Effect of Preliminary Alkali Desilication on Ammonia Pressure Leaching of Low-Grade Copper–Silver Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Experiments
3. Results and Discussion
3.1. Leaching Kinetics of the Initial Concentrate
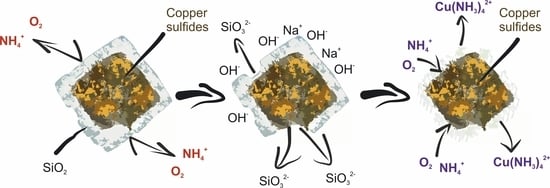
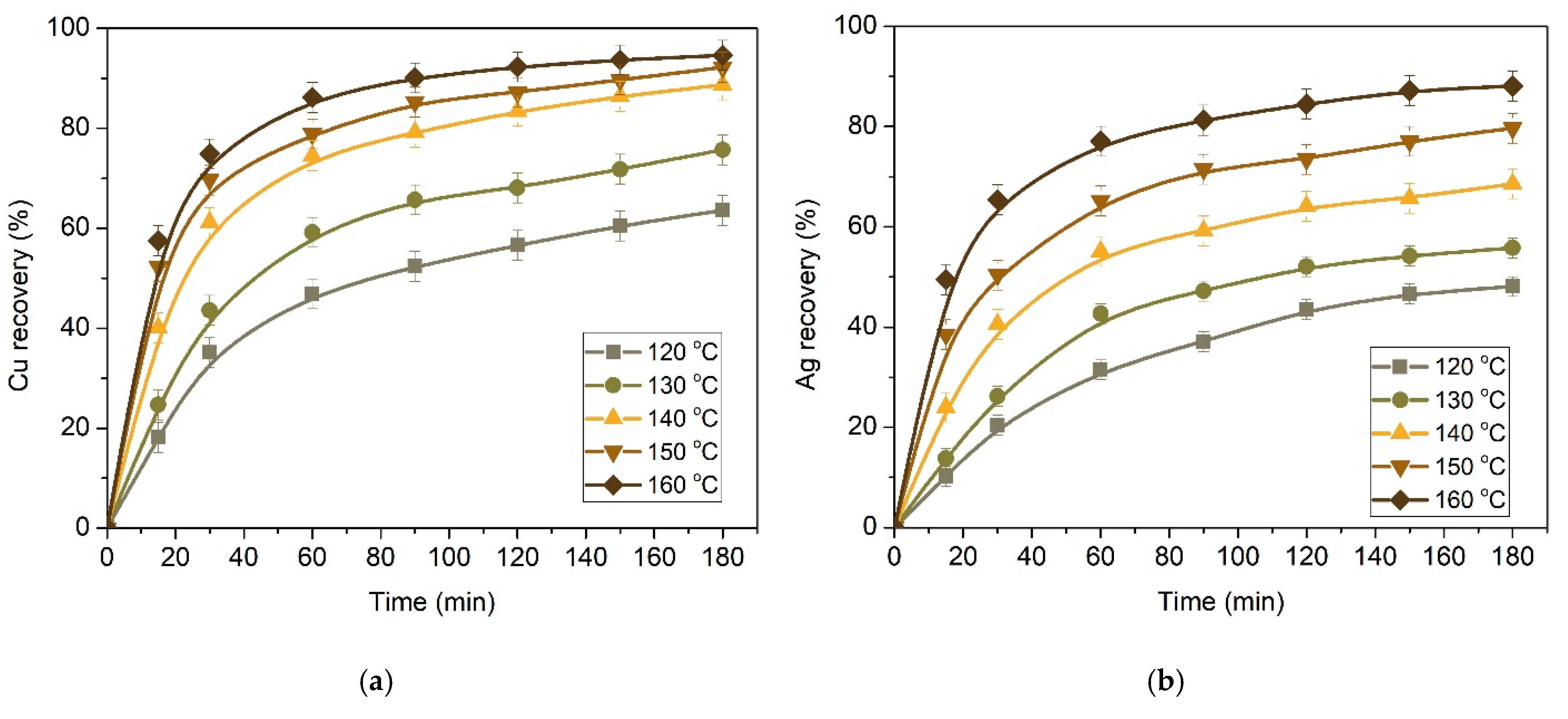
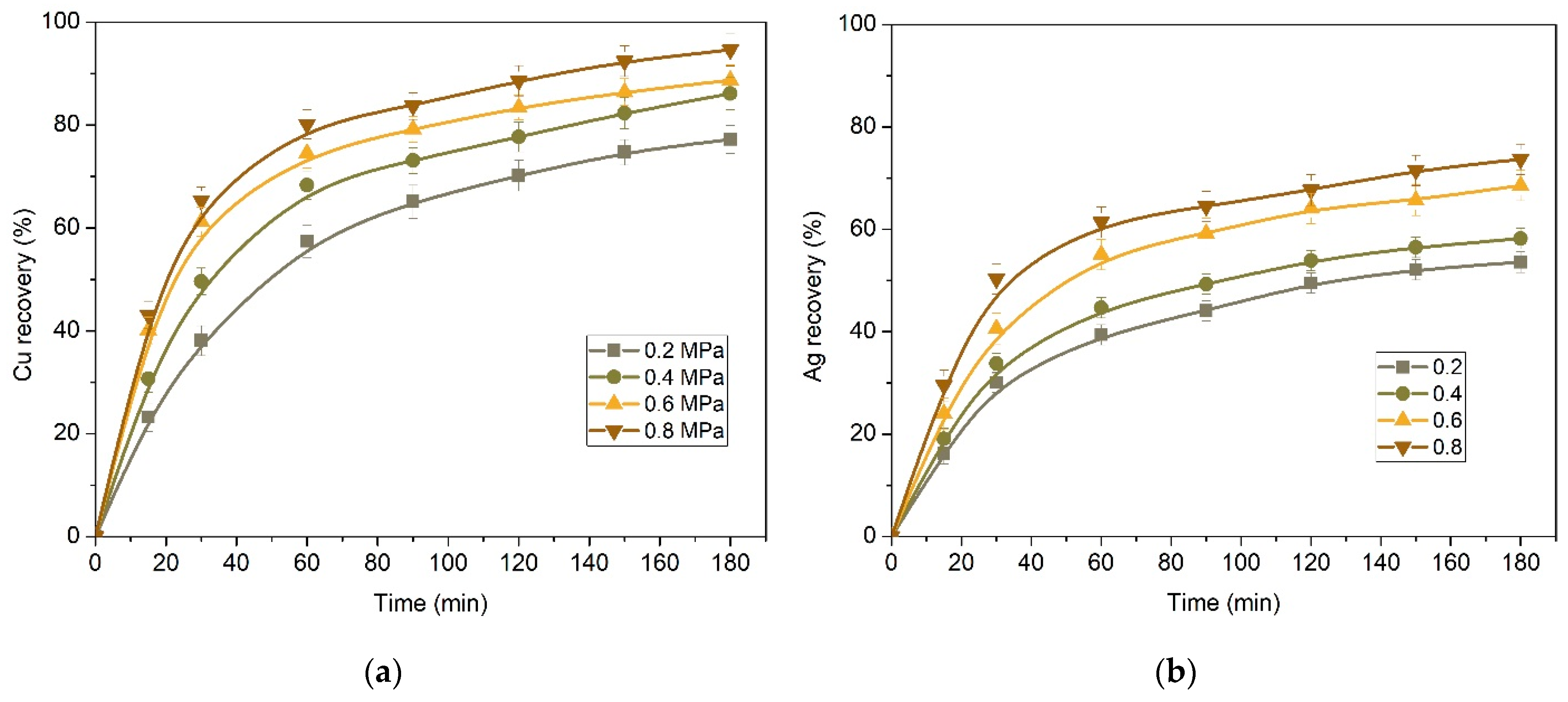
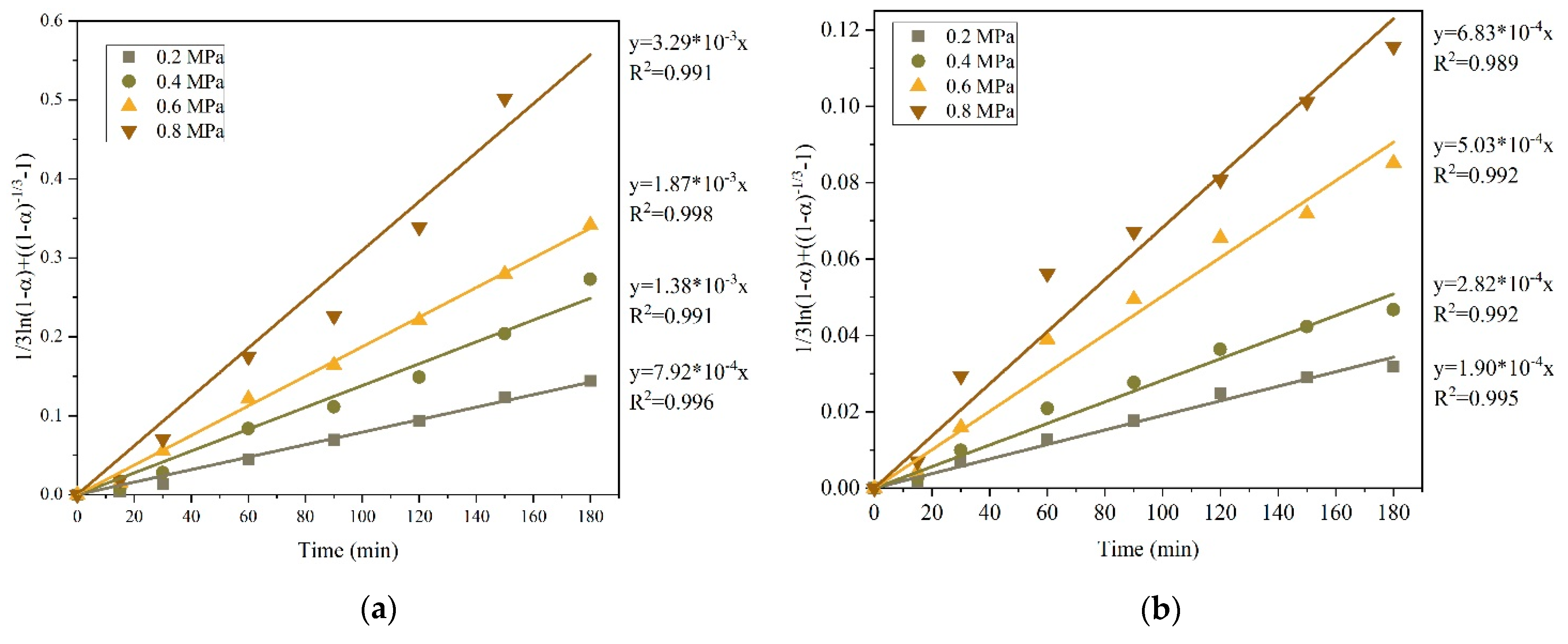
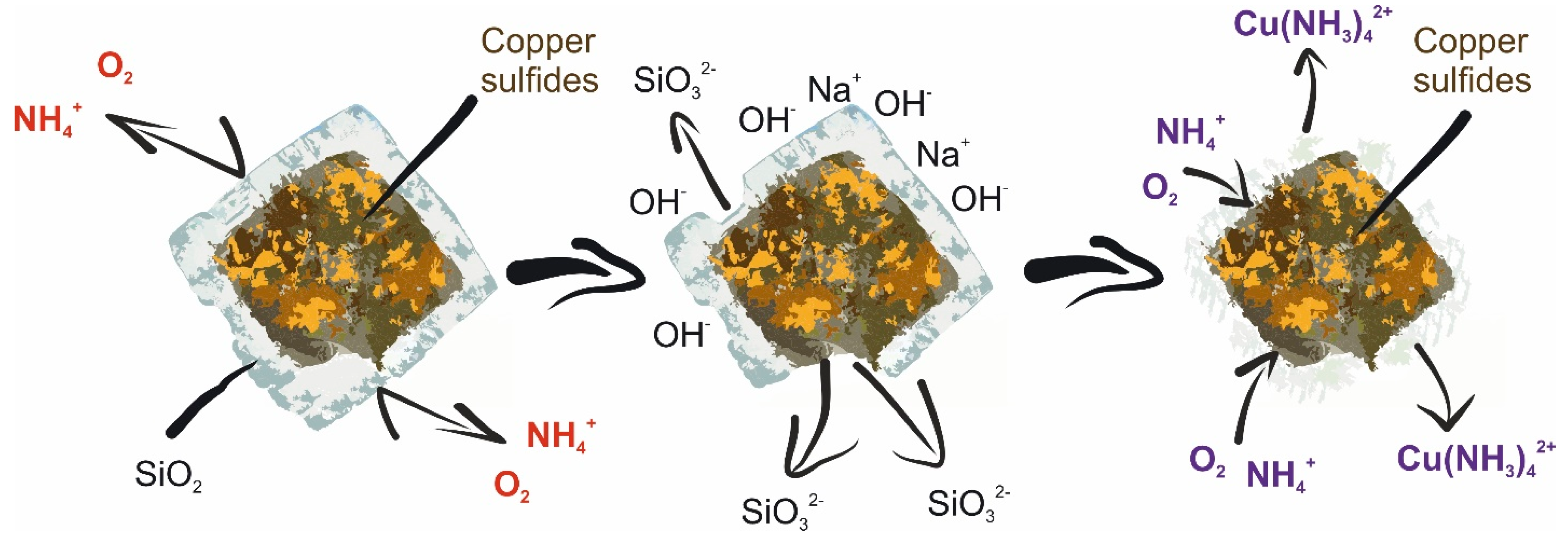
3.2. Effect of Preliminary Desilication on Ammonia Leaching of Low-Grade Copper–Silver Concentrate
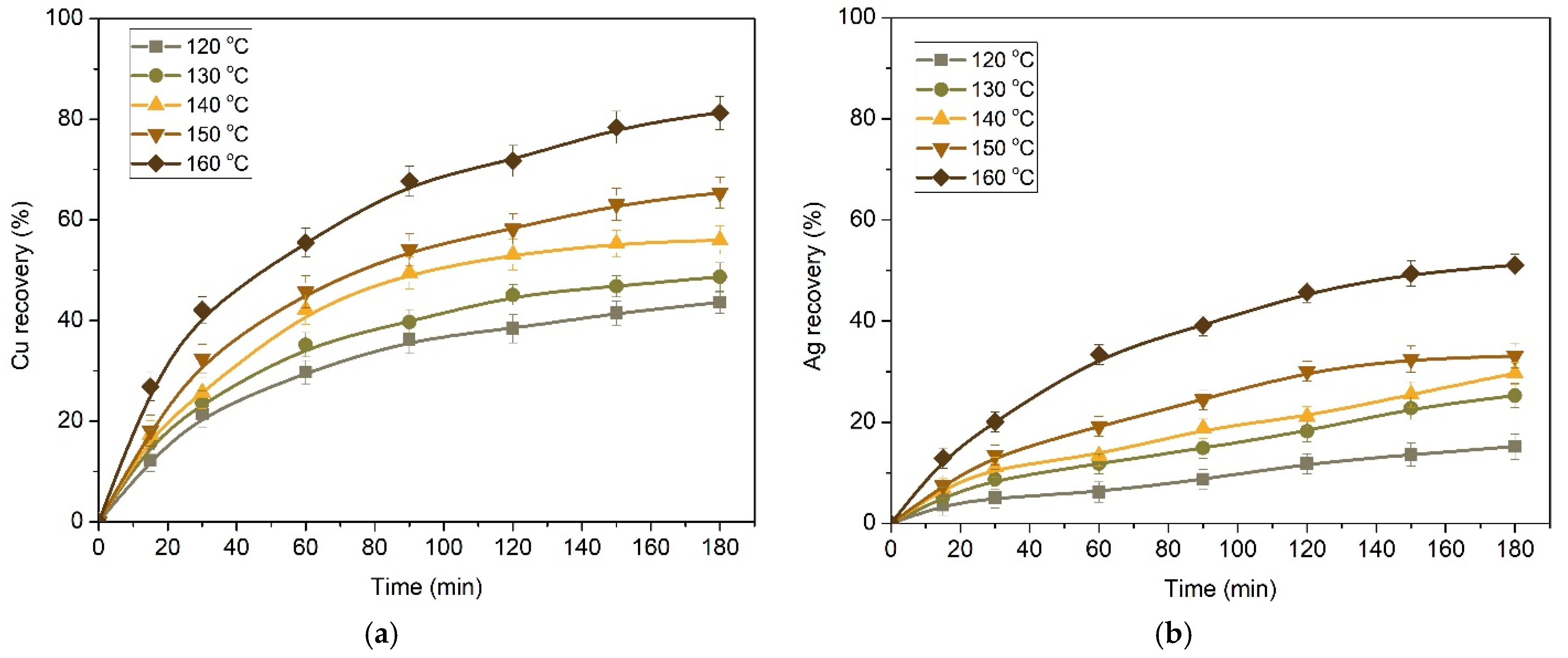
3.2.1. Kinetics of Copper and Silver Leaching from a Concentrate after Preliminary Desilication
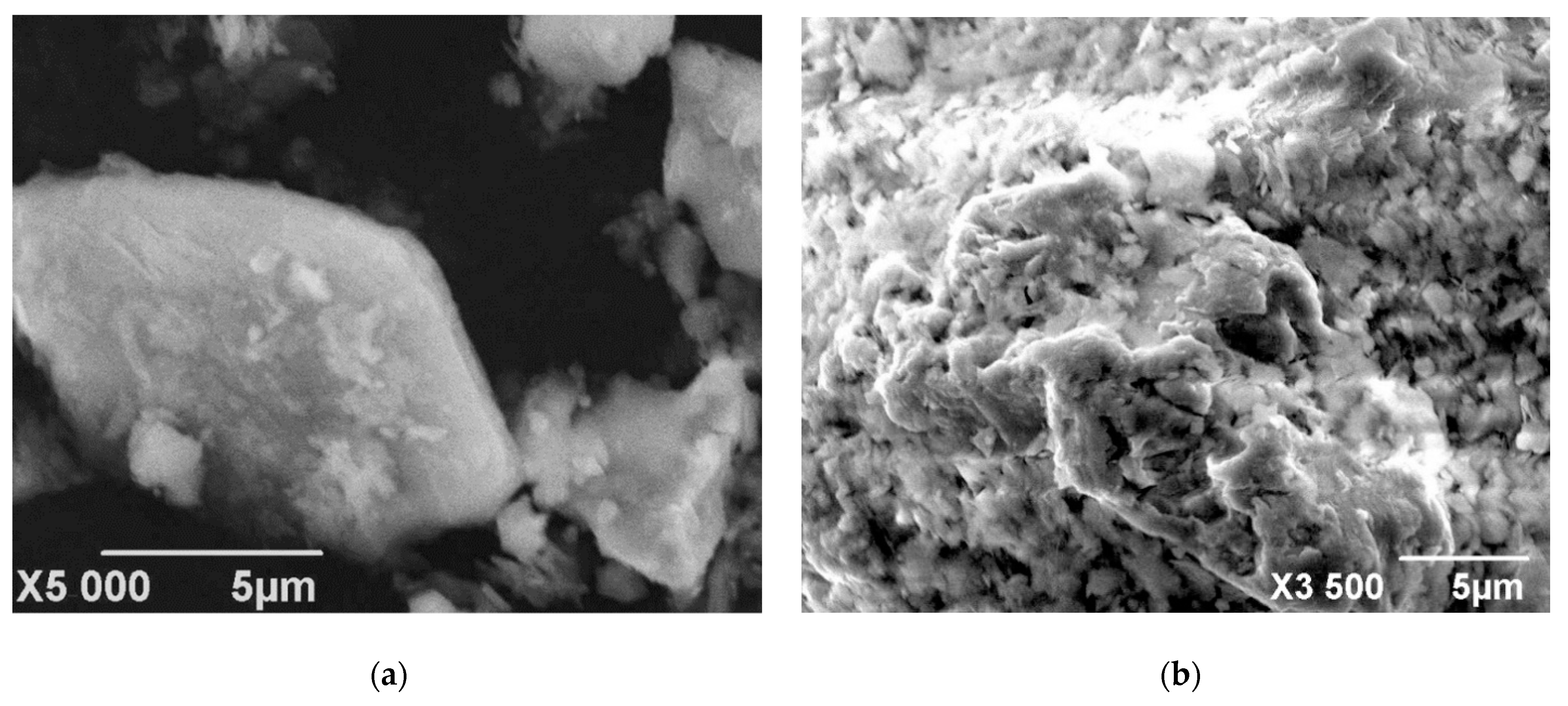
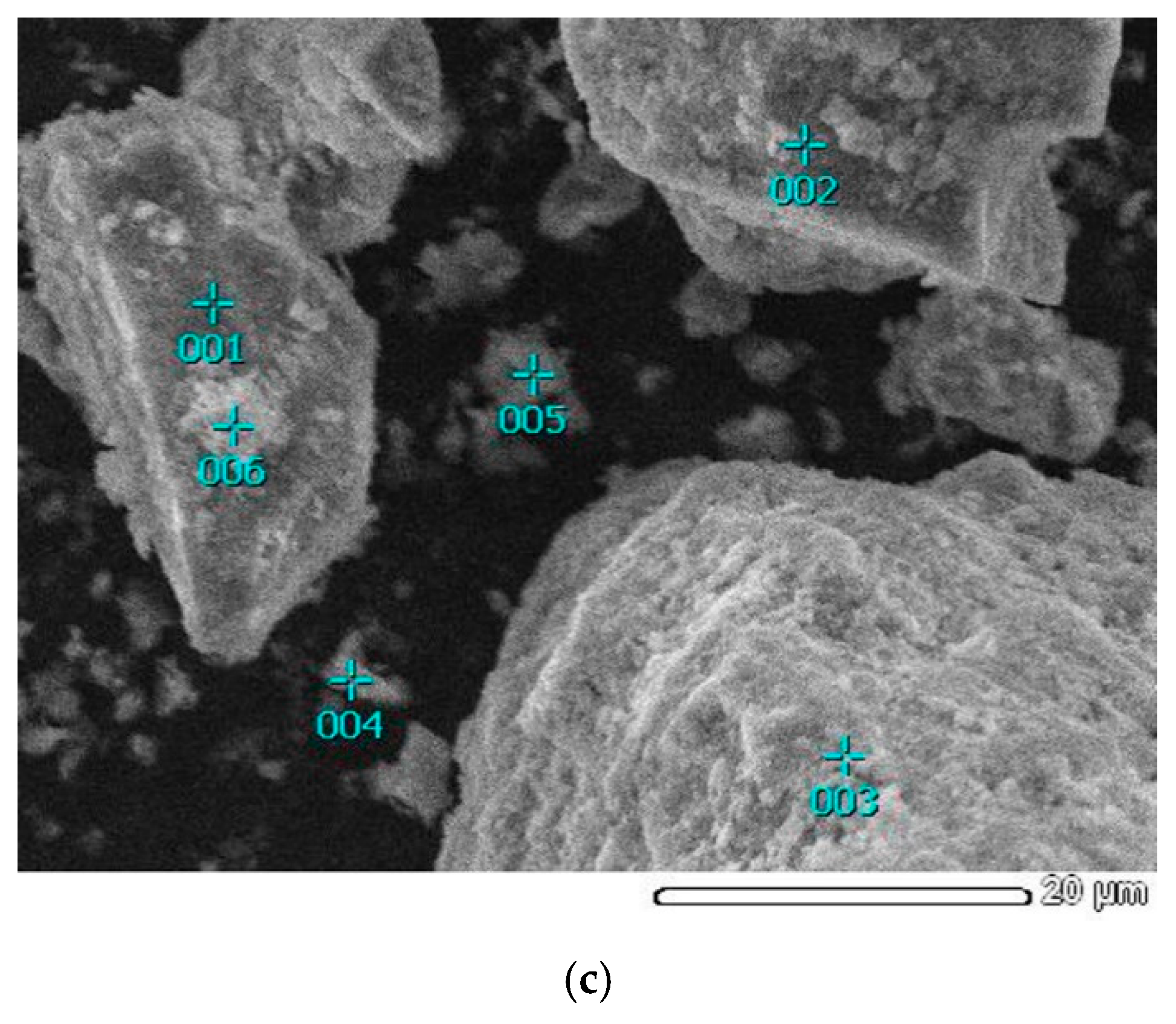
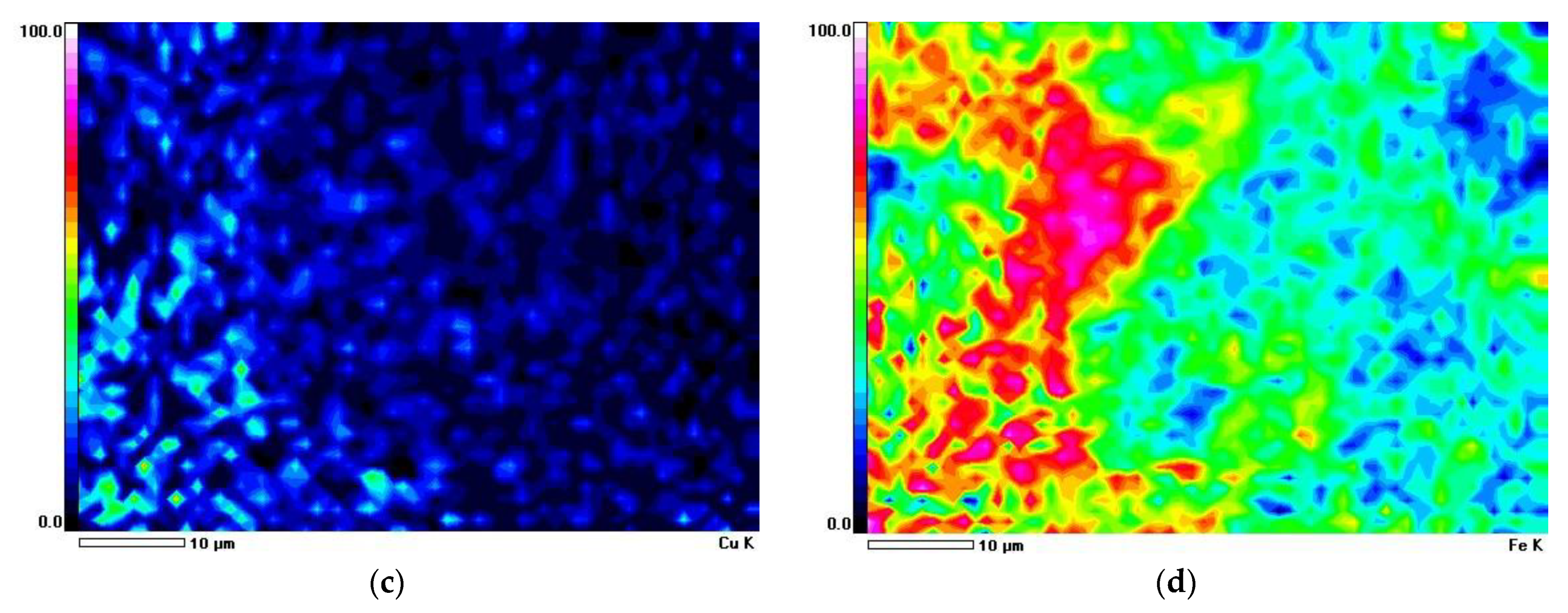
3.2.2. Characteristics of Solid Residue
4. Conclusions
- In addition to sulfide minerals of copper, the initial concentrate contains a large amount of oxidized copper and aluminosilicate compounds that coat valuable components, preventing their leaching into the solution.
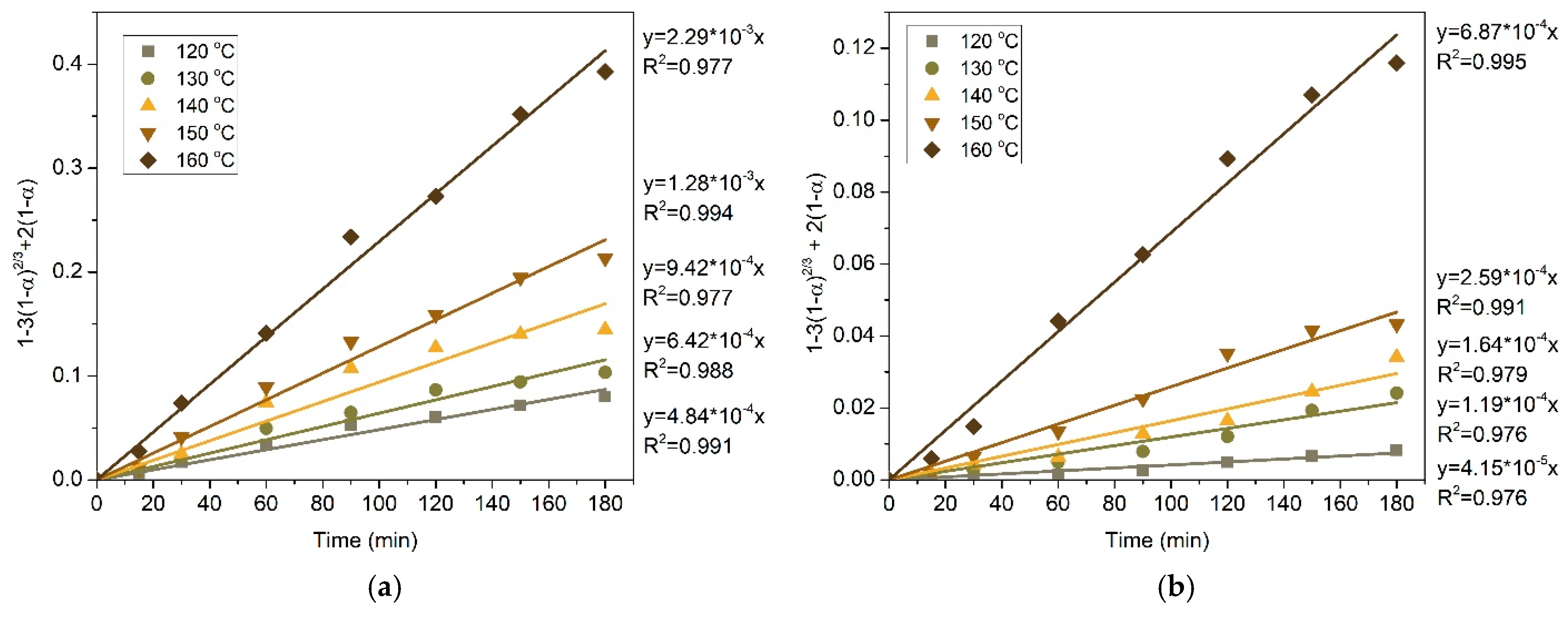
- The results of the experiments on the pressure leaching of the initial copper concentrate in an ammonium/ammonium-sulphate solution in the presence of oxygen are in good agreement with the shrinking core model in the intra-diffusion mode; the activation energies for copper and silver in this case were 53.50 kJ/mol and 90.35 kJ/mol, respectively.
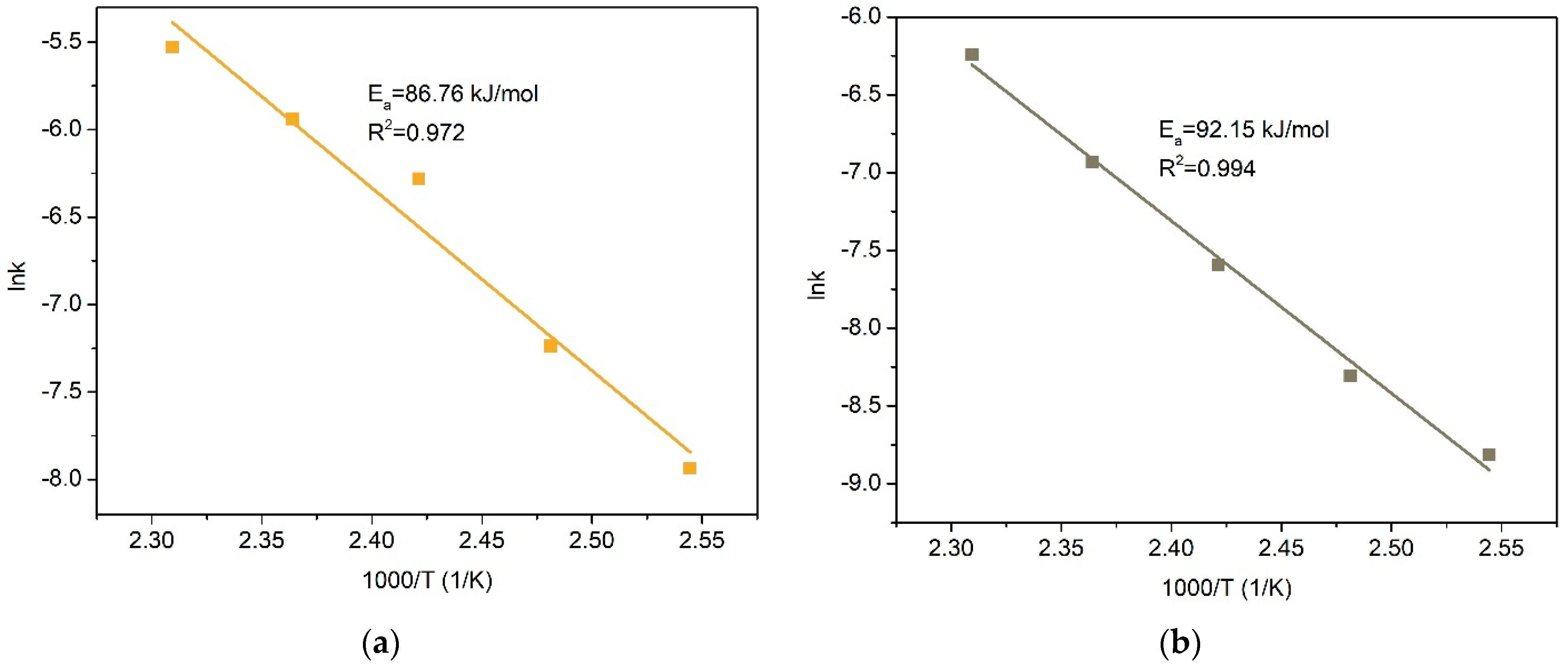
- After the preliminary alkaline desilication of the concentrate, the recovery rate for Cu and Ag increases significantly, and the new shrinking core model proves to be the most adequate, indicating that the process is limited by diffusion through the product layer and interfacial diffusion. The partial order with respect to oxygen pressure is close to 1, which may also indicate external diffusion limitations. The activation energy of the process increases to 86.76 kJ/mol for Cu and 92.15 kJ/mol for Ag.
- Using the time-to-a-given-fraction method, it was shown that a high activation energy is necessary in the later stages of the process at low temperatures, when the most refractory sulfide minerals of Cu and Ag appear to be unleached.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peters, E. Hydrometallurgical process innovation. Hydrometallurgy 1992, 29, 431–459. [Google Scholar] [CrossRef]
- Sinclair, L.; Thompson, J. In situ leaching of copper: Challenges and future prospects. Hydrometallurgy 2015, 157, 306–324. [Google Scholar] [CrossRef]
- Ignatkina, V.A.; Bocharov, V.A.; Makavetskas, A.R.; Kayumov, A.A.; Aksenova, D.D.; Khachatryan, L.S.; Fishchenko, Y.Y. Rational Processing of Refractory Copper-Bearing Ores. Russ. J. NonFerr. Metals 2018, 59, 364–373. [Google Scholar] [CrossRef]
- Singer, D.A. Future copper resources. Ore Geol. Rev. 2017, 86, 271–279. [Google Scholar] [CrossRef]
- Palaniandy, S. Impact of mechanochemical effect on chalcopyrite leaching. Int. J. Miner. Process. 2015, 136, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yang, H.; Liu, Y.; Tong, L.; Auwalu, A. Study on the mechanical activation of malachite and the leaching of complex copper ore in the Luanshya mining area, Zambia. Int. J. Miner. Metall. Mater. 2020, 27, 292–300. [Google Scholar] [CrossRef]
- Ye, X.; Gredelj, S.; Skinner, W.; Grano, S.R. Regrinding sulphide minerals—Breakage mechanisms in milling and their influence on surface properties and flotation behaviour. Powder Technol. 2010, 203, 133–147. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Xu, X.; Zhou, S. Bioleaching of different copper sulfides by Acidithiobacillus ferrooxidans and its adsorption on minerals. Hydrometallurgy 2013, 140, 42–47. [Google Scholar] [CrossRef]
- Halinen, A.-K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore. Hydrometallurgy 2009, 98, 92–100. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Anderson, C.G.; Harrison, K.D.; Krys, L.E. Theoretical considerations of sodium nitrite oxidation and fine grinding in refractory precious-metal concentrate pressure leaching. Min. Metall. Explor. 1996, 13, 4–11. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Mamyachenkov, S.V.; Anisimova, O.S. Nitric Acid Leaching of Copper-Zinc Sulfide Middlings. Metallurgist 2016, 60, 229–233. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Kolmachikhin, B.V. Polymetallic Ore Concentration Middlings Nitric Acid Leaching Kinetics. SSP 2017, 265, 1065–1070. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Y.; Yan, L.; Yang, R. Recovery of nickel, copper and cobalt from low-grade Ni–Cu sulfide tailings. Hydrometallurgy 2005, 80, 54–58. [Google Scholar] [CrossRef]
- Ukraintsev, I.V.; Petrov, G.V.; Ivanov, B.S.; Boduen, A.Y. Autoclave conditioning of a low-grade sulphide copper concentrate. Tsm 2016, 10, 43–48. [Google Scholar] [CrossRef]
- McDonald, R.G.; Muir, D.M. Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 2007, 86, 191–205. [Google Scholar] [CrossRef]
- Cui, F.; Mu, W.; Wang, S.; Xin, H.; Xu, Q.; Zhai, Y.; Luo, S. Sodium sulfate activation mechanism on co-sulfating roasting to nickel-copper sulfide concentrate in metal extractions, microtopography and kinetics. Miner. Eng. 2018, 123, 104–116. [Google Scholar] [CrossRef]
- Medvedev, A.S.; So, T.; Ptitsyn, A.M. Combined processing technology of the Udokan sulfide copper concentrate. Russ. J. NonFerr. Met. 2012, 53, 125–128. [Google Scholar] [CrossRef]
- Sun, X.; Chen, B.; Yang, X.; Liu, Y. Technological conditions and kinetics of leaching copper from complex copper oxide ore. J. Cent. South Univ. Technol. 2009, 16, 936–941. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and Their Application in Hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Boduen, A.Y.; Petrov, G.V.; Serebryakov, M.A. Investigation of ammonia autoclave leaching of silver and rhenium containing ill-conditioned copper concentrate. Tsm 2016, 10, 23–28. [Google Scholar] [CrossRef]
- Forward, F.A.; Mackiw, V.N. Chemistry of the Ammonia Pressure Process for Leaching Ni, Cu, and Co from Sherritt Gordon Sulphide Concentrates. JOM 1955, 7, 457–463. [Google Scholar] [CrossRef]
- Meng, X.; Han, K.N. The Principles and Applications of Ammonia Leaching of Metals—A Review. Mineral Process. Extr. Metall. Rev. 1996, 16, 23–61. [Google Scholar] [CrossRef]
- MetaLeach. Available online: http://www.metaleach.com/ (accessed on 23 April 2020).
- Hedjazi, F.; Monhemius, A.J. Industrial application of ammonia-assisted cyanide leaching for copper-gold ores. Miner. Eng. 2018, 126, 123–129. [Google Scholar] [CrossRef]
- Baba, A.A.; Ghosh, M.K.; Pradhan, S.R.; Rao, D.S.; Baral, A.; Adekola, F.A. Characterization and kinetic study on ammonia leaching of complex copper ore. Trans. NonFerr. Met. Soc. China 2014, 24, 1587–1595. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.; Hu, H.; Chen, Q. Dissolution kinetics of malachite in ammonia/ammonium sulphate solution. J. Cent. South Univ. Technol. 2012, 19, 903–910. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yin, Z.L.; Xiong, S.F.; Chen, Y.G.; Chen, Q.Y. Leaching and kinetic modeling of calcareous bornite in ammonia ammonium sulfate solution with sodium persulfate. Hydrometallurgy 2014, 144–145, 86–90. [Google Scholar] [CrossRef]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Tavakoli Mohammadi, M.R. Ammonia Leaching: A New Approach of Copper Industry in Hydrometallurgical Processes. J. Inst. Eng. India Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Turan, M.D.; Arslanoğlu, H.; Altundoğan, H.S. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J. Taiwan Inst. Chem. Eng. 2015, 50, 49–55. [Google Scholar] [CrossRef]
- Turan, M.D.; Altundoğan, H.S. Leaching of copper from chalcopyrite concentrate by using ammonium persulphate in an autoclave: Determination of most suitable impeller type by using response surface methodology. J. Cent. South Univ. 2013, 20, 622–628. [Google Scholar] [CrossRef]
- Baba, A.A.; Balogun, A.F.; Olaoluwa, D.T.; Bale, R.B.; Adekola, F.A.; Alabi, A.G.F. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar] [CrossRef]
- Nabizadeh, A.; Aghazadeh, V. Dissolution study of chalcopyrite concentrate in oxidative ammonia/ammonium carbonate solutions at moderate temperature and ambient pressure. Hydrometallurgy 2015, 152, 61–68. [Google Scholar] [CrossRef]
- Muir, D.M. A review of the selective leaching of gold from oxidised copper–gold ores with ammonia–cyanide and new insights for plant control and operation. Miner. Eng. 2011, 24, 576–582. [Google Scholar] [CrossRef]
- Boduen, A.Y.; Fokina, S.B.; Petrov, G.V.; Andreev, Y.V. Ammonia autoclave technology for the processing of low-grade concentrates generated in flotation concentration of cupriferous sandstones. Obogashchenie Rud 2019, 2, 33–38. [Google Scholar] [CrossRef]
- Baranov, V.F. The use of foreign experience in the development of a reconstruction option for the Zhezkazgan concentration complex. Obogashchenie Rud 2020, 1, 54–59. [Google Scholar] [CrossRef]
- Shoppert, A.A.; Loginova, I.V.; Pismak, V.N. Investigating of a Low-Grade Copper Concentrate Desilication by Alkali Pressure Leaching. MSF 2019, 946, 608–614. [Google Scholar] [CrossRef]
- Dickinson, C.F.; Heal, G.R. Solid–liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-25424-9. [Google Scholar]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching Kinetics of Sulfides from Refractory Gold Concentrates by Nitric Acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Baba, A.A.; Adekola, F.A. Hydrometallurgical processing of a Nigerian sphalerite in hydrochloric acid: Characterization and dissolution kinetics. Hydrometallurgy 2010, 101, 69–75. [Google Scholar] [CrossRef]
- Karimov, K.A.; Rogozhnikov, D.A.; Naboichenko, S.S.; Karimova, L.M.; Zakhar’yan, S.V. Autoclave Ammonia Leaching of Silver from Low-Grade Copper Concentrates. Metallurgist 2018, 62, 783–789. [Google Scholar] [CrossRef]
- Naboichenko, S.S. Avtoklavnaja Gidrometallurgija Cvetnych Metallov; GOU UGTU-UPI: Ekaterinburg, Russia, 2002; ISBN 978-5-321-00065-6. [Google Scholar]
- Hidalgo, T.; Kuhar, L.; Beinlich, A.; Putnis, A. Kinetics and mineralogical analysis of copper dissolution from a bornite/chalcopyrite composite sample in ferric-chloride and methanesulfonic-acid solutions. Hydrometallurgy 2019, 188, 140–156. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometallurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Iglesias-Gonzalez, N.; Romero, R.; Mazuelos, A.; Carranza, F. Ferric leaching of the sphalerite contained in a bulk concentrate: Kinetic study. Miner. Eng. 2018, 125, 50–59. [Google Scholar] [CrossRef]
- Smith, P. The processing of high silica bauxites—Review of existing and potential processes. Hydrometallurgy 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Zhou, K. Effects of Si-bearing minerals on the conversion of hematite into magnetite during reductive Bayer digestion. Hydrometallurgy 2019, 189, 105126. [Google Scholar] [CrossRef]









| Cu | Fe | S | SiO2 | Al2O3 | Na2O | Ag | Other |
|---|---|---|---|---|---|---|---|
| 7.0 | 8.2 | 5.7 | 55.1 | 10.5 | 2.2 | 140 ppm | 11.3 |
| Copper Form. | Content, wt. % |
|---|---|
| Primary sulfides | 14.7 |
| Secondary sulfides | 66.5 |
| Oxidized form | 18.8 |
| Total | 100 |
| # | Limiting step | Equation | R2 | ||||
|---|---|---|---|---|---|---|---|
| 120 °C | 130 °C | 140 °C | 150 °C | 160 °C | |||
| 1 | Diffusion through the product layer (sp) | 1 − 3(1 − α)2/3 + 2(1 − α) | 0.991 | 0.988 | 0.977 | 0.994 | 0.977 |
| 2 | Diffusion through the liquid film (sp) | α | 0.667 | 0.662 | 0.626 | 0.625 | 0.610 |
| 3 | Surface chemical reactions (sp) | 1 − (1 − α)1/3 | 0.750 | 0.729 | 0.805 | 0.737 | 0.845 |
| 4 | New shrinking core model | 1/3ln(1 − α) + [(1 − α)-1/3 − 1] | 0.989 | 0.993 | 0.960 | 0.989 | 0.968 |
| 5 | Hybrid (1st equation + 3rd equation) | (1 − 3(1 − α)2/3 + 2(1 − α)) + (1 − (1 − α)1/3) | 0.855 | 0.846 | 0.841 | 0.846 | 0.947 |
| Element | Al | Si | S | Fe | Cu | Ag | O | Total |
|---|---|---|---|---|---|---|---|---|
| Figure 5c. Point 001 | 2.78 | 21.07 | 2.83 | 18.39 | 18.60 | 0.15 | 36.16 | 100 |
| Figure 5c. Point 002 | 3.10 | 32.29 | 0.29 | 11.12 | 7.24 | 0.00 | 45.96 | 100 |
| Figure 5c. Point 003 | 3.58 | 40.46 | 0.00 | 2.92 | 1.89 | 0.00 | 51.15 | 100 |
| Figure 5c. Point 004 | 2.91 | 10.43 | 0.62 | 27.41 | 26.33 | 0.50 | 31.80 | 100 |
| Figure 5c. Point 005 | 1.49 | 5.61 | 0.29 | 53.19 | 7.29 | 0.00 | 32.13 | 100 |
| Figure 5c. Point 006 | 4.40 | 14.31 | 5.74 | 12.16 | 34.85 | 0.83 | 27.71 | 100 |
| Cu | Fe | S | SiO2 | Al2O3 | Na2O | Ag | Other |
|---|---|---|---|---|---|---|---|
| 10.7 | 12.6 | 8.7 | 30.5 | 15.1 | 8.8 | 210 ppm | 13.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimov, K.; Shoppert, A.; Rogozhnikov, D.; Kuzas, E.; Zakhar’yan, S.; Naboichenko, S. Effect of Preliminary Alkali Desilication on Ammonia Pressure Leaching of Low-Grade Copper–Silver Concentrate. Metals 2020, 10, 812. https://doi.org/10.3390/met10060812
Karimov K, Shoppert A, Rogozhnikov D, Kuzas E, Zakhar’yan S, Naboichenko S. Effect of Preliminary Alkali Desilication on Ammonia Pressure Leaching of Low-Grade Copper–Silver Concentrate. Metals. 2020; 10(6):812. https://doi.org/10.3390/met10060812
Chicago/Turabian StyleKarimov, Kirill, Andrei Shoppert, Denis Rogozhnikov, Evgeniy Kuzas, Semen Zakhar’yan, and Stanislav Naboichenko. 2020. "Effect of Preliminary Alkali Desilication on Ammonia Pressure Leaching of Low-Grade Copper–Silver Concentrate" Metals 10, no. 6: 812. https://doi.org/10.3390/met10060812