Effect of Short T6 Heat Treatment on the Thermal Conductivity and Mechanical Properties of Different Casting Processes Al-Si-Mg-Cu Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
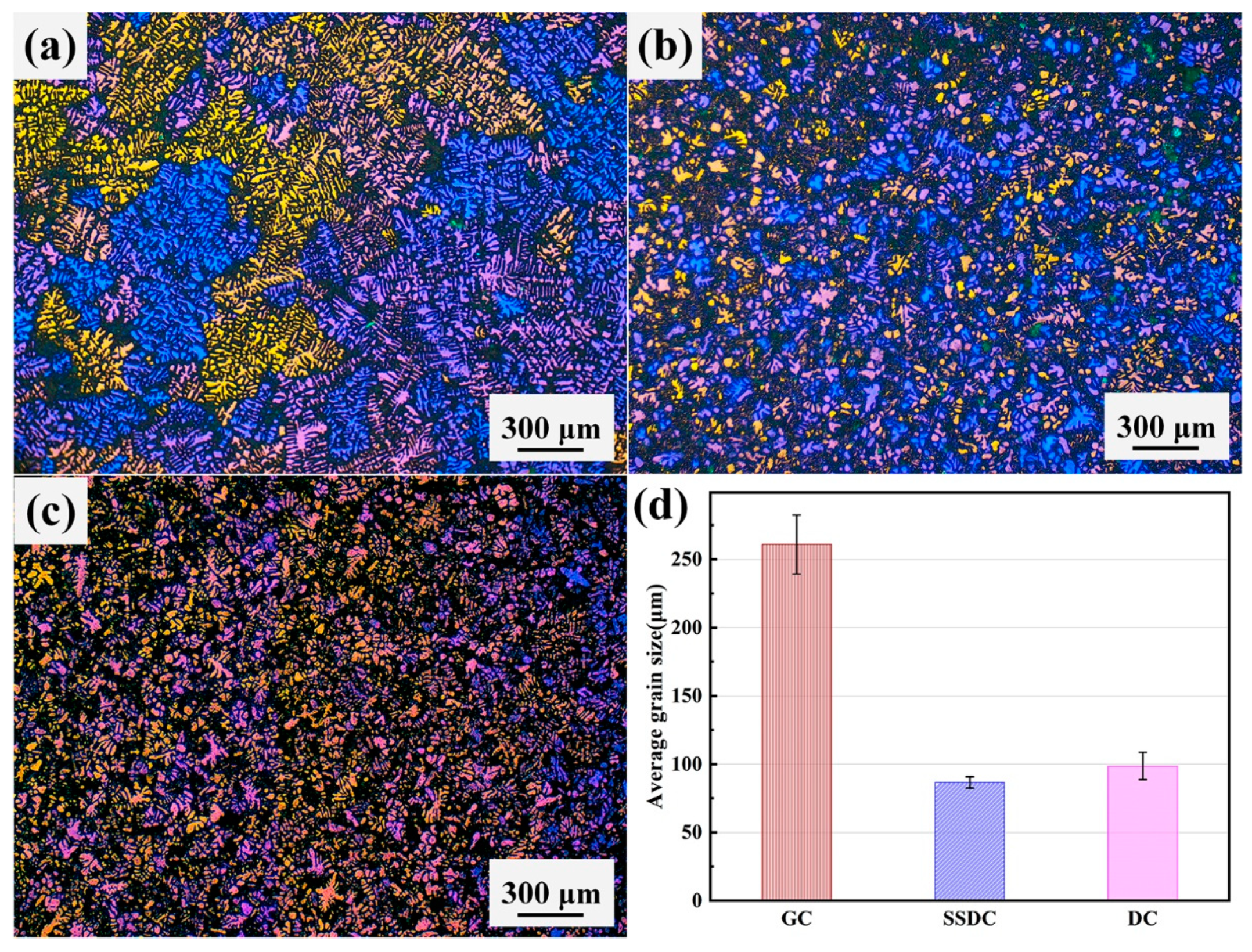
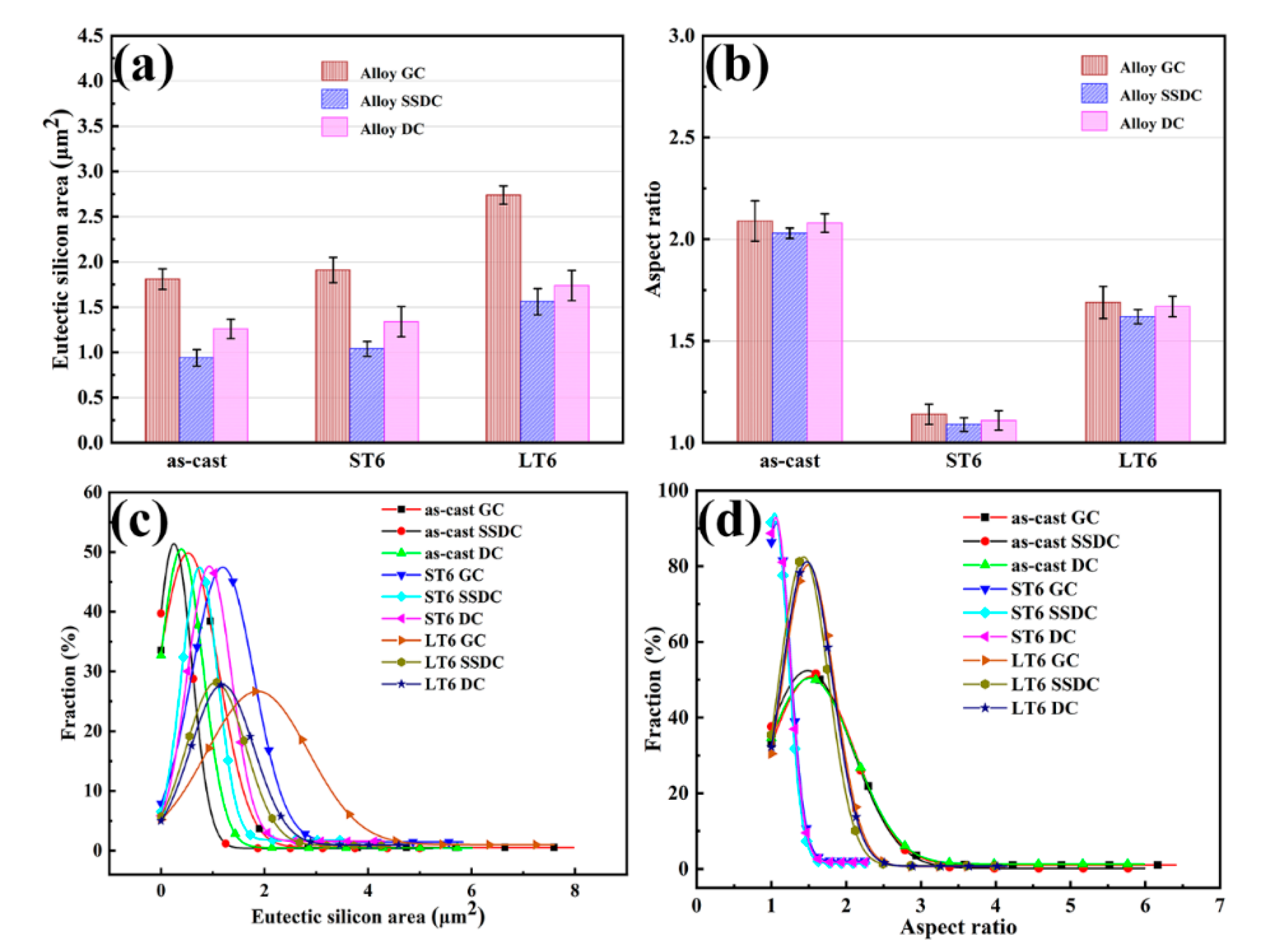
3.1. Microstructure of Alloy under Three Casting Processes
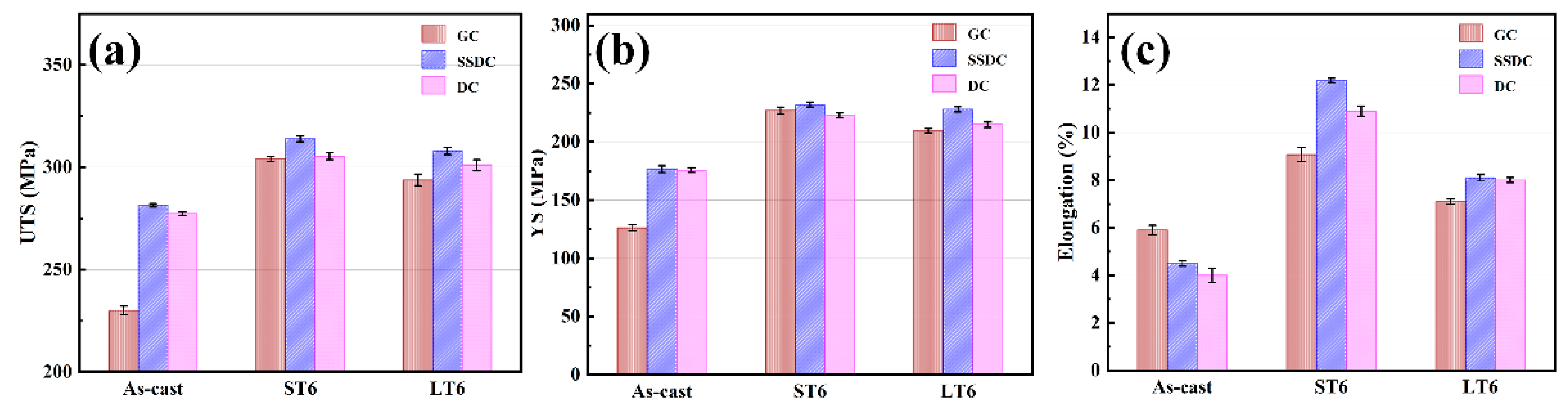
3.2. Mechanical Properties of Alloys under Three Casting Processes
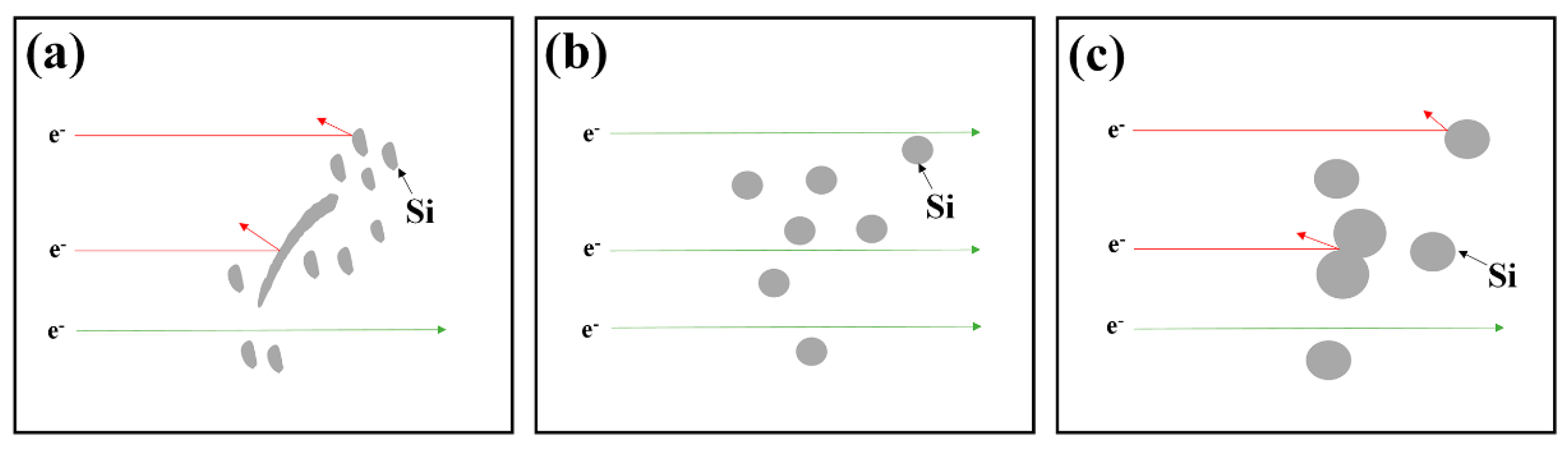
3.3. Electrical and Thermal Conductivity of Alloys under Three Casting Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sauvage, X.; Bobruk, E.V.; Murashkin, M.Y.; Nasedkina, Y.; Enikeev, N.A.; Valiev, R.Z. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al–Mg–Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Choi, S.-W.; Hong, S.-K. The behavior of thermal diffusivity change according to the heat treatment in Al-Si binary system. J. Alloys Compd. 2016, 687, 54–58. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh Strength and High Electrical Conductivity in Copper. Science 2004, 304, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiev, R.Z.; Murashkin, M.Y.; Sabirov, I. A nanostructural design to produce high-strength Al alloys with enhanced electrical conductivity. Scr. Mater. 2014, 76, 13–16. [Google Scholar] [CrossRef]
- Miyake, J.; Fine, M.E. Electrical conductivity versus strength in a precipitation hardened alloy. Acta Metall. Mater. 1992, 40, 733–741. [Google Scholar] [CrossRef]
- Sakai, Y.; Inoue, K.; Asano, T.; Wada, H.; Maeda, H. Development of high-strength, high-conductivity Cu-Ag alloys for high-field pulsed magnet use. Appl. Phys. Lett. 1991, 59, 2965–2967. [Google Scholar] [CrossRef]
- Alfonso, I.; Maldonado, C.; Gonzalez, G.; Bedolla, A. Effect of Mg content and solution treatment on the microstructure of Al-Si-Cu-Mg alloys. J. Mater. Sci. 2006, 41, 1945–1952. [Google Scholar] [CrossRef]
- Eisaabadi, B.G.; Yeom, G.Y.; Tiryakioğlu, M.; Netto, N.; Beygi, R.; Mehrizi, M.Z.; Kim, S.K. The effect of solution treatment time on the microstructure and ductility of naturally-aged A383 alloy die castings. Mater. Sci. Eng. A 2018, 722, 1–7. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, Y.-M.; Kim, Y.-C.; Kang, C.-S. Effects of alloying elements on mechanical and thermal characteristics of Al-6wt-%Si-0.4wt-%Mg–(Cu) foundry alloys. Mater. Sci. Technol. 2019, 35, 1365–1371. [Google Scholar] [CrossRef]
- Beroual, S.; Boumerzoug, Z.; Paillard, P.; Borjon-Piron, Y. Effects of heat treatment and addition of small amounts of Cu and Mg on the microstructure and mechanical properties of Al-Si-Cu and Al-Si-Mg cast alloys. J. Alloys Compd. 2019, 784, 1026–1035. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zheng, L.H.; StJohn, D.H. Effect of a short solution treatment time on microstructure and mechanical properties of modified Al–7wt.%Si–0.3wt.%Mg alloy. J. Light Met. 2002, 2, 27–36. [Google Scholar] [CrossRef]
- Pedersen, L.; Arnberg, L. The effect of solution heat treatment and quenching rates on mechanical properties and microstructures in AlSiMg foundry alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 525–532. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Arnberg, L.; Zhang, D.L. Modelling dissolution of Mg2Si and homogenisation in Al-Si-Mg casting alloys. Int. J. Cast Met. Res. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Cai, Q.; Mendis, C.L.; Chang, I.T.H.; Fan, Z. Effect of short T6 heat treatment on the microstructure and the mechanical properties of newly developed die-cast Al–Si–Mg–Mn alloys. Mater. Sci. Eng. A 2020, 788. [Google Scholar] [CrossRef]
- Menargues, S.; Martín, E.; Baile, M.T.; Picas, J.A. New short T6 heat treatments for aluminium silicon alloys obtained by semisolid forming. Mater. Sci. Eng. A 2015, 621, 236–242. [Google Scholar] [CrossRef]
- Choi, S.W.; Cho, H.S.; Kang, C.S.; Kumai, S. Precipitation dependence of thermal properties for Al–Si–Mg–Cu–(Ti) alloy with various heat treatment. J. Alloys Compd. 2015, 647, 1091–1097. [Google Scholar] [CrossRef]
- Lados, D.A.; Apelian, D.; Wang, L. Solution Treatment Effects on Microstructure and Mechanical Properties of Al-(1 to 13 pct)Si-Mg Cast Alloys. Metall. Mater. Trans. B 2010, 42, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Vandersluis, E.; Ravindran, C. Effects of solution heat treatment time on the as-quenched microstructure, hardness and electrical conductivity of B319 aluminum alloy. J. Alloys Compd. 2020, 838. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, J.M.; Kim, M.S. A new approach to the design of a low Si-added Al–Si casting alloy for optimising thermal conductivity and fluidity. J. Mater. Sci. 2015, 50, 7271–7281. [Google Scholar] [CrossRef]
- Lumley, R.N.; Deeva, N.; Larsen, R.; Gembarovic, J.; Freeman, J. The Role of Alloy Composition and T7 Heat Treatment in Enhancing Thermal Conductivity of Aluminum High Pressure Diecastings. Metall. Mater. Trans. A 2012, 44, 1074–1086. [Google Scholar] [CrossRef]
- Olafsson, P.; Sandstrom, R.; Karlsson, Å. Comparison of experimental, calculated and observed values for electrical and thermal conductivity of aluminium alloys. J. Mater. Sci. 1997, 32, 4383–4390. [Google Scholar] [CrossRef]
- Lin, Y.C.; Luo, S.-C.; Huang, J.; Yin, L.-X.; Jiang, X.-Y. Effects of solution treatment on microstructures and micro-hardness of a Sr-modified Al-Si-Mg alloy. Mater. Sci. Eng. A 2018, 725, 530–540. [Google Scholar] [CrossRef]
- Shivkumar, S.; Ricci, S.; Keller, C.; Apelian, D. Effect of solution treatment parameters on tensile properties of cast aluminum alloys. J. Heat Treat. 1990, 8, 63–70. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Wang, Z.; Gao, A. Achievement of High Strength and Ductility in Al-Si-Cu-Mg Alloys by Intermediate Phase Optimization in As-Cast and Heat Treatment Conditions. Materials 2020, 13, 647. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Xu, X.; Zhao, Y.; Wang, Z.; Wu, C.; Pan, D.; Meng, Z. Effect of the addition of Sr modifier in different conditions on microstructure and mechanical properties of T6 treated Al-Mg2Si in-situ composite. Mater. Sci. Eng. A 2018, 721, 263–273. [Google Scholar] [CrossRef]
- Dong, X.; Yang, H.; Zhu, X.; Ji, S. High strength and ductility aluminium alloy processed by high pressure die casting. J. Alloys Compd. 2019, 773, 86–96. [Google Scholar] [CrossRef]
- Chawla, N.; Shen, Y.L. Mechanical Behavior of Particle Reinforced Metal Matrix Composites. Adv. Eng. Mater. 2001, 3, 357–370. [Google Scholar] [CrossRef]
- Vandersluis, E.; Ravindran, C. Influence of solidification rate on the microstructure, mechanical properties, and thermal conductivity of cast A319 Al alloy. J. Mater. Sci. 2019, 54, 4325–4339. [Google Scholar] [CrossRef]
- Cui, X.; Cui, H.; Wu, Y.; Liu, X. The improvement of electrical conductivity of hypoeutectic Al-Si alloys achieved by composite melt treatment. J. Alloys Compd. 2019, 788, 1322–1328. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, Y.M.; Lee, K.M.; Cho, H.S.; Hong, S.K.; Kim, Y.C.; Kang, C.S.; Kumai, S. The effects of cooling rate and heat treatment on mechanical and thermal characteristics of Al–Si–Cu–Mg foundry alloys. J. Alloys Compd. 2014, 617, 654–659. [Google Scholar] [CrossRef]
- Lumley, R.N.; Polmear, I.J.; Groot, H.; Ferrier, J. Thermal characteristics of heat-treated aluminum high-pressure die-castings. Scr. Mater. 2008, 58, 1006–1009. [Google Scholar] [CrossRef]



| Si | Cu | Mg | Fe | Sr | B | Al |
|---|---|---|---|---|---|---|
| 7.933 | 0.099 | 0.512 | 0.551 | 0.080 | 0.010 | Bal. |
| Alloy | Status | Area Size (μm2) | Aspect Ratio |
|---|---|---|---|
| As-cast | 1.81 | 2.09 | |
| GC | ST6 | 1.91 | 1.14 |
| LT6 | 2.74 | 1.69 | |
| As-cast | 0.94 | 2.03 | |
| SSDC | ST6 | 1.04 | 1.09 |
| LT6 | 1.56 | 1.62 | |
| As-cast | 1.26 | 2.08 | |
| DC | ST6 | 1.34 | 1.11 |
| LT6 | 1.74 | 1.67 |
| Alloy | Status | UTS (MPa) | YS (MPa) | Elongation (%) |
|---|---|---|---|---|
| As-cast | 230.1 ± 2.1 | 126.1 ± 2.7 | 5.9 ± 0.21 | |
| GC | ST6 | 304 ± 1.2 | 227 ± 2.8 | 9.08 ± 0.32 |
| LT6 | 293.7 ± 2.7 | 209.4 ± 2.1 | 7.8 ± 0.11 | |
| As-cast | 281.4 ± 0.9 | 176.5 ± 2.8 | 4.5 ± 0.12 | |
| SSDC | ST6 | 313.87 ± 1.5 | 232 ± 2 | 12.19 ± 0.09 |
| LT6 | 308 ± 1.8 | 228 ± 2.2 | 8.1 ± 0.13 | |
| As-cast | 279.4 ± 0.9 | 175.8 ± 2 | 4 ± 0.30 | |
| DC | ST6 | 305.4 ± 1.7 | 223 ± 2.1 | 10.9 ± 0.22 |
| LT6 | 301 ± 2.7 | 215 ± 2.6 | 8 ± 0.11 |
| Alloy | Status | Electrical Conductivity (% IACS) | Thermal Conductivity (W/(m·K)) |
|---|---|---|---|
| As-cast | 36.24 ± 0.18 | 152.9 ± 0.69 | |
| GC | ST6 | 40.96 ± 0.19 | 171.2 ± 0.82 |
| LT6 | 38.79 ± 0.18 | 162.7 ± 0.68 | |
| As-cast | 37.94 ± 0.13 | 159.6 ± 0.48 | |
| SSDC | ST6 | 42.31 ± 0.2 | 176.4 ± 0.76 |
| LT6 | 40.52 ± 0.19 | 169.6 ± 0.73 | |
| As-cast | 37.07 ± 0.1 | 156.2 ± 0.42 | |
| DC | ST6 | 41.78 ± 0.1 | 174.4 ± 0.39 |
| LT6 | 39.78 ± 0.17 | 166.6 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.-Q.; Wang, J.-G.; Guan, Z.-P.; Ma, P.-K.; Zhao, P.; Li, Z.-J.; Lu, T.-S.; Yan, R.-F. Effect of Short T6 Heat Treatment on the Thermal Conductivity and Mechanical Properties of Different Casting Processes Al-Si-Mg-Cu Alloys. Metals 2021, 11, 1450. https://doi.org/10.3390/met11091450
Dong Z-Q, Wang J-G, Guan Z-P, Ma P-K, Zhao P, Li Z-J, Lu T-S, Yan R-F. Effect of Short T6 Heat Treatment on the Thermal Conductivity and Mechanical Properties of Different Casting Processes Al-Si-Mg-Cu Alloys. Metals. 2021; 11(9):1450. https://doi.org/10.3390/met11091450
Chicago/Turabian StyleDong, Zhong-Qiang, Jin-Guo Wang, Zhi-Ping Guan, Pin-Kui Ma, Po Zhao, Zhu-Jin Li, Tian-Shi Lu, and Rui-Fang Yan. 2021. "Effect of Short T6 Heat Treatment on the Thermal Conductivity and Mechanical Properties of Different Casting Processes Al-Si-Mg-Cu Alloys" Metals 11, no. 9: 1450. https://doi.org/10.3390/met11091450






