Facile Synthesis of PdCuRu Porous Nanoplates as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Pd Nanoplates
2.3. Synthesis of PdCuRu Nanoplates with Different Compositions
2.4. Phase-Transfer of PdCuRu Nanoplates into Hydrophobic Solvent
2.5. Morphological, Structural, and Compositional Characterizations
2.6. Electrochemical Measurements
2.7. Measurements of Electrochemical Active Surface Area (ECSA)
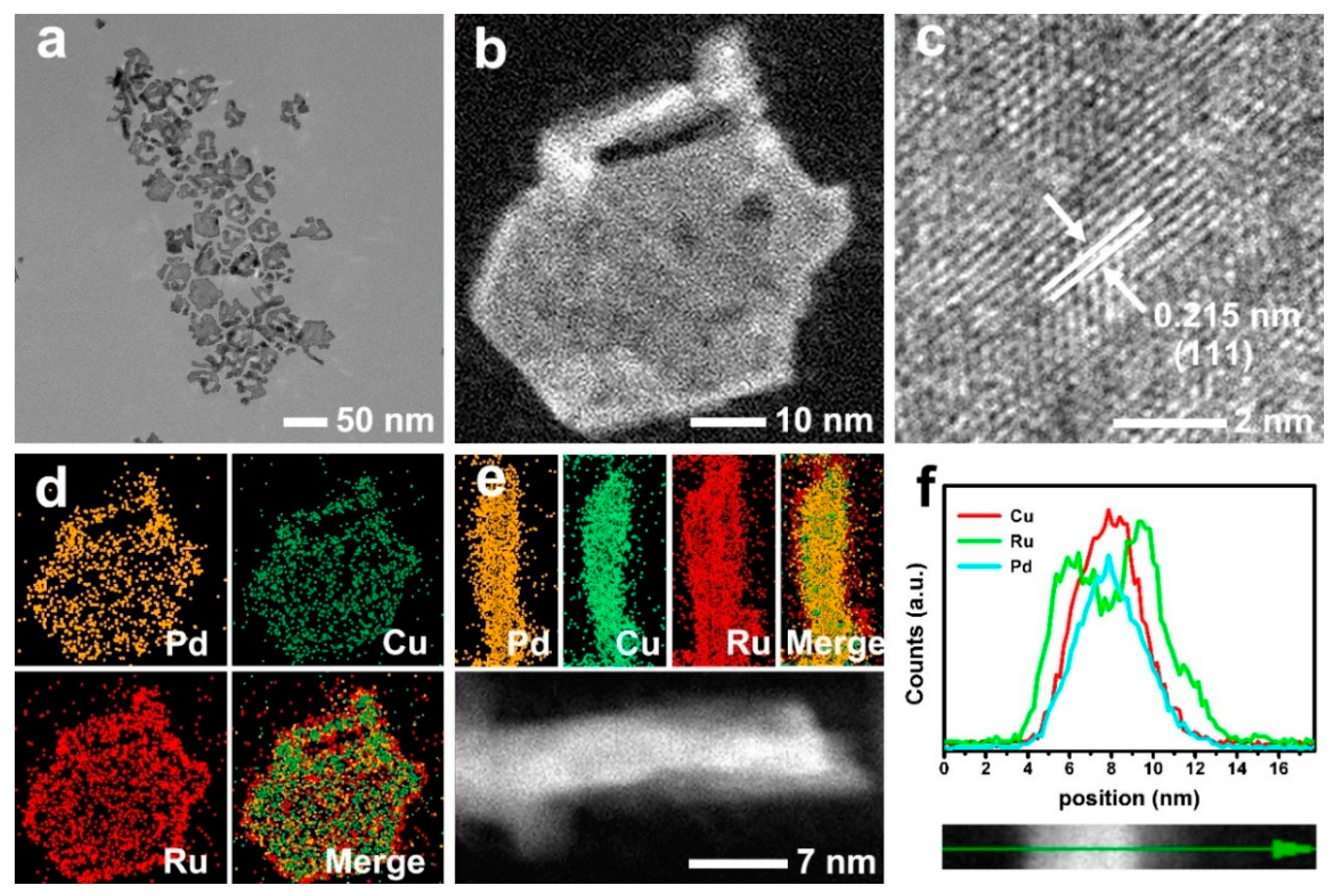
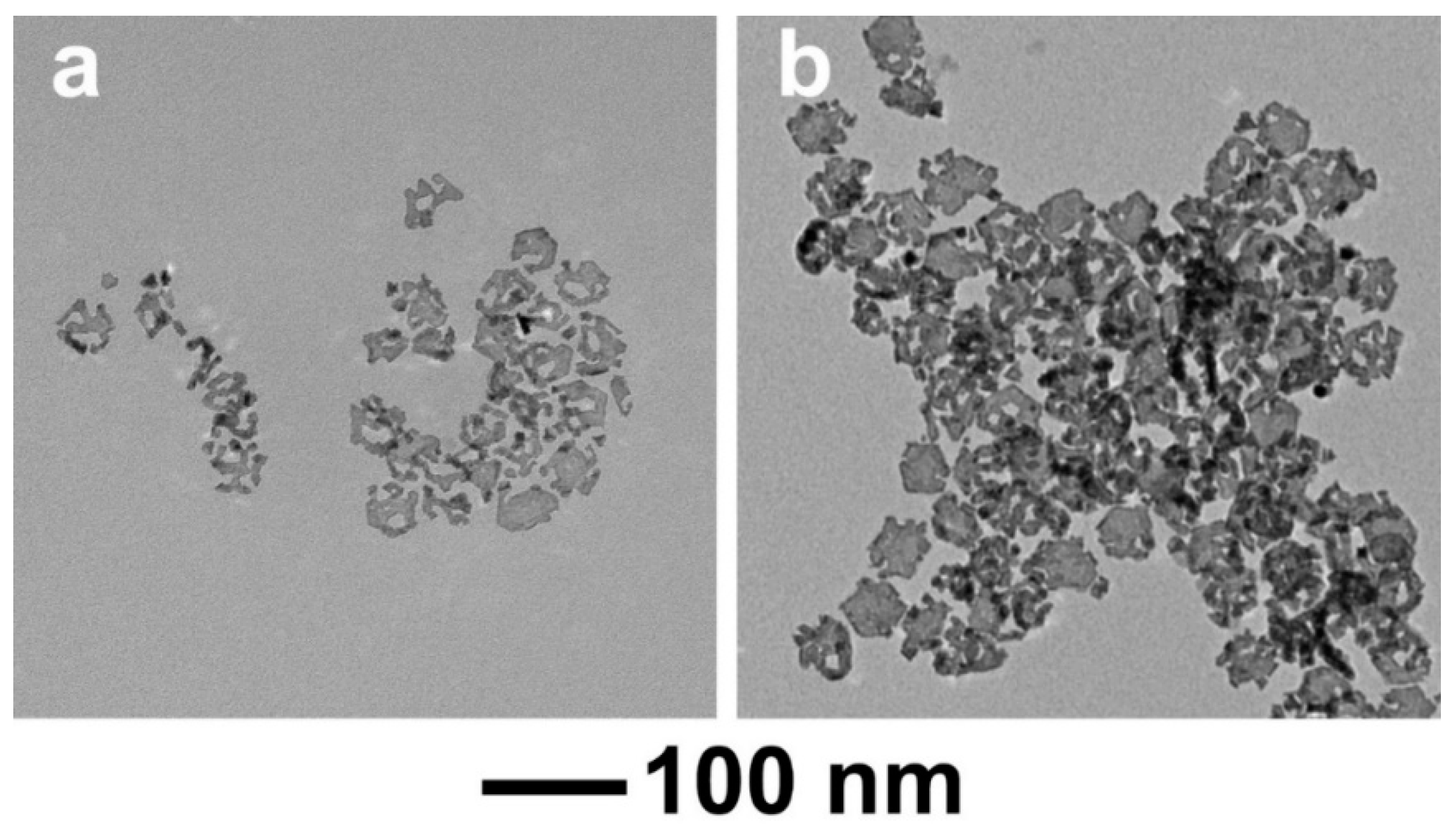
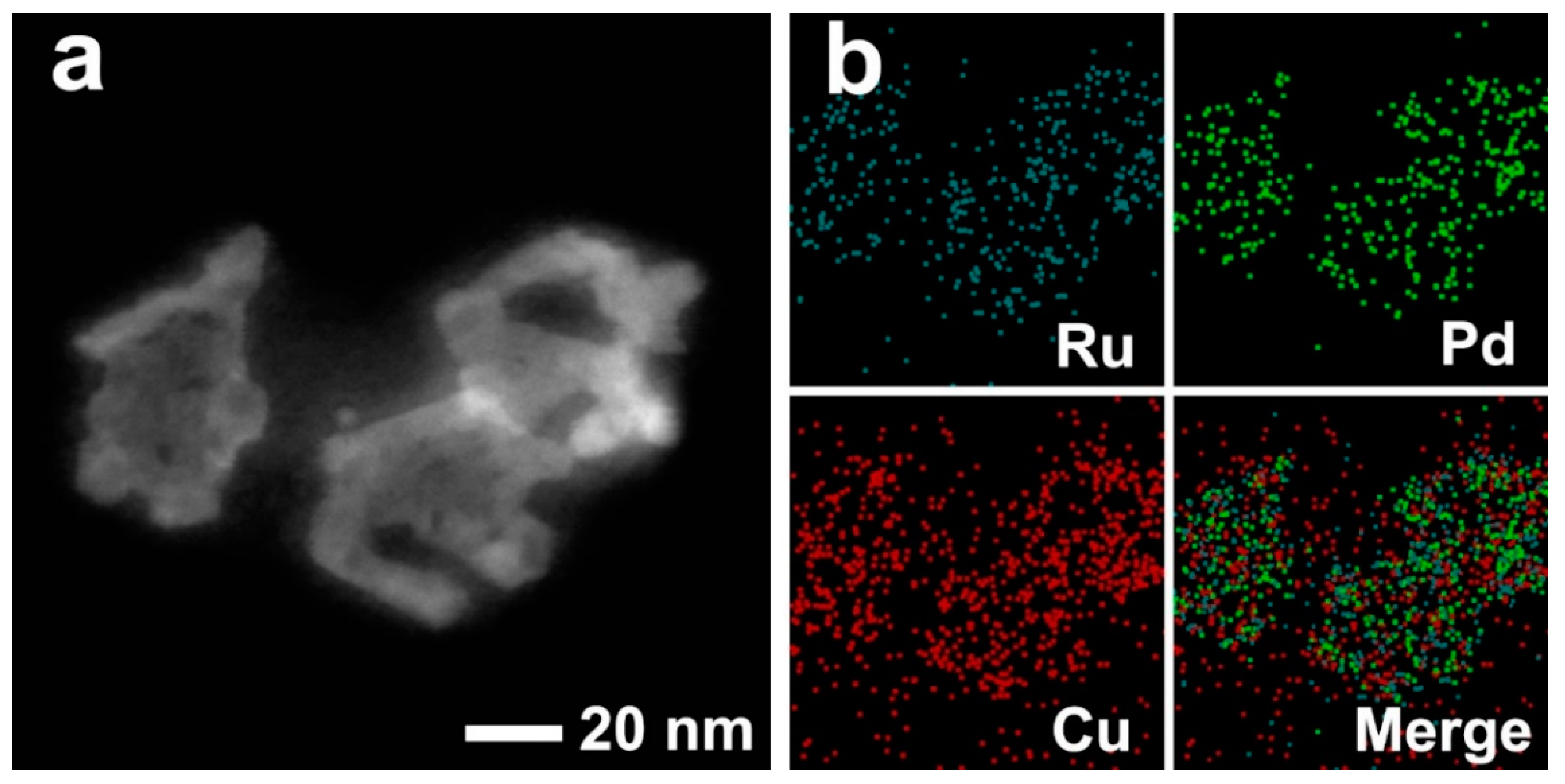
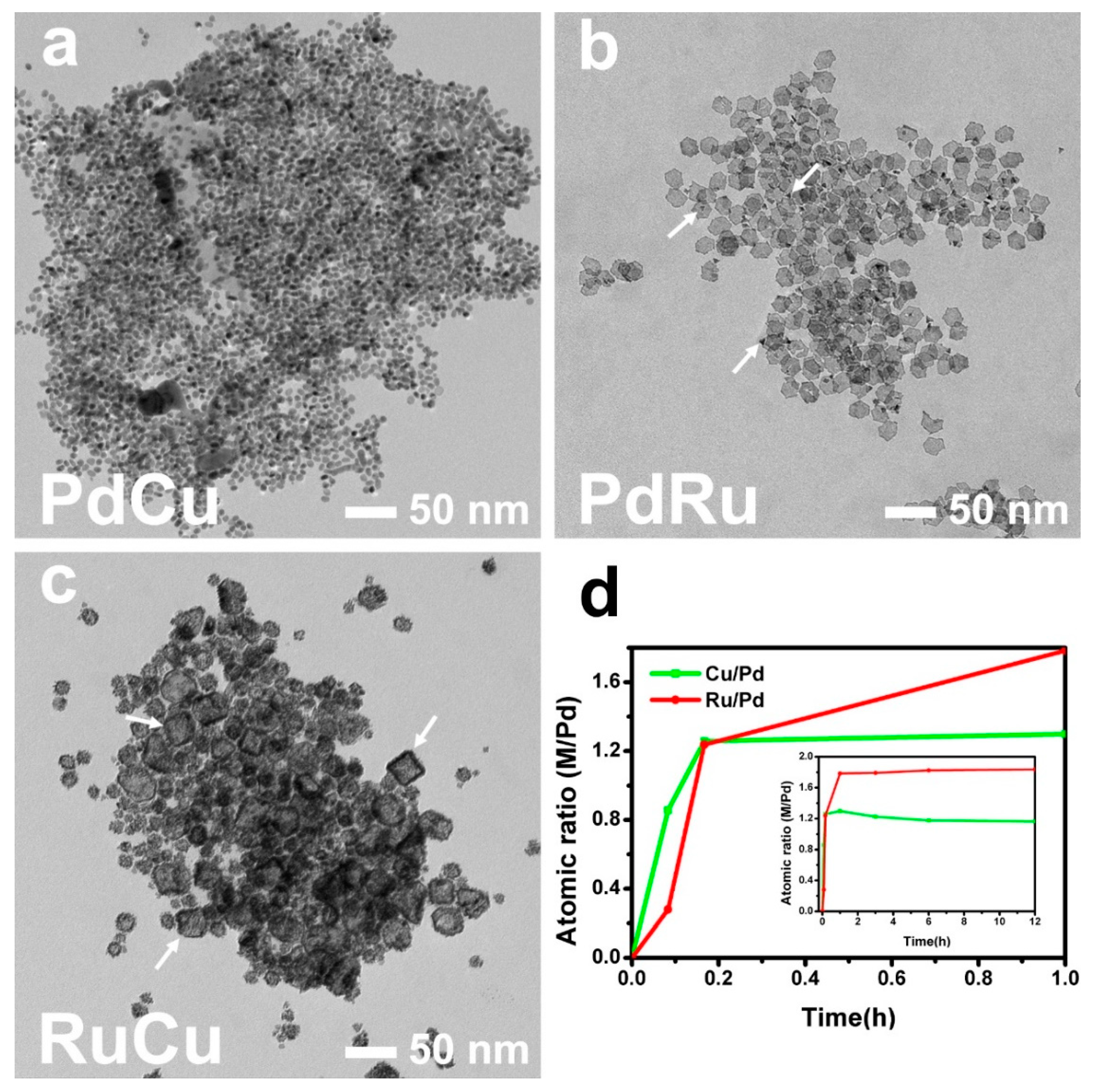
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wu, X.; Yan, Y.; Luo, S.; Li, X.; Huang, J.; Zhang, H.; Yang, D. Coupling PtNi ultrathin nanowires with MXenes for boosting electrocatalytic hydrogen evolution in both acidic and alkaline Solutions. Small 2019, 15, 1805474. [Google Scholar] [CrossRef] [PubMed]
- Ataee-Esfahani, H.; Imura, M.; Yamauchi, Y. All-metal mesoporous nanocolloids: Solution-phase synthesis of core-shell Pd@Pt nanoparticles with a designed concave surface. Angew. Chem. 2013, 52, 13611–13615. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Bhavsar, S.; Najera, M.; Solunke, R.; Veser, G. Chemical looping: To combustion and beyond. Catal. Today 2014, 228, 96–105. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Liu, T.F.; Li, J.J.; Li, B.; Liu, Y.F.; Zhang, B.S.; Xiong, D.H.; Amorim, I.; Li, W.; Liu, L.F. Boosting the hydrogen evolution performance of ruthenium clusters through synergistic coupling with cobalt phosphide. Energy Environ. Sci. 2018, 11, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Durst, J.; Siebel, A.; Simon, C.; Hasche, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- Geng, Z.; Liu, Y.; Kong, X.; Li, P.; Li, K.; Liu, Z.; Du, J.; Shu, M.; Si, R.; Zeng, J. Achieving a record-high yield rate of 120.9 μgNH3 mg−1cat h−1 for N2 electrochemical reduction over Ru single-atom catalysts. Adv. Mater. 2018, 30, 1803498. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef]
- Li, W.D.; Liu, Y.; Wu, M.; Feng, X.L.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.L.; Wu, K.F.; Liu, Z.Y.; et al. Carbon-quantum-dots-loaded ruthenium nanoparticles as an efficient electrocatalyst for hydrogen production in alkaline media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef]
- Ge, J.; He, D.; Bai, L.; You, R.; Lu, H.; Lin, Y.; Tan, C.; Kang, Y.B.; Xiao, B.; Wu, Y.; et al. Ordered porous Pd octahedra covered with mono layer Ru atoms. J. Am. Chem. Soc. 2015, 137, 14566–14569. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, B.; Wang, X. Engineering nanointerfaces for nanocatalysis. Chem. Soc. Rev. 2014, 43, 7870–7886. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Shoemaker, K.; Peles, A.; Kaneko, K.; Protsailo, L. Pt mono layer on porous Pd-Cu alloys as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 9253–9255. [Google Scholar] [CrossRef]
- Xiong, Y.; Shan, H.; Zhou, Z.; Yan, Y.; Chen, W.; Yang, Y.; Liu, Y.; Tian, H.; Wu, J.; Zhang, H.; et al. Tuning surface structure and strain in Pd–Pt core–shell nanocrystals for enhanced electrocatalytic oxygen reduction. Small 2017, 13, 1603423. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhao, H.; Huang, B.; Li, B.; Li, H.; Han, Y.; Wang, Z.; Wu, X.; Pan, Y.; Sun, Y.; et al. Advanced ultrathin RuPdM (M = Ni, Co, Fe) nanosheets electrocatalyst boosts hydrogen evolution. ACS Central Science 2019, 5, 1991–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Huang, X.; Tan, C.; Zhang, H. Thin metal nanostructures: Synthesis, properties and applications. Chem. Sci. 2015, 6, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liu, Y.; Chen, B.; Gong, Y.; Gu, L.; Fan, Z.; Yang, N.; Lai, Z.; Chen, Y.; Wang, J.; et al. Submonolayered Ru deposited on ultrathin Pd nanosheets used for enhanced catalytic applications. Adv. Mater. 2016, 28, 10282–10286. [Google Scholar] [CrossRef]
- Yin, X.; Liu, X.; Pan, Y.T.; Walsh, K.A.; Yang, H. Hanoi tower-like multi layered ultrathin palladium nanosheets. Nano Lett. 2014, 14, 7188–7194. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, Y.; Wi, D.H.; Lee, S.; Lee, S.U.; Lee, Y.W.; Choi, S.I.; Han, S.W. Ultrathin free-standing ternary-alloy nanosheets. Angew. Chem. 2016, 55, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yan, N.; Yu, R.; Chang, C.R.; Zhou, G.; Hu, H.S.; Rong, H.; Niu, Z.; Mao, J.; Asakura, H.; et al. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Li, X.; Tang, M.; Zhong, H.; Huang, J.; Bian, T.; Jiang, Y.; Han, Y.; Zhang, H.; Yang, D. Tailoring the edge sites of 2D Pd nanostructures with different fractal dimensions for enhanced electrocatalytic performance. Adv. Sci. 2018, 5, 1800430. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Shifa, T.A.; Wen, Y.; Wang, F.; Zhan, X.; Wang, Q.; Xu, K.; Huang, Y.; Yin, L.; et al. Two-dimensional non-layered materials: Synthesis, properties and applications. Adv. Funct. Mater. 2017, 27, 1603254. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Grzelczak, M. Growing anisotropic crystals at the nanoscale. Science 2017, 356, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-dimensional metal nanomaterials: Synthesis, properties, and applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef]
- Ding, J.; Shao, Q.; Feng, Y.; Huang, X. Ruthenium-nickel sandwiched nanoplates for efficient water splitting electrocatalysis. Nano Energy 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-rich RuCu nanosheets for pH-universal overall water splitting electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef]
- Yan, Y.; Shan, H.; Li, G.; Xiao, F.; Jiang, Y.; Yan, Y.; Jin, C.; Zhang, H.; Wu, J.; Yang, D. Epitaxial growth of multimetallic Pd@PtM (M = Ni, Rh, Ru) core–shell nanoplates realized by in situ-produced CO from interfacial catalytic reactions. Nano Lett. 2016, 16, 7999–8004. [Google Scholar] [CrossRef]
- Kang, Y.; Snyder, J.; Chi, M.; Li, D.; More, K.; Markovic, N.; Stamenkovic, V. Multimetallic core/interlayer/shell nanostructures as advanced electrocatalysts. Nano Lett. 2014, 14, 6361–6367. [Google Scholar] [CrossRef]
- Arán-Ais, R.; Dionigi, F.; Merzdorf, T.; Gocyla, M.; Heggen, M.; Dunin-Borkowski, R.; Gliech, M.; Solla-Gullón, J.; Herrero, E.; Feliu, J.; et al. Elemental anisotropic growth and atomic-scale structure of shape-controlled octahedral Pt-Ni-Co alloy nanocatalysts. Nano Lett. 2015, 15, 7473–7480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Wang, A.; Gong, Y.; Hao, W.; Cheng, H.; Chen, J.; Li, B.; Yang, N.; Niu, W.; Wang, J.; et al. Crystal phase-based epitaxial growth of hybrid noble metal nanostructures on 4H/fcc Au nanowires. Nat. Chem. 2018, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, Y.; Li, Y.; Zhang, H.; Li, D.; Yang, D. Size-controlled synthesis of Pd nanosheets for tunable plasmonic properties. CrystEngComm 2015, 17, 1833–1838. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Xia, K.; Zhang, W.; Jiang, Y.; Zeng, Y.; Zhang, H.; Jin, C.; Zhang, Z.; Yang, D. Ultrathin two-dimensional Pd-based nanorings as catalysts for hydrogenation with high activity and stability. Small 2015, 11, 4745–4752. [Google Scholar] [CrossRef]
- Han, Y.; Yan, Y.; Wu, Z.; Jiang, Y.; Li, X.; Xu, Q.; Yang, X.; Zhang, H.; Yang, D. Facile synthesis of Pd@Ru nanoplates with controlled thickness as efficient catalysts for hydrogen evolution reaction. CrystEngComm 2018, 20, 4230–4236. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Li, G.; Huang, B.; Pan, J.; Liu, Q.; Han, J.; Xiao, L.; Lu, J.; Zhuang, L. Pt-Ru catalyzed hydrogen oxidation in alkaline media: Oxophilic effect or electronic effect? Energy Environ. Sci. 2015, 8, 177–181. [Google Scholar] [CrossRef]
- Lu, B.; Guo, L.; Wu, F.; Peng, Y.; Lu, J.E.; Smart, T.J.; Wang, N.; Finfrock, Y.Z.; Morris, D.; Zhang, P.; et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media. Nat. Commun. 2019, 10, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Sun, J.; Zhang, Y.; Wang, B.; Li, Q.; Fan, Y. Facile synthesis of urchin-like RuCu and hollow RuCuMo nanoparticles and preliminary insight to their formation process by cyclic voltammetry. RSC Adv. 2018, 8, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, Z.; Lyu, Z.; Hood, Z.; Xie, M.; Vara, M.; Chi, M.; Xia, Y. Ru octahedral nanocrystals with a face-centered cubic structure, {111} facets, thermal stability up to 400 °C, and enhanced catalytic activity. J. Am. Chem. Soc. 2019, 141, 7028–7036. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Han, Y.; Cong, N.; Jin, L.; Tan, L.; Chen, H.; Zhai, C.; Zhou, X.; Fang, H.; Zhu, Y. Hydrothermal synthesis of spherical Ru with high efficiency hydrogen evolution activity. J. Electroanal. Chem. 2019, 848, 11320. [Google Scholar] [CrossRef]
- Bian, T.; Liu, H.; Sun, B.; Xiao, B.; Jiang, Y.; Jin, C.; Yuan, A.; Zhang, H.; Yang, D. Ion-templated fabrication of Pt-Cu alloy octahedra with controlled compositions for electrochemical detection of H2O2. J. Alloy Compd. 2019, 788, 1334–1340. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Li, X.; Qiao, X.; Wu, X.; Li, J.; Shen, R.; Yang, D.; Zhang, H. Unexpected Kirkendall effect in twinned icosahedral nanocrystals driven by strain gradient. Nano Res. 2020, 13, 2641–2649. [Google Scholar] [CrossRef]
- Chen, A.; Endres, E.; Ashberry, H.; Bueno, S.; Chen, Y.; Skrabalak, S. Galvanic replacement of intermetallic nanocrystals as a route toward complex heterostructures. Nanoscale 2021, 13, 2618–2625. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Reggiano, G.; Thota, S.; Wang, Y.; Kerns, P.; Suib, S.; Zhao, J. Au–Cu–M (M = Pt, Pd, Ag) nanorods with enhanced catalytic efficiency by galvanic replacement reaction. Chem. Commun. 2019, 55, 1249–1252. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen-evolution electrocatalyst. Angew. Chem. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Wu, X.Q.; Jiang, Y.; Yan, Y.; Li, X.; Luo, S.; Huang, J.; Li, J.; Shen, R.; Yang, D.; Zhang, H. Tuning surface structure of Pd3Pb-PtnPb nanocrystals for boosting the methanol oxidation reaction. Adv. Sci. 2019, 6, 1902249. [Google Scholar] [CrossRef] [Green Version]
- Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavičius, S.; Kirtinaitiene, M.; Trusovas, R. Substrate impact on the structure and electrocatalyst properties of molybdenum disulfide for HER from water. Metals 2020, 10, 1251. [Google Scholar] [CrossRef]
- Barman, B.; Sarkar, B.; Nanda, K. Pd-coated Ru nanocrystals supported on N-doped graphene as HER and ORR electrocatalysts. Chem. Commun. 2019, 55, 13928–13931. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Qian, N.; Li, J.; Li, X.; Yang, D.; Zhang, H. Facile Synthesis of PdCuRu Porous Nanoplates as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium. Metals 2021, 11, 1451. https://doi.org/10.3390/met11091451
Chen C, Qian N, Li J, Li X, Yang D, Zhang H. Facile Synthesis of PdCuRu Porous Nanoplates as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium. Metals. 2021; 11(9):1451. https://doi.org/10.3390/met11091451
Chicago/Turabian StyleChen, Changhong, Ningkang Qian, Junjie Li, Xiao Li, Deren Yang, and Hui Zhang. 2021. "Facile Synthesis of PdCuRu Porous Nanoplates as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium" Metals 11, no. 9: 1451. https://doi.org/10.3390/met11091451






