández, F.J.; González González, J.E. XRD and SEM studies of the layer of corrosion products for carbon steel in various different environments in the province of Las Palmas (The Canary Islands, Spain). Corros. Sci. 2002, 44, 2425–2438. [Google Scholar] [CrossRef]


| Test Site | Elevation (m) | Geographic Coordinate | Atmosphere | |
|---|---|---|---|---|
| North Latitude | West Longitude | |||
| Power station of Jinámar | 30 | 28° 02′ 30″ N | 15° 24′ 39″ W | Industrial marine |
| Composition (wt. %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | C | Mn | Zn | Ti | Cu | Mg | Al | Others | Fe |
| 0.08 | 99.47 | 0.06 | 0.37 | - | - | - | - | - | 0.02 | balance |
| Exposure Time (day) | SO2 (mg/(m2·day)) | Cl− (mg/(m2·day)) | TOW (h/year) |
|---|---|---|---|
| 60 | 2.99 | 85.43 | 862 |
| 123 | 2.17 | 78.10 | 1552 |
| 182 | 4.47 | 80.86 | 1897 |
| 243 | 6.74 | 68.45 | 2673 |
| 305 | 7.12 | 64.68 | 3553 |
| 396 | 7.33 | 72.36 | 4312 |
| Time (day) | rcorr (µm/year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Angle | Orientation | ||||||||||
| 30° | 45° | 60° | N | NE | E | SE | S | SW | W | NW | |
| 60 | 71.1 | 56.1 | 55.1 | 61.38 | 62.60 | 56.15 | 54.40 | 64.22 | 56.91 | 57.07 | 58.53 |
| 123 | 52.4 | 51.7 | 46.9 | 52.52 | 51.72 | 46.29 | 51.82 | 47.19 | 52.03 | 52.50 | 51.26 |
| 182 | 44.6 | 39.5 | 38.8 | 41.77 | 39.46 | 39.70 | 45.86 | 40.88 | 45.86 | 31.08 | 39.60 |
| 243 | 39.3 | 37.5 | 33.4 | 39.78 | 37.51 | 36.22 | 36.91 | 34.87 | 37.57 | 27.48 | 35.10 |
| 305 | 35.7 | 31.4 | 29.6 | 32.25 | 31.44 | 30.19 | 28.63 | 27.81 | 29.78 | 25.89 | 32.96 |
| 396 | 31.9 | 24.7 | 26.0 | 25.18 | 28.76 | 23.50 | 22.92 | 22.72 | 23.33 | 24.91 | 26.48 |
| Constants | Exposure Angle | Orientation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30° | 45° | 60° | N | NE | E | SE | S | SW | W | NW | |
| k | 33.0920 (0.049) | 30.3580 (2.3768) | 28.7564 (1.2736) | 31.1281 (2.1182) | 30.6990 (1.3036) | 28.8354 (1.6732) | 30.4171 (3.4883) | 27.0422 (1.3639) | 30.7166 (3.1321) | 25.0282 (3.0355) | 30.3778 (1.7038) |
| n | −0.4236 (0.0012) | −0.3714 (0.06505) | −0.3789 (0.03663) | −0.3993 (0.0556) | −0.4079 (0.0345) | −0.3877 (0.04774) | −0.3687 (0.0954) | −0.4901 (0.0392) | −0.3810 (0.0842) | −0.4847 (0.0944) | −0.3848 (0.0462) |
| 0.9999 | 0.8902 | 0.9629 | 0.9273 | 0.9710 | 0.9419 | 0.7935 | 0.9795 | 0.8392 | 0.8664 | 0.9441 | |
| Variables | Model (6) | Model (7) | Model (8) | Model (9) | Model (10) | Model (11) | Model (12) | Model (13) | Model (14) | Model (15) | Model (16) | Model (17) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | 4.2192 * (0.0258) | 4.2484 * (0.0353) | 3.3580 * (0.0190) | 3.3943 * (0.0379) | 4.2499 * (0.2587) | 4.2862 * (0.1851) | 3.8302 * (0.2637) | 3.8666 * (0.1887) | 5.2157 * (0.7226) | 5.2521 * (0.5119) | 4.7733 * (0.6890) | 4.8097 * (0.4880) |
| TEXP | −0.9193 * (0.0385) | −0.9193 * (0.0289) | - | - | - | - | - | - | - | - | - | - |
| SO2 | - | - | - | - | −0.0726 ** (0.0383) | −0.0726 * (0.0271) | −0.1092 * (0.0345) | −0.1092 * (0.0245) | - | - | - | - |
| CL | - | - | - | - | −0.0133 (0.0082) | −0.0133 ** (0.0058) | −0.0287 * (0.0093) | −0.0287 * (0.0066) | - | - | - | - |
| TOW | - | - | - | - | −0.8768 * (0.2996) | −0.8768 * (0.2121) | - | - | - | - | −0.9763 * (0.2796) | −0.9763 * (0.1978) |
| LTEXP | - | - | −0.4463 * (0.0206) | −0.4463 * (0.0167) | −0.2363 * (0.0616) | −0.2363 * (0.0435) | 0.1289 (0.1760) | 0.1289 (0.1247) | 0.2260 (0.1800) | 0.2260 *** (0.1274) | −0.2211 * (0.0612) | −0.2211 * (0.0395) |
| LSO2 | - | - | - | - | - | - | - | - | −0.1547 * (0.0486) | −0.1547 * (0.0332) | −0.0963 *** (0.0489) | −0.0963 * (0.0346) |
| LCL | - | - | - | - | - | - | - | - | −0.7264 * (0.2455) | −0.7264 * (0.01737) | −0.2828 (0.2066) | −0.2828 *** (0.1461) |
| LTOW | - | - | - | - | - | - | −0.6523 * (0.2236) | −0.6523 * (0.1584) | −0.7762 * (0.2225) | −0.7762 * (0.1574) | - | - |
| O2 | - | −0.0006 (0.0434) | - | −0.0006 (0.0511) | - | −0.0006 (0.0396) | - | −0.0006 (0.0396) | - | −0.0006 (0.0439) | - | −0.0006 (0.0395) |
| O3 | - | −0.0825 ** (0.0434) | - | −0.0824 (0.0511) | - | −0.0824 ** (0.0396) | - | −0.0824 ** (0.0396) | - | −0.0824 ** (0.0439) | - | −0.0824 ** (0.0395) |
| O4 | - | −0.0548 (0.0434) | - | −0.0548 (0.0511) | - | −0.0548 (0.0396) | - | −0.0548 (0.0396) | - | −0.0548 (0.0441) | - | −0.0548 (0.0395) |
| O5 | - | −0.0777 *** (0.0434) | - | −0.0776 (0.0511) | - | −0.0776 *** (0.0396) | - | −0.0776 *** (0.0396) | - | −0.0776 *** (0.0439) | - | −0.0776 *** (0.0395) |
| O6 | - | −0.0341 (0.0434) | - | −0.0341 (0.0511) | - | −0.0341 (0.0396) | - | −0.0341 (0.0396) | - | −0.0341 (0.0439) | - | −0.0341 (0.0395) |
| O7 | - | −0.1615 * (0.0434) | - | −0.1615 * (0.0511) | - | −0.1615 * (0.0396) | - | −0.1615 * (0.0396) | - | −0.1615 * (0.0439) | - | −0.1615 * (0.0395) |
| O8 | - | −0.0297 (0.0434) | - | −0.0297 (0.0511) | - | −0.0297 (0.0396) | - | −0.0297 (0.0396) | - | −0.0297 (0.0439) | - | −0.0297 (0.0395) |
| I1 | - | 0.0899 * (0.0377) | - | 0.0899 * (0.0443) | - | 0.0899 ** (0.0343) | - | 0.0899 ** (0.0343) | - | 0.0899 ** (0.0343) | - | 0.0899 ** (0.0342) |
| I2 | - | −0.0864 ** (0.0377) | - | −0.0864 * (0.0443) | - | −0.0864 ** (0.0343) | - | −0.0864 ** (0.0343) | - | −0.0864 ** (0.0343) | - | −0.0864 ** (0.0342) |
| 0.8908 | 0.9373 | 0.8684 | 0.9133 | 0.8964 | 0.9480 | 0.8964 | 0.9480 | 0.8966 | 0.9482 | 0.8966 | 0.9483 | |
| N | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, J.J.; Cano, V.; Vasconcelos, H.C.; Souto, R.M. The Influence of Test-Panel Orientation and Exposure Angle on the Corrosion Rate of Carbon Steel. Mathematical Modelling. Metals 2020, 10, 196. https://doi.org/10.3390/met10020196
Santana JJ, Cano V, Vasconcelos HC, Souto RM. The Influence of Test-Panel Orientation and Exposure Angle on the Corrosion Rate of Carbon Steel. Mathematical Modelling. Metals. 2020; 10(2):196. https://doi.org/10.3390/met10020196
Chicago/Turabian StyleSantana, Juan J., Víctor Cano, Helena C. Vasconcelos, and Ricardo M. Souto. 2020. "The Influence of Test-Panel Orientation and Exposure Angle on the Corrosion Rate of Carbon Steel. Mathematical Modelling" Metals 10, no. 2: 196. https://doi.org/10.3390/met10020196






