Synergistic Effect of rhBMP-2 Protein and Nanotextured Titanium Alloy Surface to Improve Osteogenic Implant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Sample Preparation
2.2. Surface Characterization of Titanium Samples
2.3. In Vitro Cell Culture
2.3.1. C2C12 Cell Line
Cellular Adhesion
Cell Viability
Evaluation of Cell Differentiation: Alkaline Phosphatase Activity
Evaluation of Cell Mineralization: Calcium Deposits Production
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Irradiated Titanium Samples
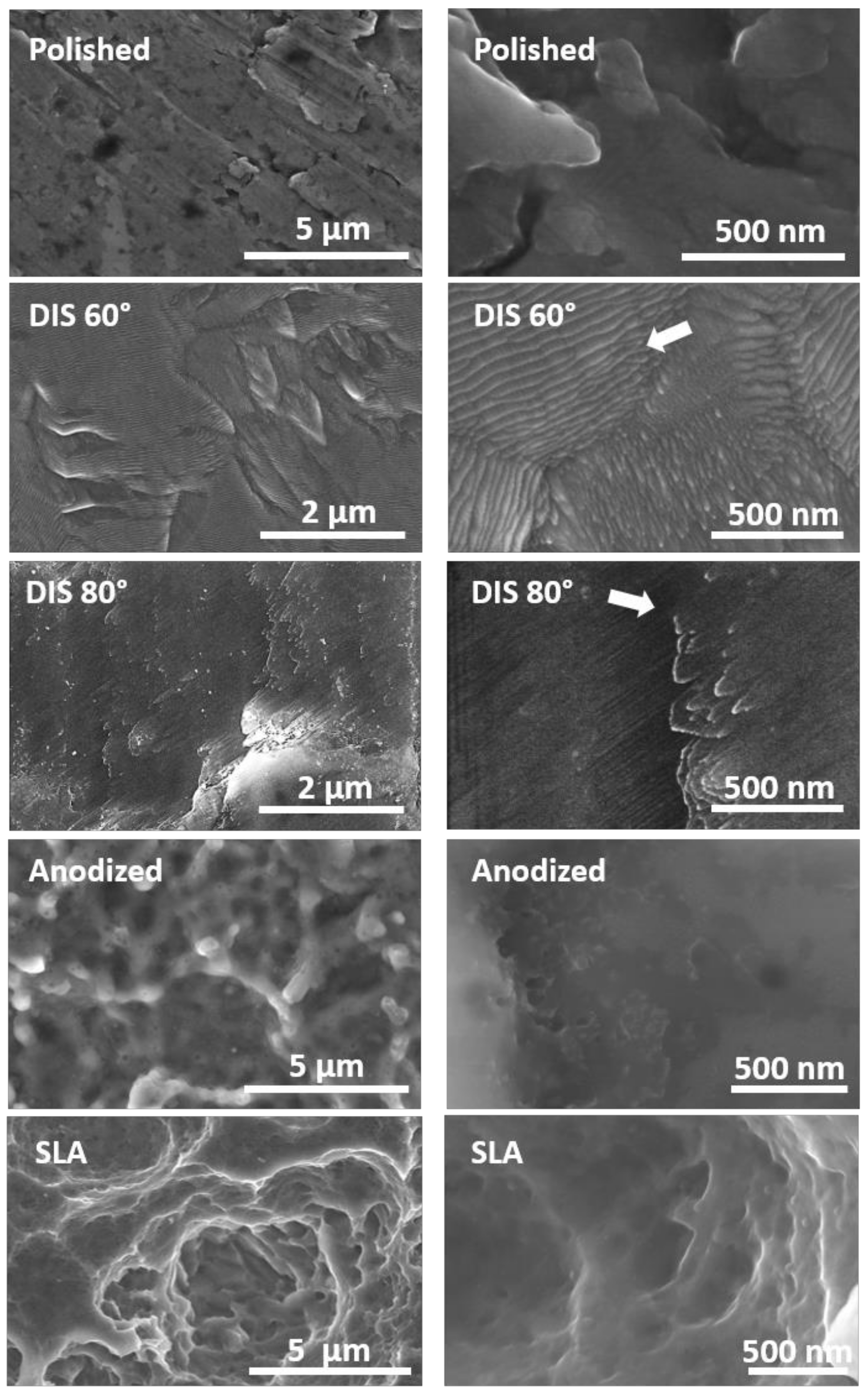
3.1.1. Evaluation of Surface Topography of Irradiated Titanium Samples
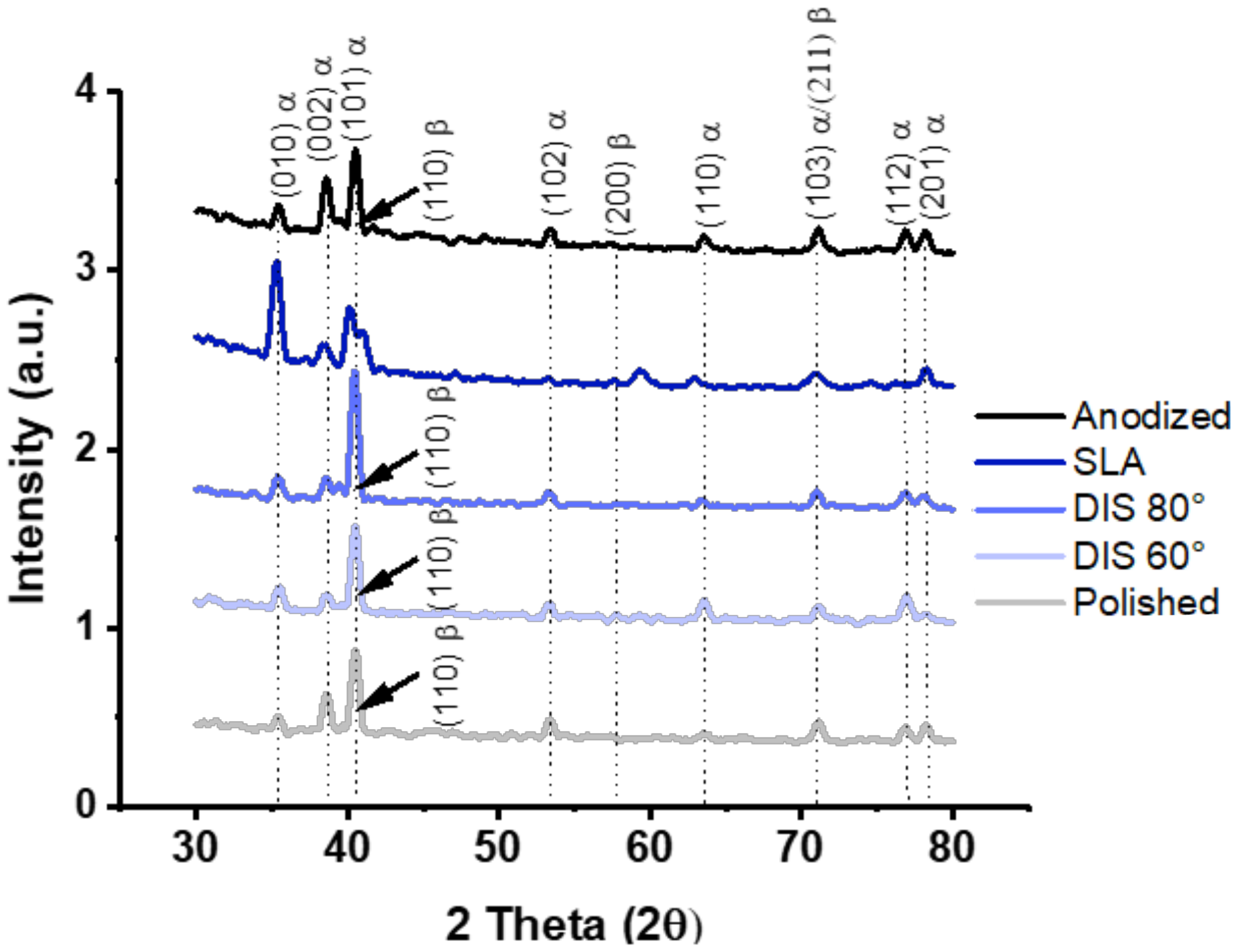
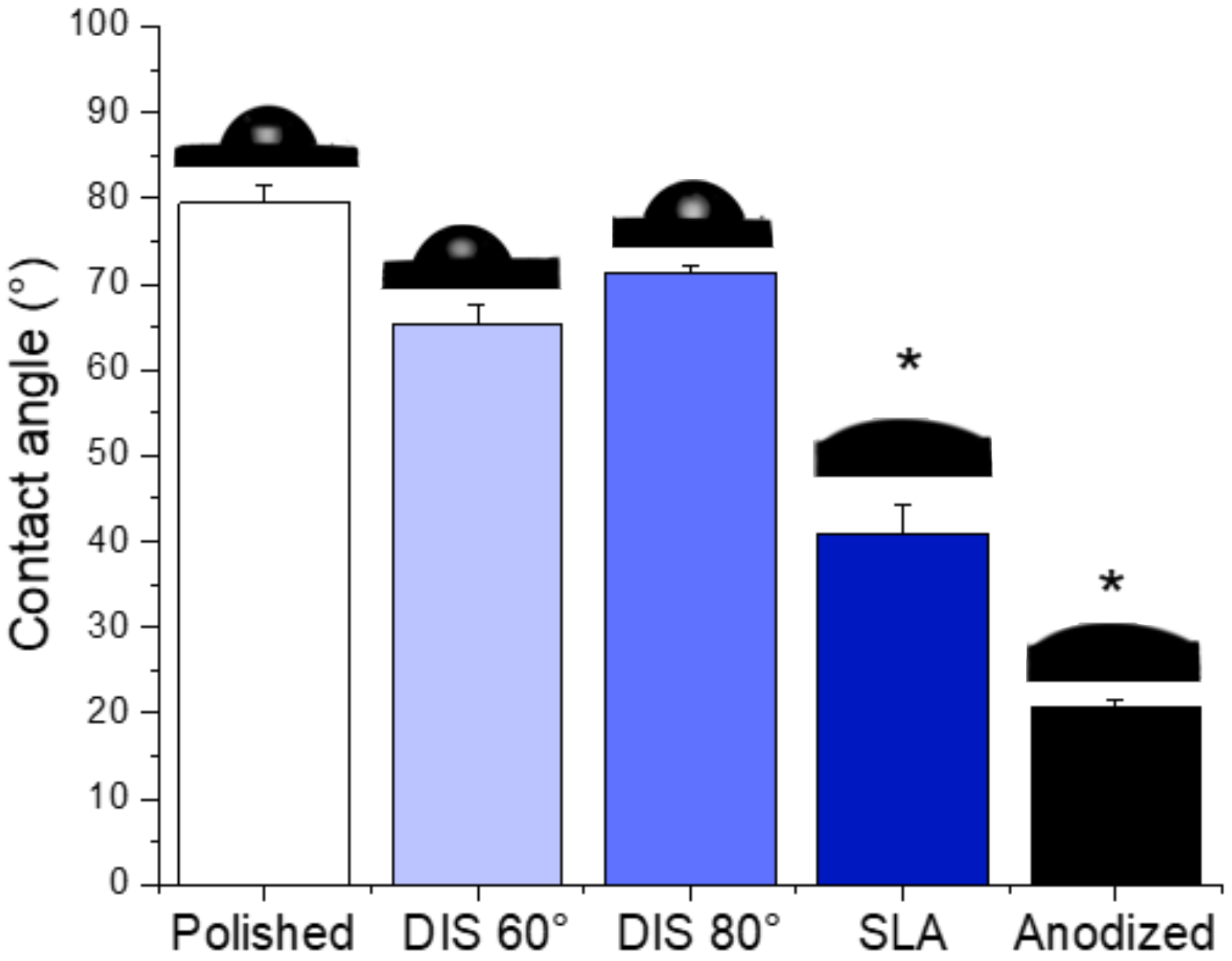
3.1.2. Evaluation of Surface Chemistry and Wettability of Irradiated Titanium Alloy Specimens
3.2. In Vitro Biological Characterization of Titanium Samples
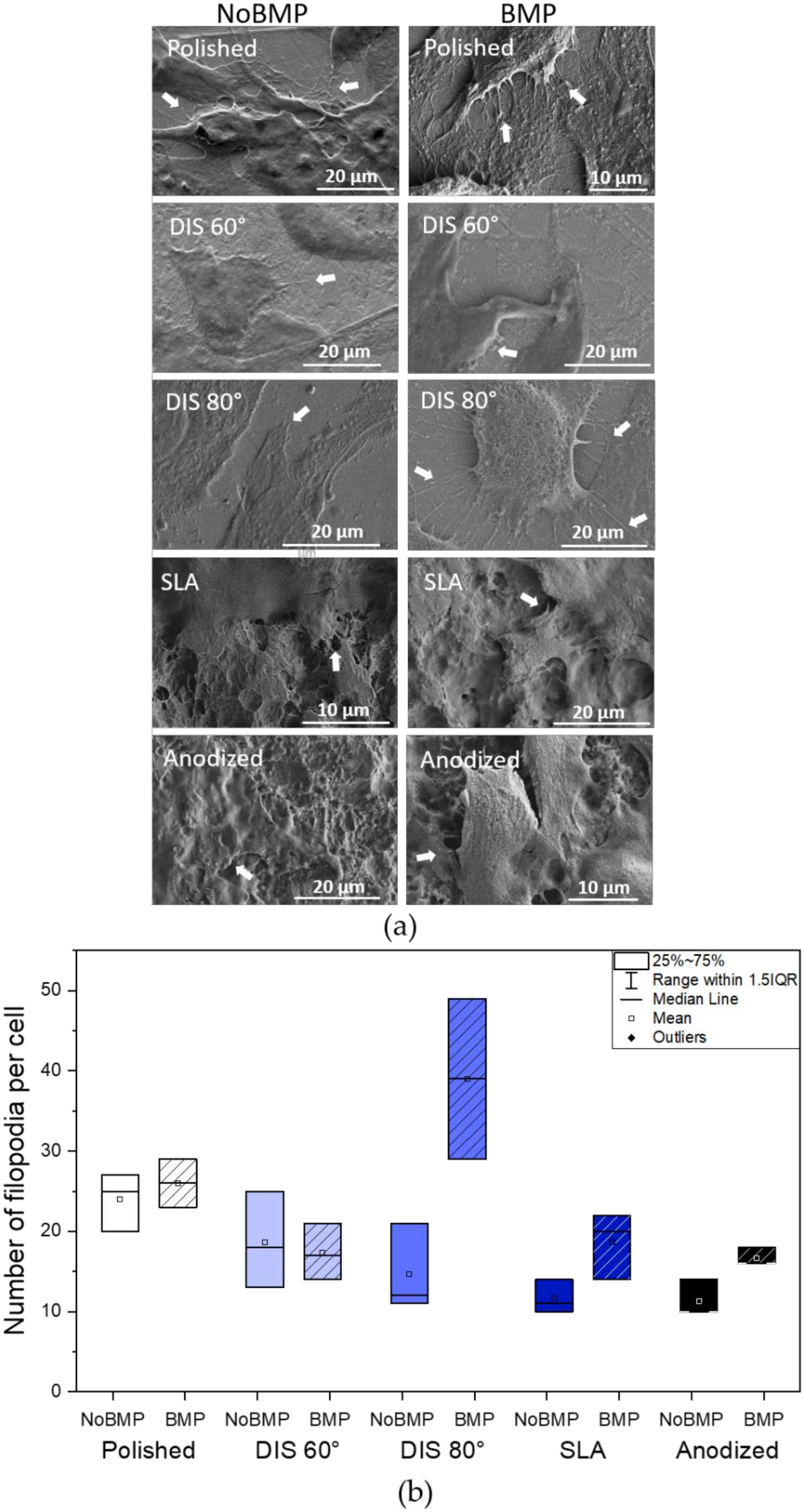
3.2.1. Evaluation of the Cellular Attachment on Irradiated Titanium Samples
3.2.2. Evaluation of Osteogenic Differentiation and Mineralization on Irradiated Titanium Samples
4. Conclusions
- DIS allowed the design of nanostructures on titanium alloys surfaces, resulting in nanocones and nanowalls of size below 50 nm by changing the incidence angle from 60 to 80 degrees with high fluences.
- The crystalline structure of DIS samples was unmodified; and although no statistical differences were observed in terms of wettability, DIS samples seemed more hydrophilic than polished samples.
- The presence of BMP-2 plays an important role in cellular adhesion and spreading. In this study, BMP-2 addition seemed to increase filopodia number per cell and vinculin expression in most surfaces and cell spreading on DIS 80° and polished surfaces compared to surfaces without BMP-2. However, surface topography or nanopatterning by itself does not contribute significantly to these processes, except by increasing slightly vinculin expression in DIS 80° nanocone-patterned surfaces.
- DIS 80° nanocone-patterned surfaces in conjunction with BMP-2 increase almost 1.2-fold cell spreading and 2-fold vinculin expression, reaching values similar to polished samples with BMP-2. Moreover, we observed a 2.25-fold increase in the number of filopodia per cells (39 ± 20) in these surfaces compared to all surfaces with or without BMP-2, suggesting a synergistic effect in cell adhesion when we combine DIS 80° treatment with BMP-2.
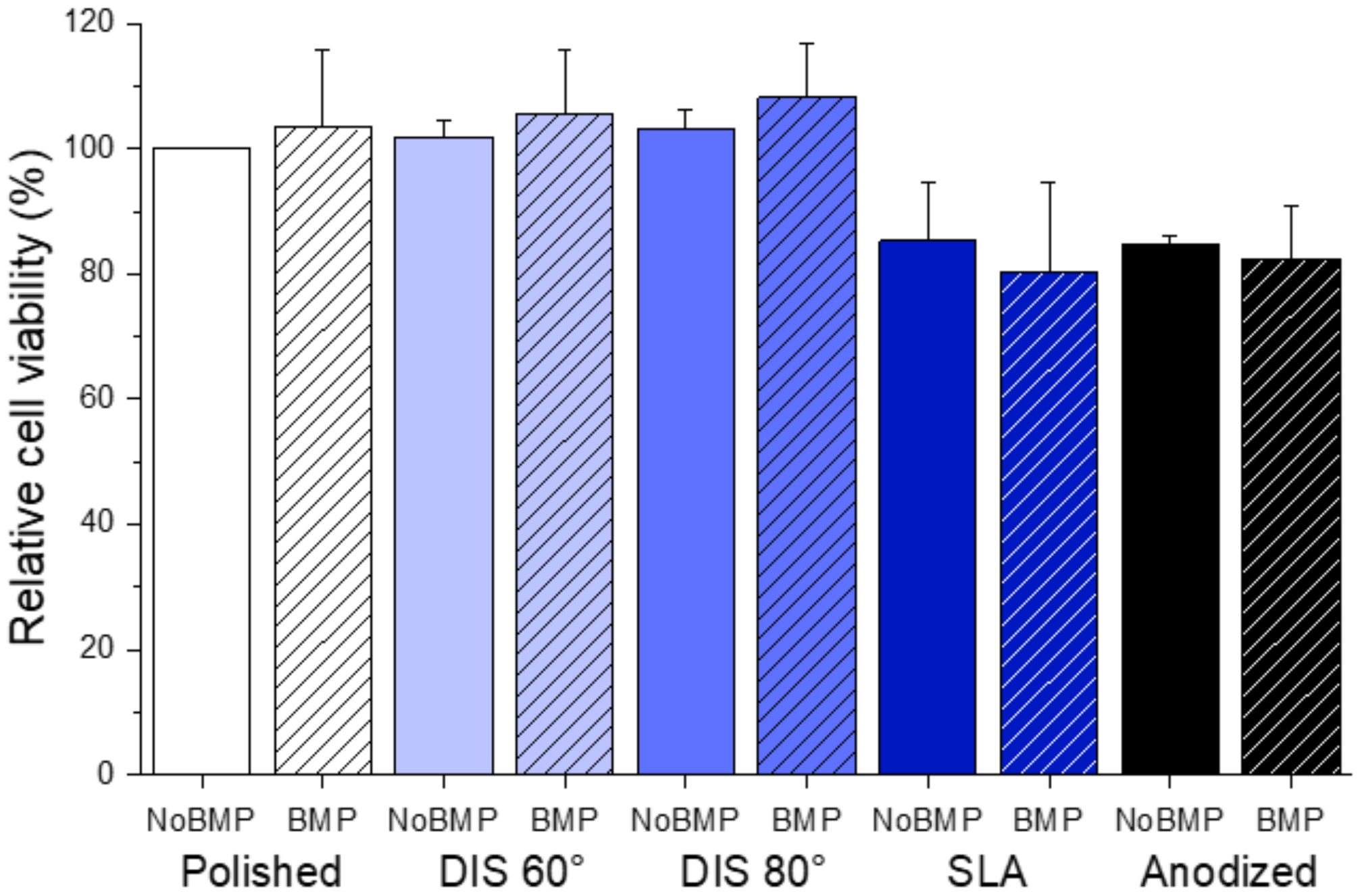
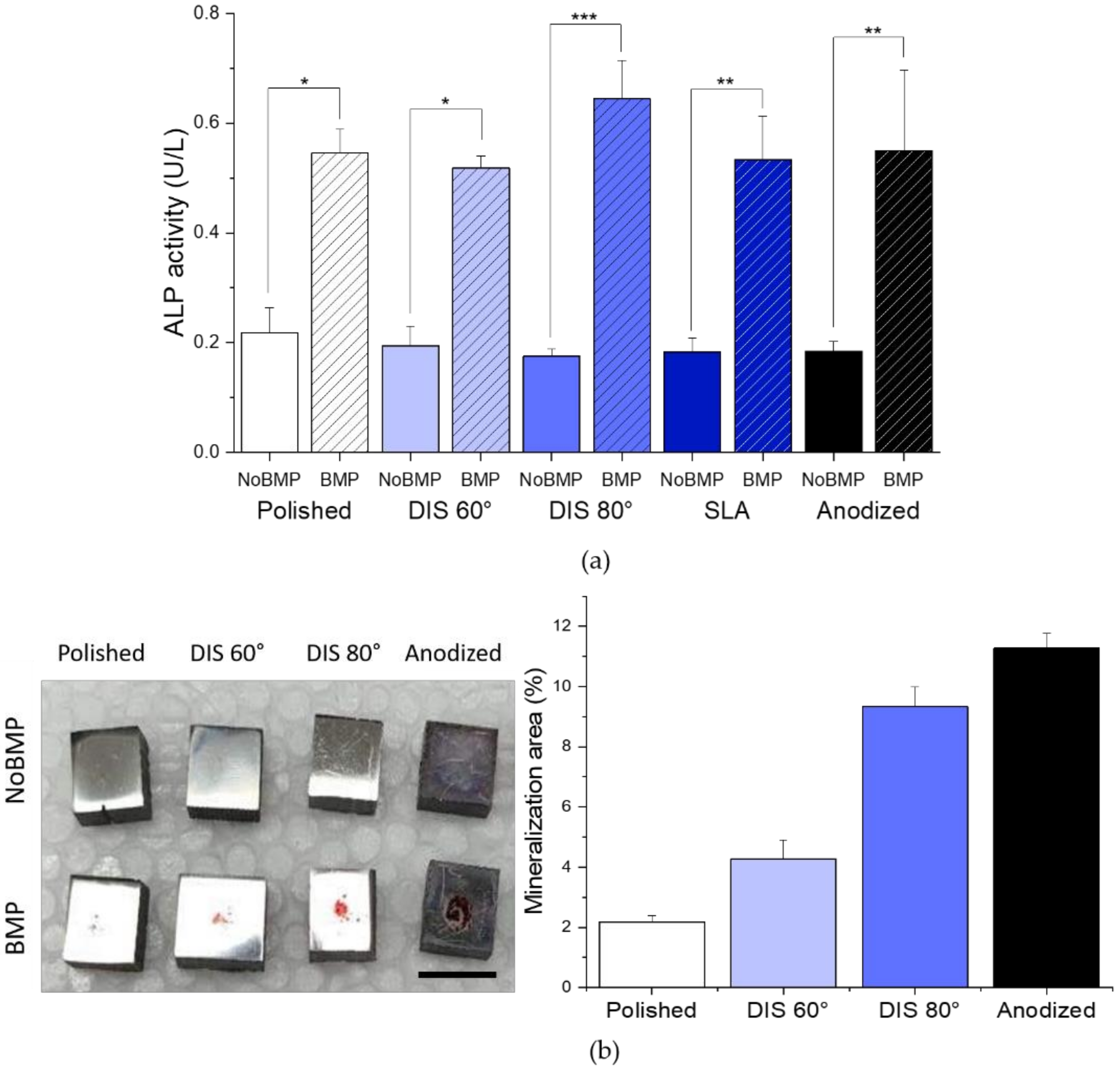
- Cell differentiation and mineralization, determined by ALP activity and calcium nodules formation, respectively, were enhanced in the presence of BMP-2 for all samples. In particular, we observed that this effect was more pronounced on DIS 80° and Anodized samples with BMP-2 (>2-fold increase in ALP activity compared to their counterparts without BMP-2 and >3.3-fold increase in cell mineralization compared to polished samples with BMP-2).
- Finally, the nanocone-like structures generated at an incidence angle of 80° in combination with BMP-2 have shown a stronger synergistic effect in modulating cellular processes when compared to DIS 60° and polished observing this nanocone topography more suitable to improve cellular interactions. Thus, DIS treatment in conjunction with BMP-2 may improve Ti implants osseointegration by guiding cell differentiation toward bone formation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Chu, P.K. Orthopedic implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–439. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-alloys and surface modifications to improve the mechanical properties and the biological response to orthopedic and dental implants: A review. BioMed Res. Int. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ramazanoglu, M.; Oshi, Y. Osseointegration and bioscience of implant surfaces—Current concepts at bone-implant interface. In Implant Dentistry—A Rapidly Evolving Practice; Turkyilmaz, I., Ed.; InTech: Rijeka, Croatia, 2011; Available online: http://www.intechopen.com/books/implant-dentistry-a-rapidly-evolving-practice/osseointegration-and-bioscience-of-implant-surfaces-current-concepts-at-bone-implant-interface (accessed on 4 October 2014).
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.G.; Webster, T.J. Nanostructured titanium: The ideal material for improving orthopedic implant efficacy? Nanomedicine 2012, 7, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Mansoorianfar, M.; Khataee, A.; Riahi, Z.; Shahin, K.; Asadnia, M.; Razmjou, A.; Hojjati-Najafabadi, A.; Mei, C.; Orooji, Y.; Li, D. Scalable fabrication of tunable titanium nanotubes via sonoelectrochemical process for biomedical applications. Ultrason. Sonochem. 2020, 64, 104783. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2009, 7, S93–S105. [Google Scholar] [CrossRef] [Green Version]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavenus, S.; Louarn, G.; Layrolle, P. Nanotechnology and dental implants. Int. J. Biomater. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Aminuddin, N.I.; Ahmad, R.; Akbar, S.A.; Pingguan-Murphy, B. Osteoblast and stem cell response to nanoscale topographies: A review. Sci. Technol. Adv. Mater. 2016, 17, 698–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Souza, J.C.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Tao, B.; Deng, Y.; Song, L.; Ma, W.; Qian, Y.; Lin, C.; Yuan, Z.; Lu, L.; Chen, M.; Yang, X.; et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surfaces B Biointerfaces 2019, 177, 242–252. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface immobilization of TiO2 nanotubes with bone morphogenetic protein-2 synergistically enhances initial Preosteoblast adhesion and osseointegration. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigmosta, T.B.; Popat, K.C.; Kipper, M.J. BMP -2 delivery from polyelectrolyte multilayers enhancesosteogenic activityon nanostructured titania. J. Biomed. Mater. Res. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.F.; Arruda, I.R.; Silva, G.M.; Machado, G.; Coelho, L.C.; Correia, M.T. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater. Sci. Eng. C 2017, 81, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Y.; Tai, I.C.; Ho, M.L.; Wang, J.W.; Weng, L.W.; Wang, Y.J.; Wang, M.W.; Tseng, C.C. Controlled release of BMP-2 from titanium with electrodeposition modification enhancing critical size bone formation. Mater. Sci. Eng. C 2019, 105, 109879. [Google Scholar] [CrossRef]
- Wu, C.; Lu, H. Smad signal pathway in BMP-2-induced osteogenesis a mini review. J. Dent. Sci. 2008, 3, 13–21. [Google Scholar]
- Teng, F.Y.; Chen, W.C.; Wang, Y.L.; Hung, C.C.; Tseng, C.C. Effects of osseointegration by bone morphogenetic protein-2 on titanium implants in vitro and in vivo. Bioinorg. Chem. Appl. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Ren, X.; Yuan, X.; Ma, L.; Xie, Y.; Bian, Z.; Zuo, J.; Wang, X.; Yu, Z.; Zhou, K.; et al. The effects of combined micron-scale surface and different nanoscale features on cell response. Adv. Mater. Sci. Eng. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Greer, A.I.; Goriainov, V.; Kanczler, J.; Black, C.R.; Turner, L.A.; Meek, R.M.; Burgess, K.; MacLaren, I.; Dalby, M.J.; Oreffo, R.O.; et al. Nanopatterned titanium implants accelerate bone formation in vivo. ACS Appl. Mater. Interfaces 2020, 12, 33541–33549. [Google Scholar] [CrossRef]
- Allain, J.P.; Shetty, A. Unraveling atomic-level self-organization at the plasma-material interface. J. Phys. D Appl. Phys. 2017, 50, 283002. [Google Scholar] [CrossRef]
- Averback, R. Ion-irradiation studies of cascade damage in metals. J. Nucl. Mater. 1982, 108–109, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Civantos, A.; Barnwell, A.; Shetty, A.R.; Pavón, J.J.; El-Atwani, O.; Arias, S.L.; Lang, E.; Reece, L.M.; Chen, M.; Allain, J.P. Designing nanostructured Ti6Al4V bioactive interfaces with directed irradiation synthesis toward cell stimulation to promote host–tissue-implant integration. ACS Biomater. Sci. Eng. 2019, 5, 3325–3339. [Google Scholar] [CrossRef]
- Civantos, A.; Allain, J.P.; Pavón, J.J.; Shetty, A.; El-Atwani, O.; Walker, E.; Arias, S.L.; Gordon, E.; Rodríguez-Ortiz, J.A.; Chen, M.; et al. Directed irradiation synthesis as an advanced plasma technology for surface modification to activate porous and “as-received” titanium surfaces. Metals 2019, 9, 1349. [Google Scholar] [CrossRef] [Green Version]
- Krasheninnikov, A.V.; Nordlund, K. Ion and electron irradiation-induced effects in nanostructured materials. J. Appl. Phys. 2010, 107, 071301. [Google Scholar] [CrossRef]
- Ghasali, E.; Baghchesaraee, K.; Orooji, Y. Study of the potential effect of spark plasma sintering on the preparation of complex FGM/laminated WC-based cermet. Int. J. Refract. Met. Hard Mater. 2020, 92, 105328. [Google Scholar] [CrossRef]
- de Gorter, D.J.; van Dinther, M.; Dijke, P.T. Measurement of constitutive activity of BMP type I receptors. Methods Enzymol. 2010. [Google Scholar] [CrossRef]
- Mancini, A.; El Bounkari, O.; Norrenbrock, A.F.; Scherr, M.; Schaefer, D.; Eder, M.; Banham, A.H.; Pulford, K.; Lyne, L.; Whetton, A.D.; et al. FMIP controls the adipocyte lineage commitment of C2C12 cells by downmodulation of C/EBPalpha. Oncogene 2006, 26, 1020–1027. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, Y.; Chiba-Ohkuma, R.; Karakida, T.; Onuma, K.; Yamamoto, R.; Fujii-Abe, K.; Saito, M.M.; Yamakoshi, Y.; Kawahara, H. Combined effect of midazolam and bone morphogenetic protein-2 for differentiation induction from C2C12 myoblast cells to osteoblasts. Pharmaceutics 2020, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Mishra, I.; Joshi, S.R.; Majumder, S.; Manna, A.K.; Varma, S. Low energy ion irradiation of TiO 2 (110)—Understanding evolution of surface morphology and scaling studies. Radiat. Eff. Defects Solids 2016, 171, 594–605. [Google Scholar] [CrossRef]
- Xue, L. Laser Consolidation—A rapid manufacturing process for making net-shape functional components. In Advances in Laser Materials Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 461–505. [Google Scholar] [CrossRef] [Green Version]
- Pederson, R.; Babushkin, O.; Skystedt, F.; Warren, R. Use of high temperature X-ray diffractometry to study phase transitions and thermal expansion properties in Ti-6Al-4V. Mater. Sci. Technol. 2003, 19, 1533–1538. [Google Scholar] [CrossRef]
- Albertini, M.; Yagüe, M.-A.F.; Lázaro, P.; Herrero-Climent, M.; Rios-Santos, J.-V.; Bullon, P.; Gil Mur, F.J. Advances in surfaces and osseointegration in implantology. Biomimetic surfaces. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e316–e325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Wehrle-Haller, B.; Vogel, V. Maturation of filopodia shaft adhesions is upregulated by local cycles of lamellipodia advancements and retractions. PLoS ONE 2014, 9, e107097. [Google Scholar] [CrossRef] [Green Version]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell–cell and cell–matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Bertrand, A.A.; Malapati, S.H.; Yamaguchi, D.T.; Lee, J.C. The intersection of mechanotransduction and regenerative osteogenic materials. Adv. Healthc. Mater. 2020, 9, 2000709. [Google Scholar] [CrossRef]
- Fourel, L.; Valat, A.; Faurobert, E.; Guillot, R.; Bourrin-Reynard, I.; Ren, K.; Lafanechère, L.; Planus, E.; Picart, C.; Albiges-Rizo, C. β3 integrin–mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J. Cell Biol. 2016, 212, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Y.; Li, T.; Ding, S.; Zhang, W.; Gu, Y.; Liu, C. Facilitated receptor-recognition and enhanced bioactivity of bone morphogenetic protein-2 on magnesium-substituted hydroxyapatite surface. Sci. Rep. 2016, 6, 24323. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Xie, Y.; Zhang, Z.; Li, K.; Hu, D.; Zheng, X.; Fan, Q.; Tang, T. YAP-mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs on hierarchical structure. Colloids Surf. B Biointerfaces 2017, 152, 344–353. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2Nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Selhuber-Unkel, C.; Erdmann, T.; López-García, M.; Kessler, H.; Schwarz, U.; Spatz, J. Cell adhesion strength is controlled by intermolecular spacing of adhesion receptors. Biophys. J. 2010, 98, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef] [Green Version]
- Babchenko, O.; Kromka, A.; Hruska, K.; Kalbacova, M.H.; Broz, A.; Vanecek, M. Fabrication of nano-structured diamond films for SAOS-2 cell cultivation. Phys. Status Solidi A 2009, 206, 2033–2037. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Babchenko, O.; Kromka, A. Study on cellular adhesion of human osteoblasts on nano-structured diamond films. Phys. Status Solidi B 2009, 246, 2774–2777. [Google Scholar] [CrossRef]
- Ferreira, M.R.W.; Fernandes, R.R.; Assis, A.F.; Dernowsek, J.A.; Passos, G.A.; Variola, F.; Bombonato-Prado, K.F. Oxidative nanopatterning of titanium surface influences mRNA and MicroRNA expression in human alveolar bone osteoblastic cells. Int. J. Biomater. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- James, A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- Karperien, M.; Roelen, B.A.; Poelmann, R.E.; Groot, A.C.G.-D.; Hierck, B.P.; DeRuiter, M.C.; Meijer, D.; Gibbs, S. Tissue formation during embryogenesis. Tissue Eng. 2015. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Vaes, B.L.T.; Dechering, K.J.; Feijen, A.; Hendriks, J.M.A.; Lefévre, C.; Mummery, C.L.; Olijve, W.; Van Zoelen, E.J.J.; Steegenga, W.T. Comprehensive microarray analysis of bone morphogenetic protein 2-induced osteoblast differentiation resulting in the identification of novel markers for bone development. J. Bone Miner. Res. 2002, 17, 2106–2118. [Google Scholar] [CrossRef]
- Mesa-Restrepo, A.; Alzate, J.F.; Patiño-Gonzalez, E. Bone morphogenetic protein 2: Heterologous expression and potential in bone regeneration. Actual. Biól. 2021, 43, 1–10, in press. [Google Scholar] [CrossRef]
- Xiao, M.; Biao, M.; Chen, Y.; Xie, M.; Yang, B.; Xiao, M.; Biao, M.; Chen, Y.; Xie, M.; Yang, B. Regulating the osteogenic function of rhBMP 2 by different titanium surface properties. J. Biomed. Mater. Res. Part A 2016, 104, 1882–1893. [Google Scholar] [CrossRef]
- Biao, M.N.; Chen, Y.M.; Xiong, S.B.; Wu, B.Y.; Yang, B.C. Synergistic effects of fibronectin and bone morphogenetic protein on the bioactivity of titanium metal. J. Biomed. Mater. Res. Part A 2017, 105, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti-Adam, E.A.; Aydin, D.; Hirschfeld-Warneken, V.C.; Spatz, J.P. Cell adhesion and response to synthetic nanopatterned environments by steering receptor clustering and spatial location. HFSP J. 2008, 2, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.; Kato, R.; Raucci, L.C.; Teixeira, L.; De Oliveira, F.; Bellesini, L.; De Oliveira, P.; Hassan, M.; Beloti, M. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J. Cell. Biochem. 2014, 115, 540–548. [Google Scholar] [CrossRef]
- Lotz, E.M.; Olivares-Navarrete, R.; Berner, S.; Boyan, B.D.; Schwartz, Z. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2016, 104, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Yagüe, M.; Antoñanzas, R.P.; Roa, J.J.; Biggs, M.; Gil, F.J.; Pegueroles, M. Enhanced osteoconductivity on electrically charged titanium implants treated by physicochemical surface modifications methods. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.B.; Freitas, G.P.; Elias, C.N.; Tye, C.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Rosa, A.L.; Beloti, M.M. Participation of integrin β3 in osteoblast differentiation induced by titanium with nano or microtopography. J. Biomed. Mater. Res. Part A 2019, 107, 1303–1313. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Nejadnik, M.R. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa-Restrepo, A.; Civantos, A.; Allain, J.P.; Patiño, E.; Alzate, J.F.; Balcázar, N.; Montes, R.; Pavón, J.J.; Rodríguez-Ortiz, J.A.; Torres, Y. Synergistic Effect of rhBMP-2 Protein and Nanotextured Titanium Alloy Surface to Improve Osteogenic Implant Properties. Metals 2021, 11, 464. https://doi.org/10.3390/met11030464
Mesa-Restrepo A, Civantos A, Allain JP, Patiño E, Alzate JF, Balcázar N, Montes R, Pavón JJ, Rodríguez-Ortiz JA, Torres Y. Synergistic Effect of rhBMP-2 Protein and Nanotextured Titanium Alloy Surface to Improve Osteogenic Implant Properties. Metals. 2021; 11(3):464. https://doi.org/10.3390/met11030464
Chicago/Turabian StyleMesa-Restrepo, Andrea, Ana Civantos, Jean Paul Allain, Edwin Patiño, Juan Fernando Alzate, Norman Balcázar, Robinson Montes, Juan José Pavón, José Antonio Rodríguez-Ortiz, and Yadir Torres. 2021. "Synergistic Effect of rhBMP-2 Protein and Nanotextured Titanium Alloy Surface to Improve Osteogenic Implant Properties" Metals 11, no. 3: 464. https://doi.org/10.3390/met11030464





